Accumulation of Agmatine, Spermidine, and Spermine in Sprouts and Microgreens of Alfalfa, Fenugreek, Lentil, and Daikon Radish
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Seed Sprouting
2.3. Growing Microgreens
2.4. Sample Preparation
2.4.1. Extraction Procedure
2.4.2. Freezing and Thawing
2.4.3. Fenugreek Sprouts as a Source of Amine Oxidases
2.5. Preparation of Standard Solutions and Derivatization
2.5.1. Internal Standard
2.5.2. Amine Standards and Calibration Solutions
2.5.3. Derivatization Procedure with Dansyl Chloride (DNS–Cl)
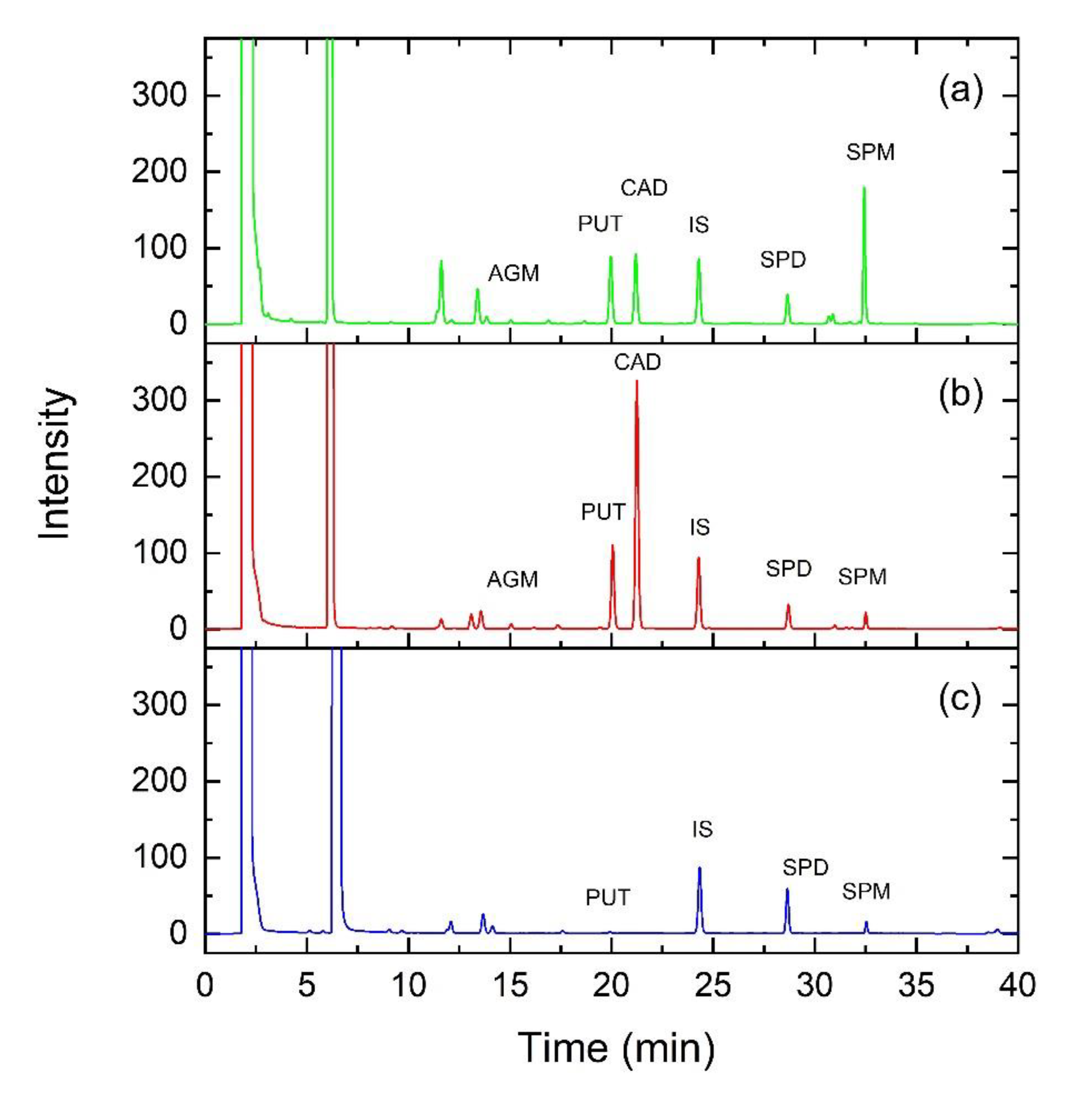
2.6. HPLC Analyses
2.7. Statistical Analysis
3. Results and Discussion
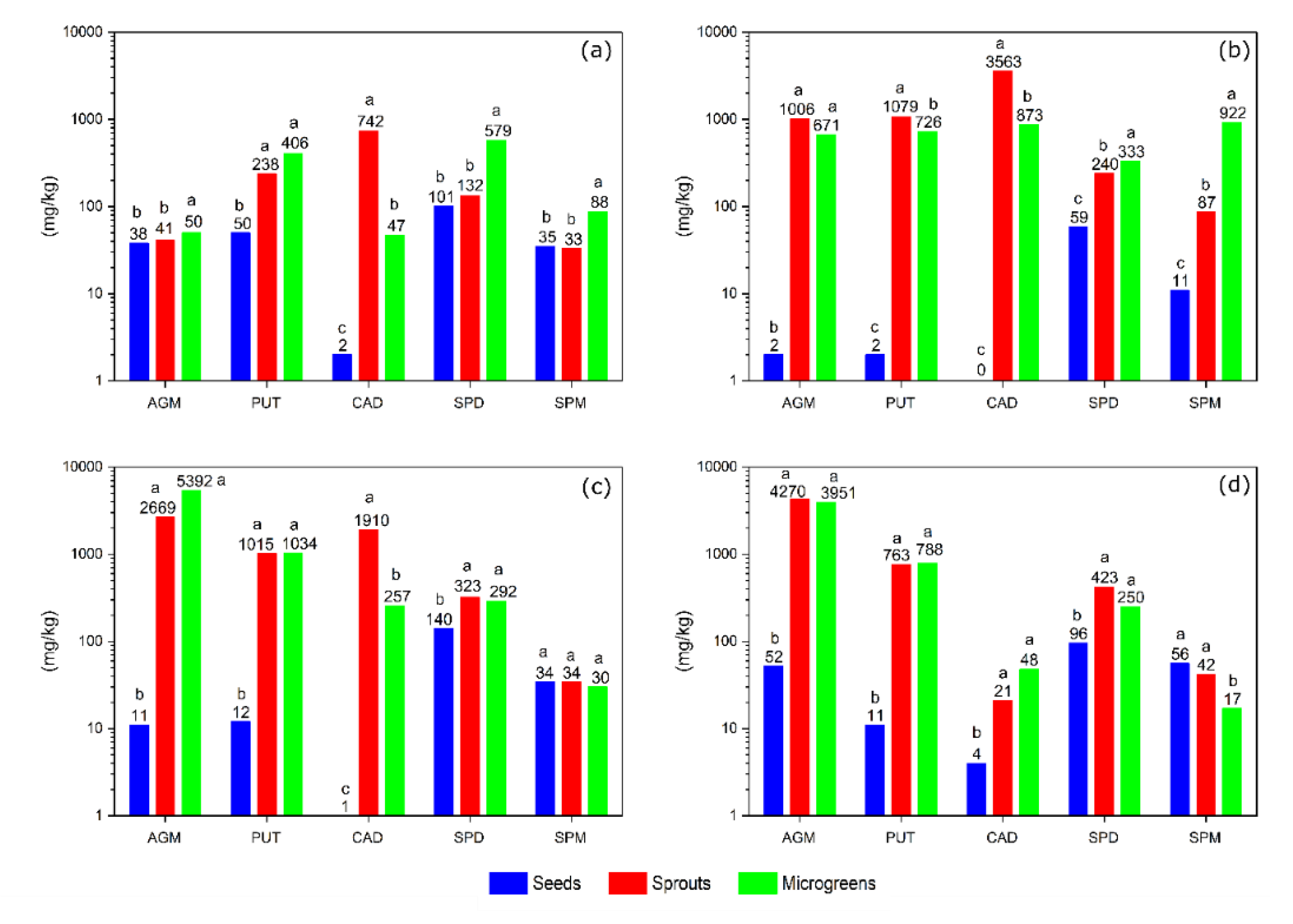
3.1. Polyamine Content in Sprouts and Microgreens of Lentil, Fenugreek, Alfalfa, and Daikon Radish
3.1.1. Lentil
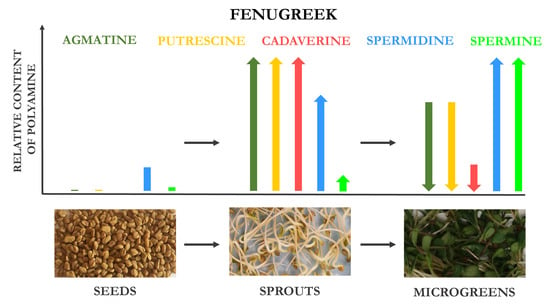
3.1.2. Fenugreek
3.1.3. Alfalfa
3.1.4. Daikon Radish
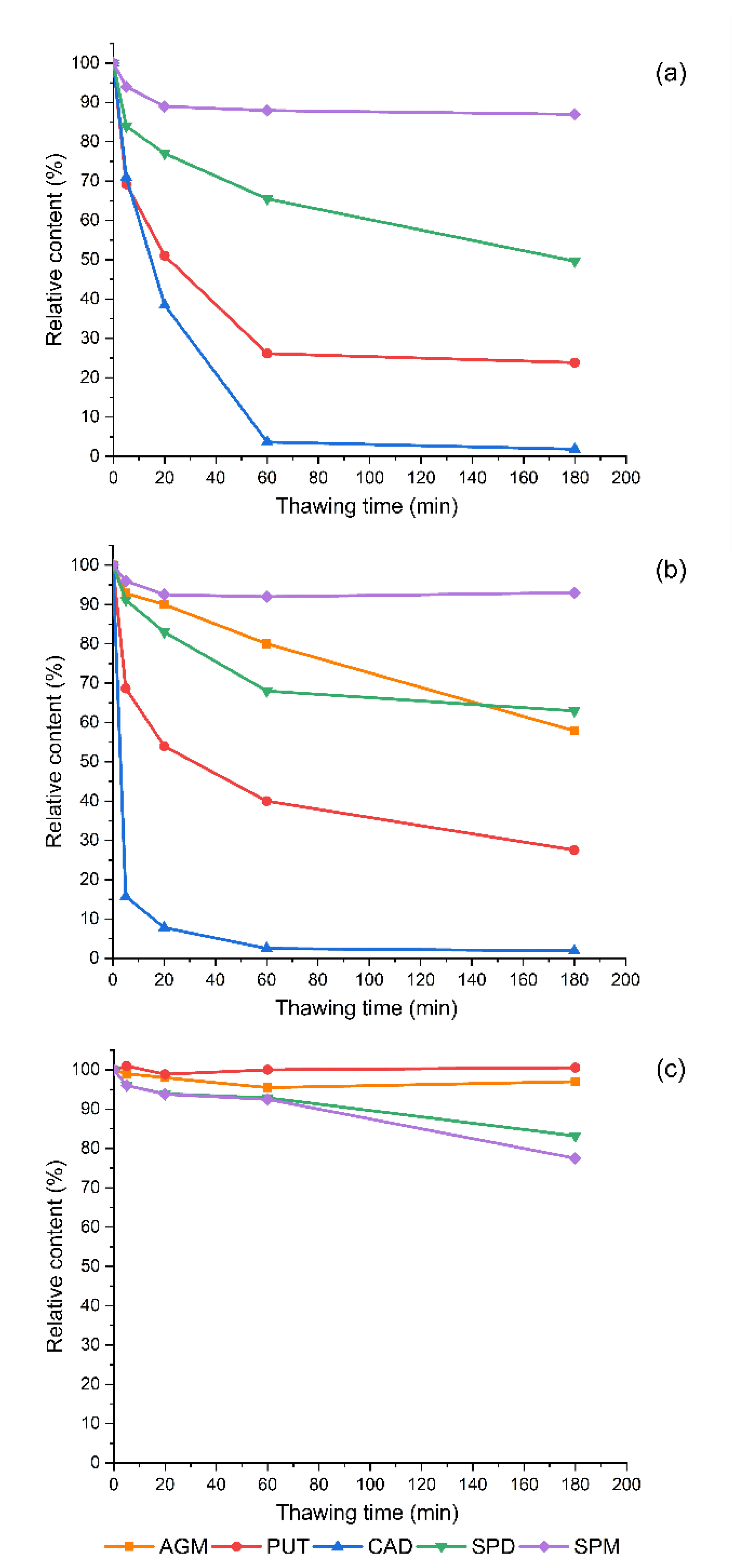
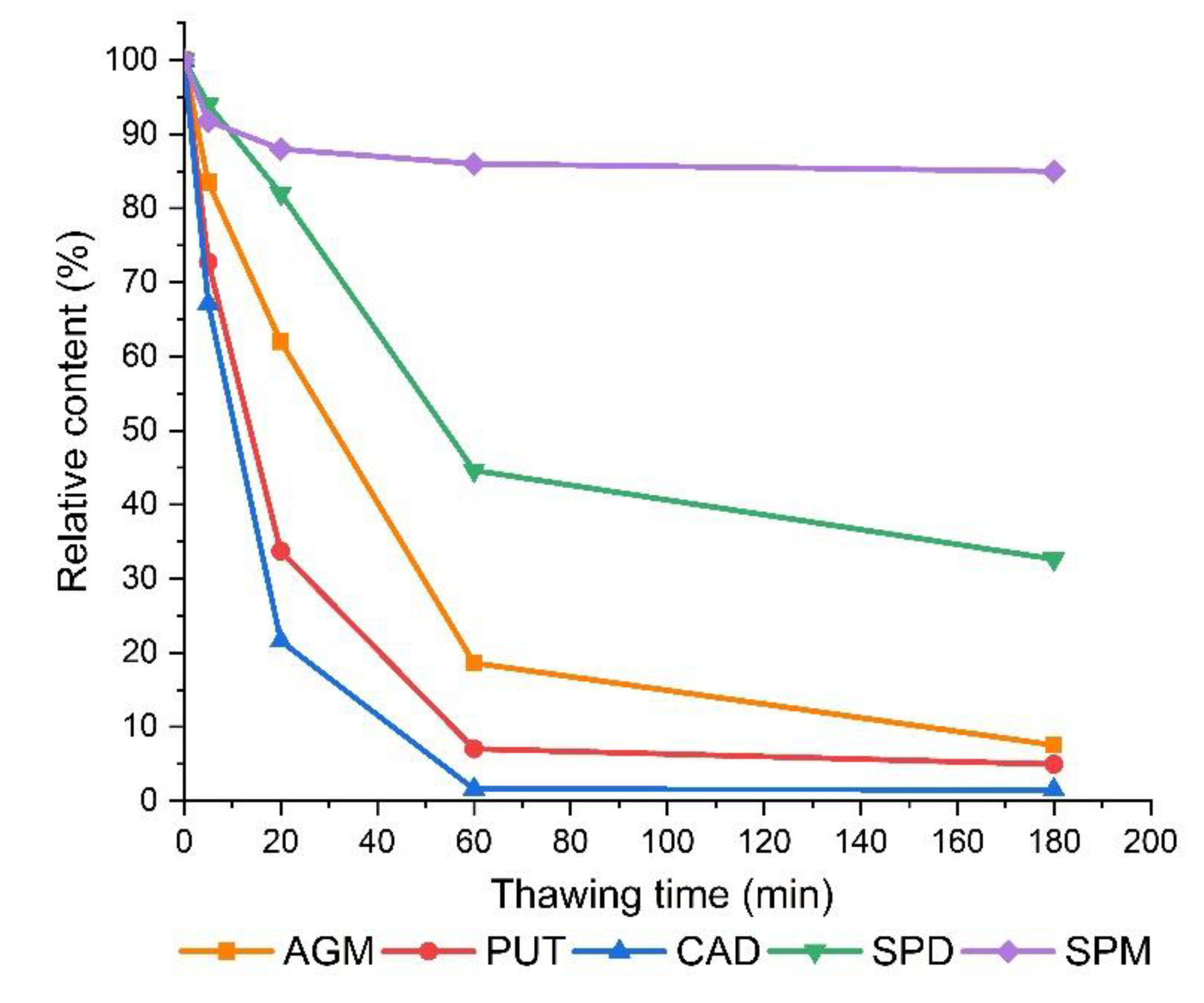
3.2. The Effect of Thawing on The Determined Polyamine Contents in Frozen Sprouts
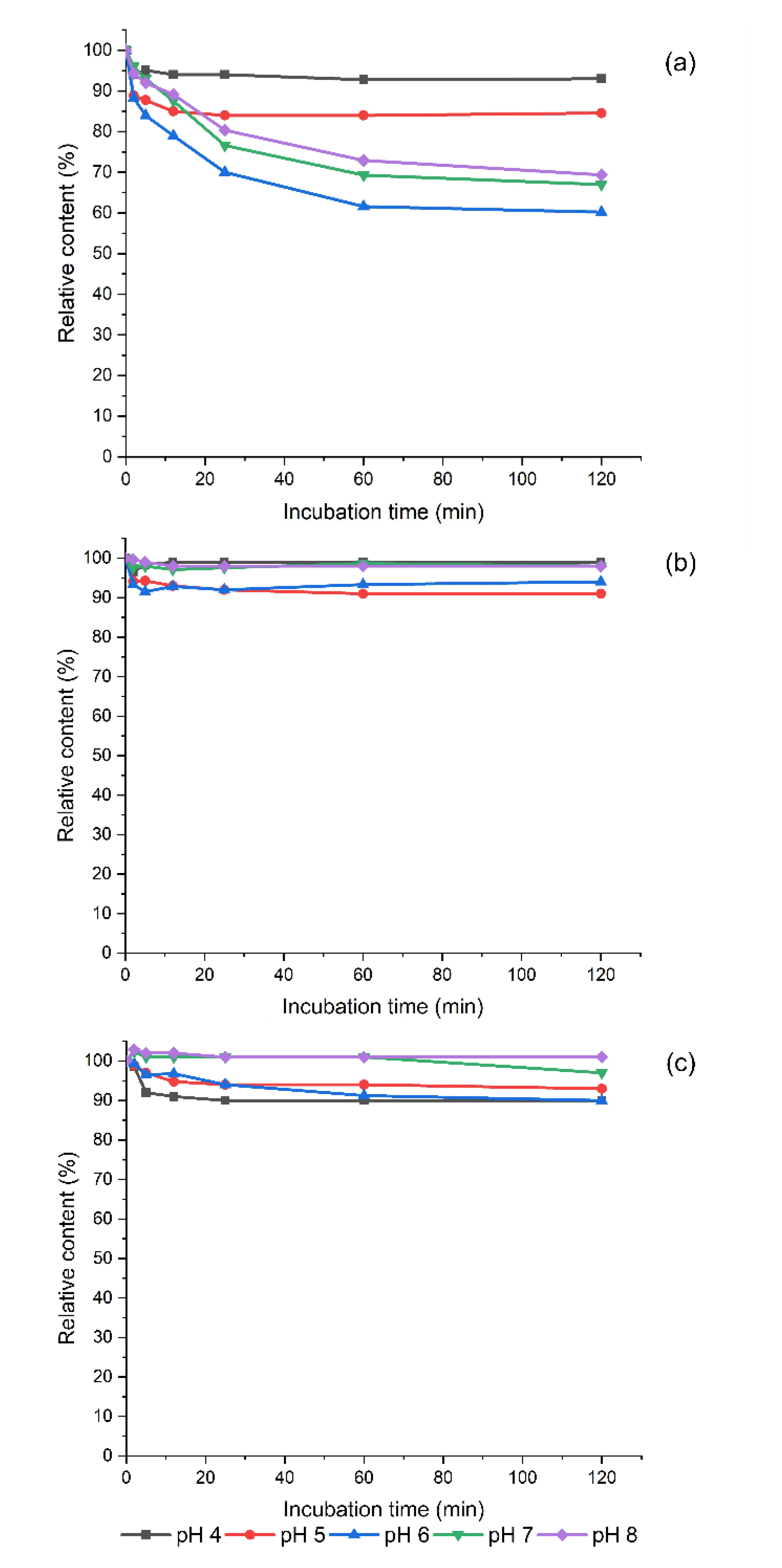
3.3. Degradation of Exogeneous Biogenic Amines by Homogenized Sprouts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.Y.; Lui, W.Y.; Wu, K.; Chan, C.L.; Dai, S.H.; Sui, Z.Q.; Corke, H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional quality in novel food sources: Genotypic variation in the nutritive and phytochemical composition of thirteen microgreens species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Microgreens: Production, shelf life, and bioactive components. Crit. Rev. Food Sci. Nutr. 2017, 57, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Butkute, B.; Taujenis, L.; Norkevičiene, E. Small-seeded legumes as a novel food source. Variation of nutritional, mineral and phytochemical profiles in the chain: Raw seeds-sprouted seeds-microgreens. Molecules 2019, 24, 133. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Viršilė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Duchovskis, P. Nutrient Levels in Brassicaceae Microgreens Increase under Tailored Light-Emitting Diode Spectra. Front. Plant Sci. 2019, 10, 1475. [Google Scholar] [CrossRef]
- Di Gioia, F.; Petropoulos, S.A.; Ozores-Hampton, M.; Morgan, K.; Rosskopf, E.N. Zinc and Iron Agronomic Biofortification of Brassicaceae Microgreens. Agronomy 2019, 9, 677. [Google Scholar] [CrossRef]
