Geographical Origin Authentication of Agri-Food Products: A Review
Abstract
1. Introduction
2. Methodology
3. Descriptive Findings
4. Techniques, Statistically Research, and Applicability of Research
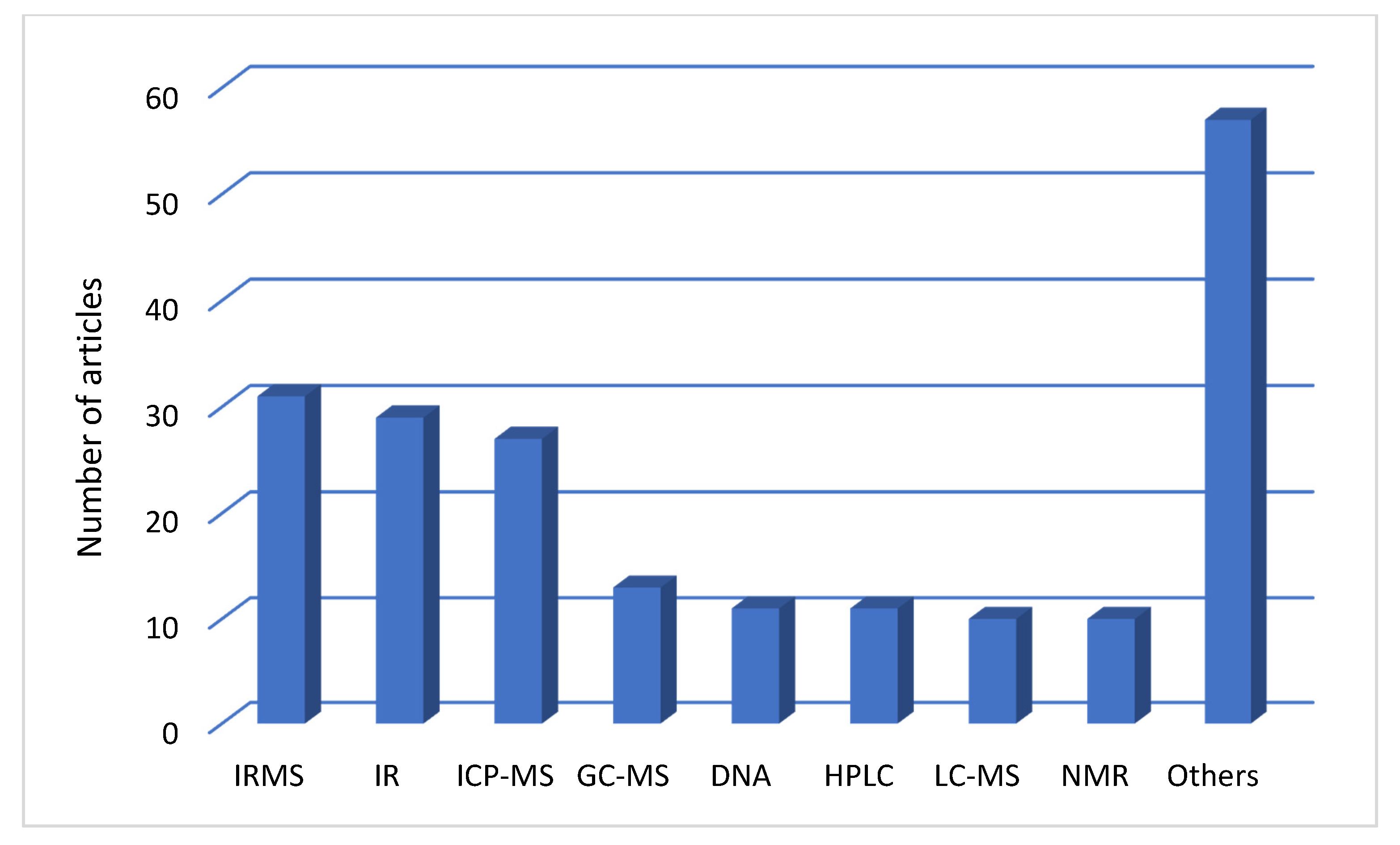
4.1. Analytical Techniques Applied in Food Authentication
4.1.1. Elemental Analysis
4.1.2. Multiple Isotope Ratio
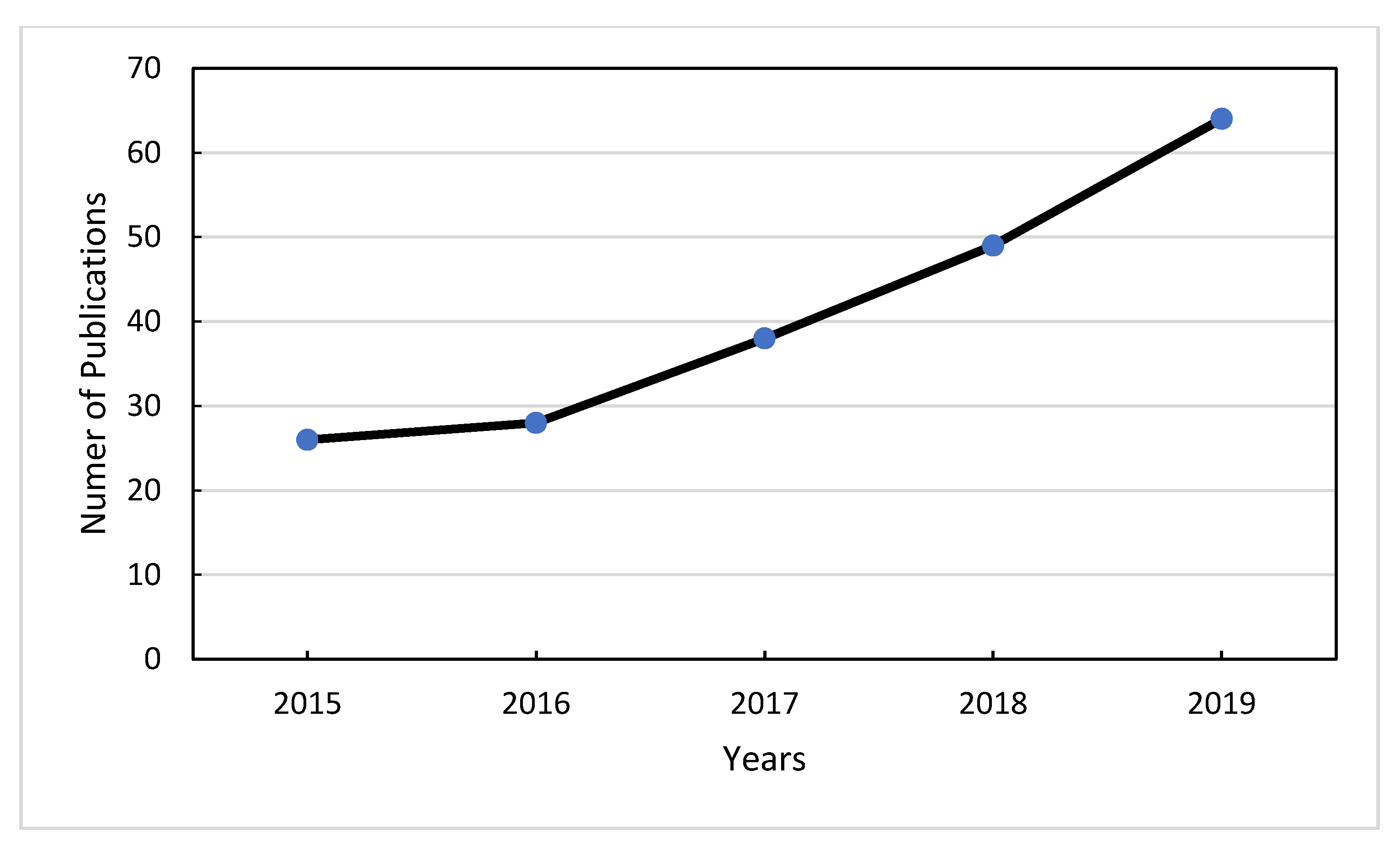
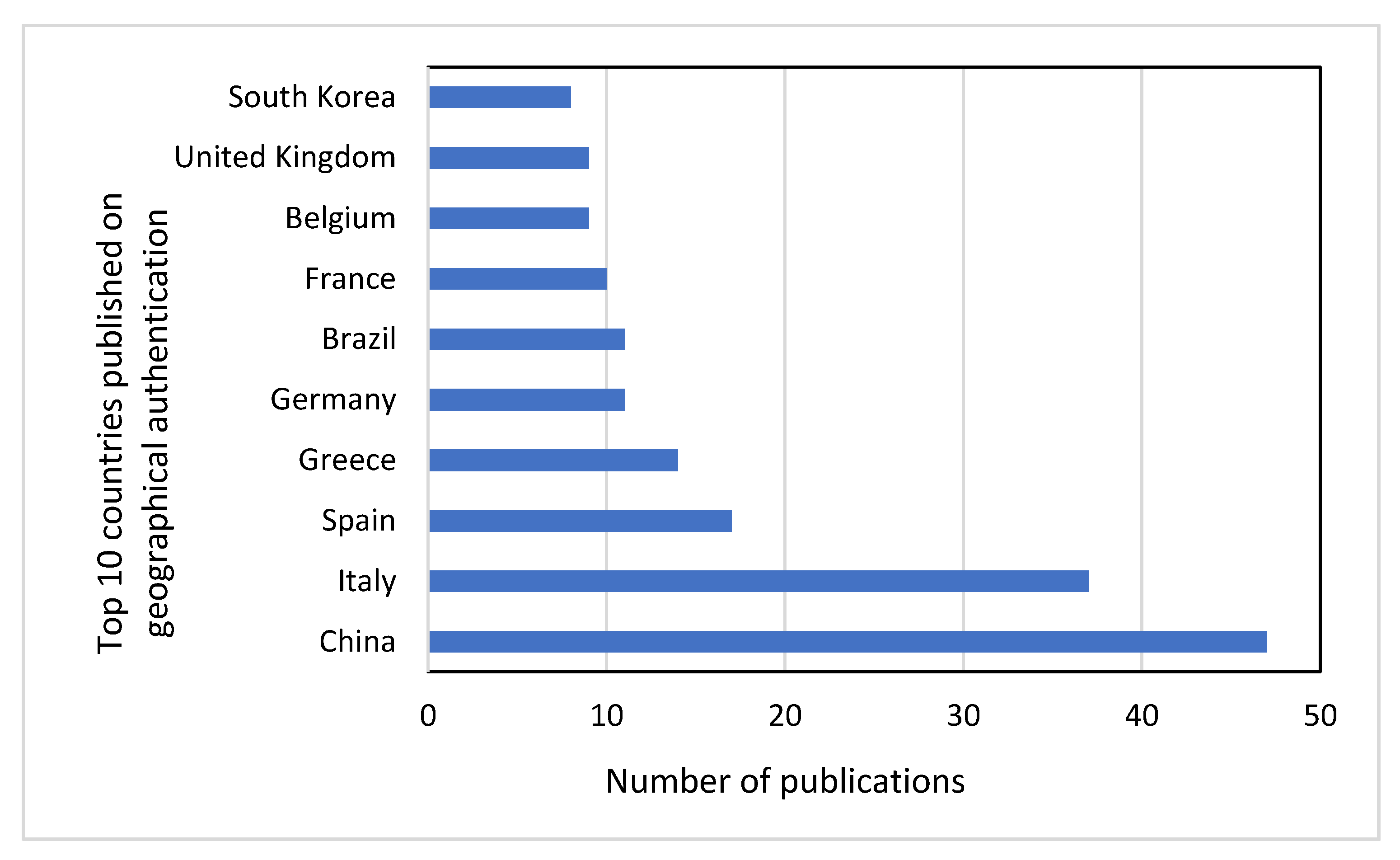
4.2. Statistically Research for