- Islam, M.Z.; Park, B.J.; Kang, H.M.; Lee, Y.T. Influence of selenium biofortification on the bioactive compounds and antioxidant activity of wheat microgreen extract. Food Chem. 2020, 309, 125763. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, A.F.; Alcázar, R. Potential applications of polyamines in agriculture and plant biotechnology. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1694, pp. 489–508. [Google Scholar]
- Glória, M.B.A.; Tavares-Neto, J.; Labanca, R.A.; Carvalho, M.S. Influence of cultivar and germination on bioactive amines in soybeans (Glycine max L. Merril). J. Agric. Food Chem. 2005, 53, 7480–7485. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Villaluenga, C.; Gulewicz, P.; Pérez, A.; Frías, J.; Vidal-Valverde, C. Influence of lupin (Lupinus luteus L. cv. 4492 and Lupinus angustifolius L. var. zapaton) and fenugreek (Trigonella foenum-graecum L.) germination on microbial population and biogenic amines. J. Agric. Food Chem. 2006, 54, 7391–7398. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, A.R. Changes in biogenic amines in mature and germinating legume seeds and their behavior during cooking. Food Nahrung 2000, 44, 23–27. [Google Scholar] [CrossRef]
- Simon-Sarkadi, L.; Holzapfel, W.H. Biogenic amines and microbial quality of sprouts. Z. Lebensm. Unters. Forsch. 1995, 200, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, C.M.; Evangelista, W.P.; Gloria, M.B.A. Bioactive amines in fresh, canned and dried sweet corn, embryo and endosperm and germinated corn. Food Chem. 2012, 131, 1355–1359. [Google Scholar] [CrossRef]
- Wanasundara, P.K.J.P.D.; Shahidi, F.; Brosnan, M.E. Changes in flax (Linum usitatissmum) seed nitrogenous compounds during germination. Food Chem. 1999, 65, 289–295. [Google Scholar] [CrossRef]
- Pinto, E.; Ferreira, I.M.P.L.V.O. Changes in the content of free and conjugated polyamines during Lettuce (Lactuca sativa) growth. J. Agric. Food Chem. 2015, 63, 440–446. [Google Scholar] [CrossRef]
- Tavladoraki, P.; Cona, A.; Angelini, R. Copper-containing amine oxidases and FAD-dependent polyamine oxidases are key players in plant tissue differentiation and organ development. Front. Plant Sci. 2016, 7, 824. [Google Scholar] [CrossRef]
- Paschalidis, K.; Tsaniklidis, G.; Wang, B.-Q.; Delis, C.; Trantas, E.; Loulakakis, K.; Makky, M.; Sarris, P.F.; Ververidis, F.; Liu, J.-H. The Interplay among Polyamines and Nitrogen in Plant Stress Responses. Plants 2019, 8, 315. [Google Scholar] [CrossRef]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Laurenzi, M.; Tipping, A.J.; Marcus, S.E.; Knox, J.P.; Federico, R.; Angelini, R.; McPherson, M.J. Analysis of the distribution of copper amine oxidase in cell walls of legume seedlings. Planta 2001, 214, 37–45. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Jing, W.; Liu, Y.; Zhang, W. Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J. Exp. Bot. 2008, 59, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Scoccianti, V.; Torrigiani, P.; Bagni, N. Distribution of diamine oxidase activity and polyamine pattern in bean and soybean seedlings at different stages of germination. Physiol. Plant. 1990, 80, 515–519. [Google Scholar] [CrossRef]
- Yang, R.; Chen, H.; Gu, Z. Factors influencing diamine oxidase activity and γ-aminobutyric acid content of fava bean (Vicia faba L.) during germination. J. Agric. Food Chem. 2011, 59, 11616–11620. [Google Scholar] [CrossRef]
- Torrigiani, P.; Scoccianti, V. Regulation of cadaverine and putrescine levels in different organs of chick-pea seed and seedlings during germination. Physiol. Plant. 1995, 93, 512–518. [Google Scholar] [CrossRef]
- Izquierdo-Casas, J.; Comas-Basté, O.; Latorre-Moratalla, M.L.; Lorente-Gascón, M.; Duelo, A.; Soler-Singla, L.; Vidal-Carou, M.C. Diamine oxidase (DAO) supplement reduces headache in episodic migraine patients with DAO deficiency: A randomized double-blind trial. Clin. Nutr. 2019, 38, 152–158. [Google Scholar] [CrossRef]
- Jumarie, C.; Séïde, M.; Marcocci, L.; Pietrangeli, P.; Mateescu, M.A. Diamine Oxidase from White Pea (Lathyrus sativus) Combined with Catalase Protects the Human Intestinal Caco-2 Cell Line from Histamine Damage. Appl. Biochem. Biotechnol. 2017, 182, 1171–1181. [Google Scholar] [CrossRef]
- Wunderlichová, L.; Buňková, L.; Koutný, M.; Jančová, P.; Buňka, F. Formation, Degradation, and Detoxification of Putrescine by Foodborne Bacteria: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1012–1030. [Google Scholar] [CrossRef]
- del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. The biogenic amines putrescine and cadaverine show in vitro cytotoxicity at concentrations that can be found in foods. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Bulushi, I.A.; Poole, S.; Deeth, H.C.; Dykes, G.A. Biogenic Amines in Fish: Roles in Intoxication, Spoilage, and Nitrosamine Formation—A Review. Crit. Rev. Food Sci. Nutr. 2009, 49, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Laube, G.; Bernstein, H.G. Agmatine: Multifunctional arginine metabolite and magic bullet in clinical neuroscience? Biochem. J. 2017, 474, 2619–2640. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef]
- Kiechl, S.; Pechlaner, R.; Willeit, P.; Notdurfter, M.; Paulweber, B.; Willeit, K.; Werner, P.; Ruckenstuhl, C.; Iglseder, B.; Weger, S.; et al. Higher spermidine intake is linked to lower mortality: A prospective population-based study. Am. J. Clin. Nutr. 2018, 108, 371–380. [Google Scholar] [CrossRef]
- Kalač, P. Health effects and occurrence of dietary polyamines: A review for the period 2005–mid 2013. Food Chem. 2014, 161, 27–39. [Google Scholar] [CrossRef]
- Muñoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Polyamines in food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef]
- Galgano, F.; Caruso, M.; Condelli, N.; Favati, F. Focused review: Agmatine in fermented foods. Front. Microbiol. 2012, 3, 199. [Google Scholar] [CrossRef]
- Ali, M.A.; Poortvliet, E.; Strömberg, R.; Yngve, A. Polyamines in foods: Development of a food database. Food Nutr. Res. 2011, 55, 5572. [Google Scholar]
- EFSA Panel on Biological Hazards. Scientific Opinion on risk based control of biogenic amine formation in fermented foods. Panel on Biological Hazards (BIOHAZ). EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- Freitas, A.E.; Neis, V.B.; Rodrigues, A.L.S. Agmatine, a potential novel therapeutic strategy for depression. Eur. Neuropsychopharmacol. 2016, 26, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- Shopsin, B. The clinical antidepressant effect of exogenous agmatine is not reversed by parachlorophenylalanine: A pilot study. Acta Neuropsychiatr. 2013, 25, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Keynan, O.; Mirovsky, Y.; Dekel, S.; Gilad, V.H.; Gilad, G.M. Safety and efficacy of dietary agmatine sulfate in lumbar disc-associated radiculopathy. An open-label, dose-escalating study followed by a randomized, double-blind, placebo-controlled trial. Pain Med. 2010, 11, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zheng, J.; Wu, X.; Xu, X.; Jia, G.; Zhao, H.; Chen, X.; Wu, C.; Tian, G.; Wang, J. Putrescine enhances intestinal immune function and regulates intestinal bacteria in weaning piglets. Food Funct. 2019, 10, 4134–4142. [Google Scholar] [CrossRef] [PubMed]
- Soda, K.; Dobashi, Y.; Kano, Y.; Tsujinaka, S.; Konishi, F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp. Gerontol. 2009, 44, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, T.J.; Gioscia-Ryan, R.A.; Hearon, C.M.; Seals, D.R. The autophagy enhancer spermidine reverses arterial aging. Mech. Ageing Dev. 2013, 134, 314–320. [Google Scholar] [CrossRef]
- Okumura, S.; Teratani, T.; Fujimoto, Y.; Zhao, X.; Tsuruyama, T.; Masano, Y.; Kasahara, N.; Iida, T.; Yagi, S.; Uemura, T.; et al. Oral administration of polyamines ameliorates liver ischemia/reperfusion injury and promotes liver regeneration in rats. Liver Transplant. 2016, 22, 1231–1244. [Google Scholar] [CrossRef]
- Sadasivan, S.K.; Vasamsetti, B.; Singh, J.; Marikunte, V.V.; Oommen, A.M.; Jagannath, M.R.; Pralhada Rao, R. Exogenous administration of spermine improves glucose utilization and decreases bodyweight in mice. Eur. J. Pharmacol. 2014, 729, 94–99. [Google Scholar] [CrossRef]
- Wu, X.; Cao, W.; Jia, G.; Zhao, H.; Chen, X.; Wu, C.; Tang, J.; Wang, J.; Liu, G. New insights into the role of spermine in enhancing the antioxidant capacity of rat spleen and liver under oxidative stress. Anim. Nutr. 2017, 3, 85–90. [Google Scholar] [CrossRef]
- Cao, W.; Wu, X.; Jia, G.; Zhao, H.; Chen, X.; Wu, C.; Tang, J.; Wang, J.; Cai, J.; Liu, G. New insights into the role of dietary spermine on inflammation, immune function and related-signalling molecules in the thymus and spleen of piglets. Arch. Anim. Nutr. 2017, 71, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Gilad, G.M.; Gilad, V.H. Long-term (5 years), high daily dosage of dietary agmatine-Evidence of safety: A case report. J. Med. Food 2014, 17, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Til, H.P.; Falke, H.E.; Prinsen, M.K.; Willems, M.I. Acute and subacute toxicity of tyramine, spermidine, spermine, putrescine and cadaverine in rats. Food Chem. Toxicol. 1997, 35, 337–348. [Google Scholar] [CrossRef]
- Schwarz, C.; Stekovic, S.; Wirth, M.; Benson, G.; Royer, P.; Sigrist, S.J.; Pieber, T.; Dammbrueck, C.; Magnes, C.; Eisenberg, T.; et al. Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging (Albany. N. Y.) 2018, 10, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Soda, K.; Kano, Y.; Chiba, F.; Koizumi, K.; Miyaki, Y. Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1,2-dimethylhydrazine-induced tumorigenesis. PLoS ONE 2013, 8, e64357. [Google Scholar] [CrossRef]
- Arnon, D.I.; Hoagland, D.R. Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci. 1940, 50, 463–484. [Google Scholar]
- Ben-Gigirey, B.; De Sousa, J.; Villa, T.G.; Barros-Velazquez, J. Changes in biogenic amines and microbiological analysis in albacore (Thunnus alalunga) muscle during frozen storage. J. Food Prot. 1998, 61, 608–615. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Granato, D.; de Araújo Calado, V.Ô.M.; Jarvis, B. Observations on the use of statistical methods in Food Science and Technology. Food Res. Int. 2014, 55, 137–149. [Google Scholar] [CrossRef]
- Bardócz, S.; Grant, G.; Brown, D.S.; Ralph, A.; Pusztai, A. Polyamines in food-implications for growth and health. J. Nutr. Biochem. 1993, 4, 66–71. [Google Scholar] [CrossRef]
- Cipolla, B.G.; Havouis, R.; Moulinoux, J.P. Polyamine contents in current foods: A basis for polyamine reduced diet and a study of its long term observance and tolerance in prostate carcinoma patients. Amino Acids 2007, 33, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Zoumas-Morse, C.; Rock, C.L.; Quintana, E.L.; Neuhouser, M.L.; Gerner, E.W.; Meyskens, F.L. Development of a Polyamine Database for Assessing Dietary Intake. J. Am. Diet. Assoc. 2007, 107, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Ouzir, M.; El Bairi, K.; Amzazi, S. Toxicological properties of fenugreek (Trigonella foenum graecum). Food Chem. Toxicol. 2016, 96, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.K.; Fiechter, G. UHPLC analysis of biogenic amines in different cheese varieties. Food Control 2018, 93, 9–16. [Google Scholar] [CrossRef]
- Okamoto, A.; Sugi, E.; Koizumi, Y.; Yanagida, F.; Udaka, S. Polyamine Content of Ordinary Foodstuffs and Various Fermented Foods. Biosci. Biotechnol. Biochem. 1997, 61, 1582–1584. [Google Scholar] [CrossRef] [PubMed]
- Kalač, P.; Špička, J.; Křížek, M.; Steidlová, Š.; Pelikánová, T. Concentrations of seven biogenic amines in sauerkraut. Food Chem. 1999, 67, 275–280. [Google Scholar] [CrossRef]
- Frías, J.; Martínez-Villaluenga, C.; Gulewicz, P.; Perez-Romero, A.; Pilarski, R.; Gulewicz, K.; Vidal-Valverde, C. Biogenic amines and HL60 citotoxicity of alfalfa and fenugreek sprouts. Food Chem. 2007, 105, 959–967. [Google Scholar] [CrossRef]
- Vale, A.P.; Santos, J.; Brito, N.V.; Marinho, C.; Amorim, V.; Rosa, E.; Oliveira, M.B.P.P. Effect of refrigerated storage on the bioactive compounds and microbial quality of Brassica oleraceae sprouts. Postharvest Biol. Technol. 2015, 109, 120–129. [Google Scholar] [CrossRef]
- Shukla, S.; Park, H.K.; Kim, J.K.; Kim, M. Determination of biogenic amines in Korean traditional fermented soybean paste (Doenjang). Food Chem. Toxicol. 2010, 48, 1191–1195. [Google Scholar] [CrossRef]
- Piletz, J.E.; May, P.J.; Wang, G.; Zhu, H. Agmatine Crosses the Blood-Brain Barrier. Ann. N. Y. Acad. Sci. 2003, 1009, 64–74. [Google Scholar] [CrossRef]
- Takeda, Y.; Yoza, K.-I.; Nogata, Y.; Ohta, H. Changes of Polyamines in Young Seedlings of Kaiware-daikon (Raphanus sativus L.). Engei Gakkai Zasshi 1993, 62, 655–660. [Google Scholar] [CrossRef][Green Version]
- Martínez-Villaluenga, C.; Frías, J.; Gulewicz, P.; Gulewicz, K.; Vidal-Valverde, C. Food safety evaluation of broccoli and radish sprouts. Food Chem. Toxicol. 2008, 46, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Gökmen, V.; Kahraman, N.; Demir, N.; Acar, J. Enzymatically validated liquid chromatographic method for the determination of ascorbic and dehydroascorbic acids in fruit and vegetables. J. Chromatogr. A 2000, 881, 309–316. [Google Scholar] [CrossRef]
- Toro-Funes, N.; Bosch-Fuste, J.; Latorre-Moratalla, M.L.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Biologically active amines in fermented and non-fermented commercial soybean products from the Spanish market. Food Chem. 2015, 173, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Feng, L.; Wang, S.; Yu, N.; Gu, Z. Accumulation of γ-aminobutyric acid in soybean by hypoxia germination and freeze-thawing incubation. J. Sci. Food Agric. 2016, 96, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Hui, Q.; Feng, X.; Feng, L.; Gu, Z.; Wang, P. The mechanism of freeze-thawing induced accumulation of γ-aminobutyric acid in germinated soybean. J. Sci. Food Agric. 2020, 100, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhu, Z.; Sun, D.W. Effects of freezing on cell structure of fresh cellular food materials: A review. Trends Food Sci. Technol. 2018, 75, 46–55. [Google Scholar] [CrossRef]
- Yang, R.; Chen, H.; Han, Y.; Gu, Z. Purification of diamine oxidase and its properties in germinated fava bean (Vicia faba L.). J. Sci. Food Agric. 2012, 92, 1709–1715. [Google Scholar] [CrossRef]
- Pietrangeli, P.; Nocera, S.; Federico, R.; Mondovi, B.; Morpurgo, L. Inactivation of copper-containing amine oxidases by turnover products. Eur. J. Biochem. 2004, 271, 146–152. [Google Scholar] [CrossRef]
- Medda, R.; Padiglia, A.; Floris, G. Plant copper-amine oxidases. Phytochemistry 1995, 39, 1–9. [Google Scholar] [CrossRef]
- Cogoni, A.; Padiglia, A.; Medda, R.; Segni, P.; Floris, G. Oxidation of spermine by an amine oxidase from lentil seedlings. Plant Physiol. 1991, 95, 477–479. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kenten, R.H.; Mann, P.J. The oxidation of amines by extracts of pea seedlings. Biochem. J. 1952, 50, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Özogul, Y.; Özogul, F. Biogenic Amines Formation, Toxicity, Regulations in Food. In Food Chemistry, Function and Analysis; Royal Society of Chemistry: London, UK, 2020; Chapter 1; pp. 1–17. [Google Scholar]
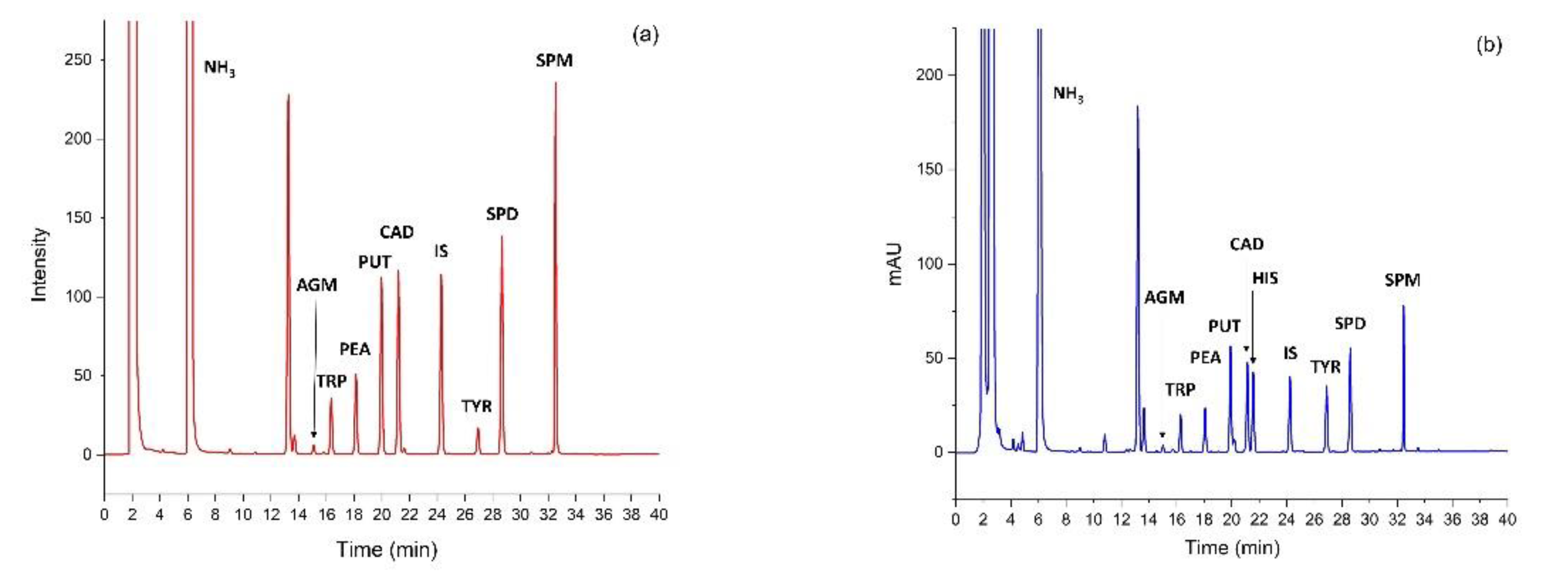

| Agmatine (AGM) | Putrescine (PUT) | Cadaverine (CAD) | Spermidine (SPD) | Spermine (SPM) | |
|---|---|---|---|---|---|
| Biosynthesis in humans | Putative From arginine (arginine decarboxylase) | Yes From ornithine/agmatine (ornithine decarboxylase/ agmatinase) | No | Yes From putrescine and S-adenosylmethioninamine (spermidine synthase) | Yes From spermidine and S-adenosylmethioninamine (spermine synthase) |
| Main dietary sources | Fermented food, various sprouts [16,39] | Fermented foods, Citrus fruits and vegetables, legumes [38,40,41] | Fermented foods, legume sprouts [16,42] | Legumes, brassica vegetables, mushrooms, cheese [38,40] | Liver, meat, legumes, brassica vegetables, cheese [38,40] |
| Oral intake Positive health effects Animal/human studies | / |
|
| ||
| Oral intake Toxicity and negative health effects Animal/human studies |
|
| |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kralj Cigić, I.; Rupnik, S.; Rijavec, T.; Poklar Ulrih, N.; Cigić, B. Accumulation of Agmatine, Spermidine, and Spermine in Sprouts and Microgreens of Alfalfa, Fenugreek, Lentil, and Daikon Radish. Foods 2020, 9, 547. https://doi.org/10.3390/foods9050547
Kralj Cigić I, Rupnik S, Rijavec T, Poklar Ulrih N, Cigić B. Accumulation of Agmatine, Spermidine, and Spermine in Sprouts and Microgreens of Alfalfa, Fenugreek, Lentil, and Daikon Radish. Foods. 2020; 9(5):547. https://doi.org/10.3390/foods9050547
Chicago/Turabian StyleKralj Cigić, Irena, Sašo Rupnik, Tjaša Rijavec, Nataša Poklar Ulrih, and Blaž Cigić. 2020. "Accumulation of Agmatine, Spermidine, and Spermine in Sprouts and Microgreens of Alfalfa, Fenugreek, Lentil, and Daikon Radish" Foods 9, no. 5: 547. https://doi.org/10.3390/foods9050547
APA StyleKralj Cigić, I., Rupnik, S., Rijavec, T., Poklar Ulrih, N., & Cigić, B. (2020). Accumulation of Agmatine, Spermidine, and Spermine in Sprouts and Microgreens of Alfalfa, Fenugreek, Lentil, and Daikon Radish. Foods, 9(5), 547. https://doi.org/10.3390/foods9050547