Published Data Concerning Geographical Origin Authentication for Years 2015–2019
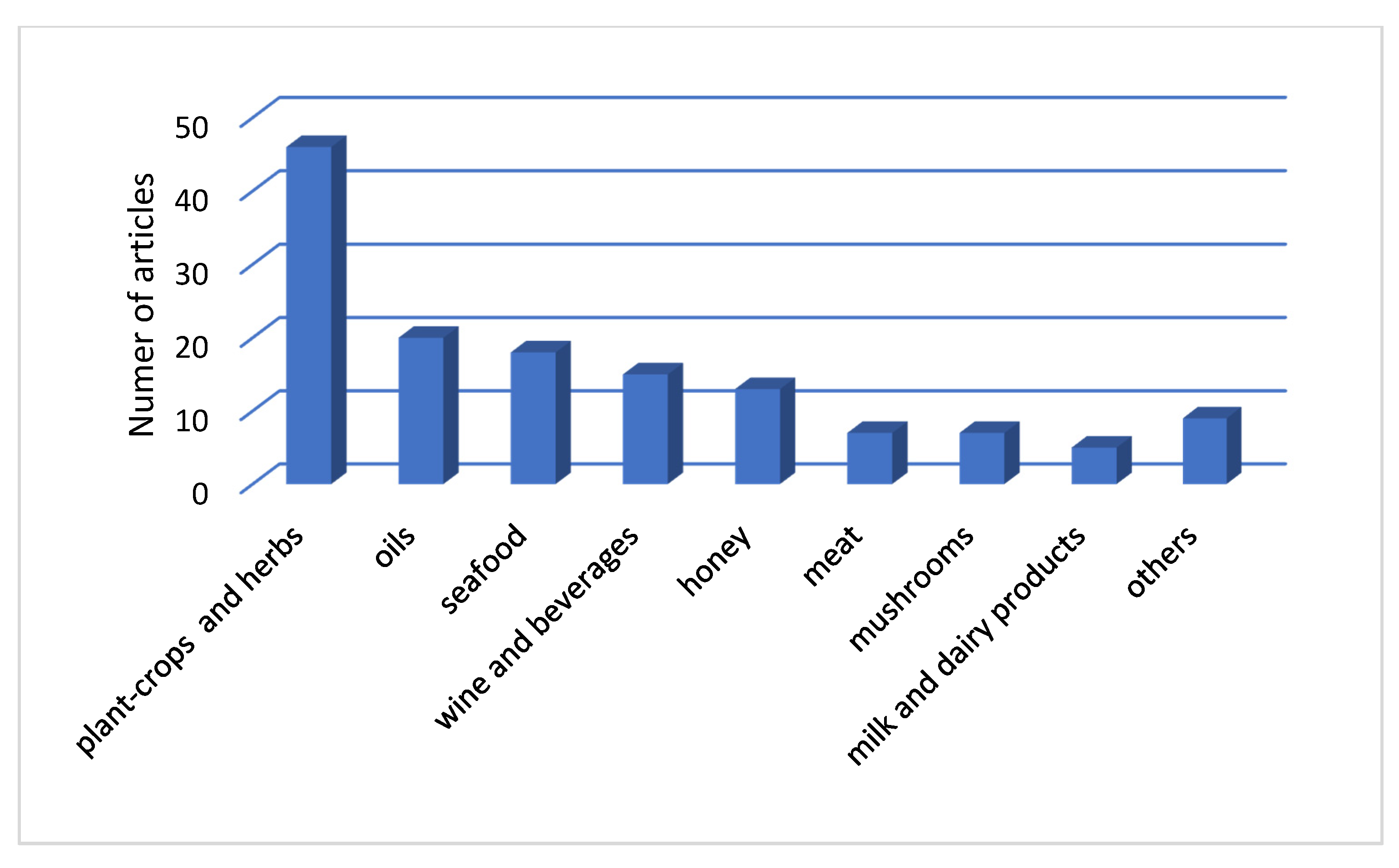
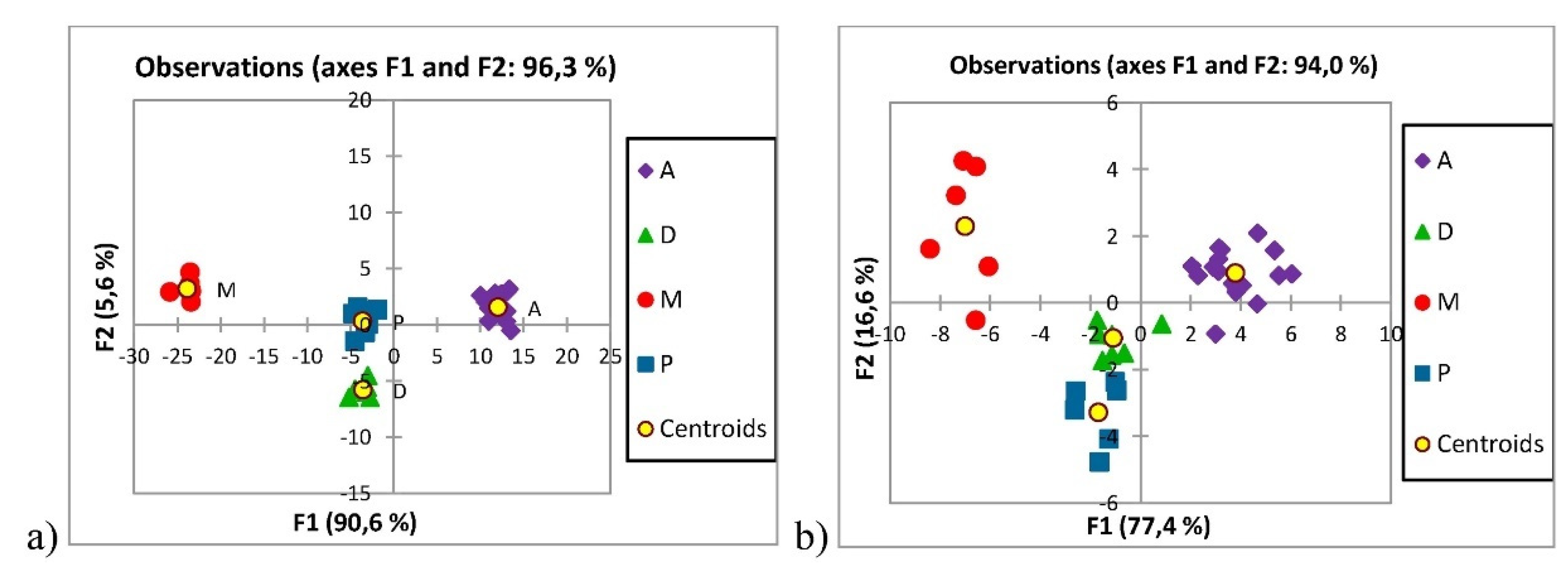
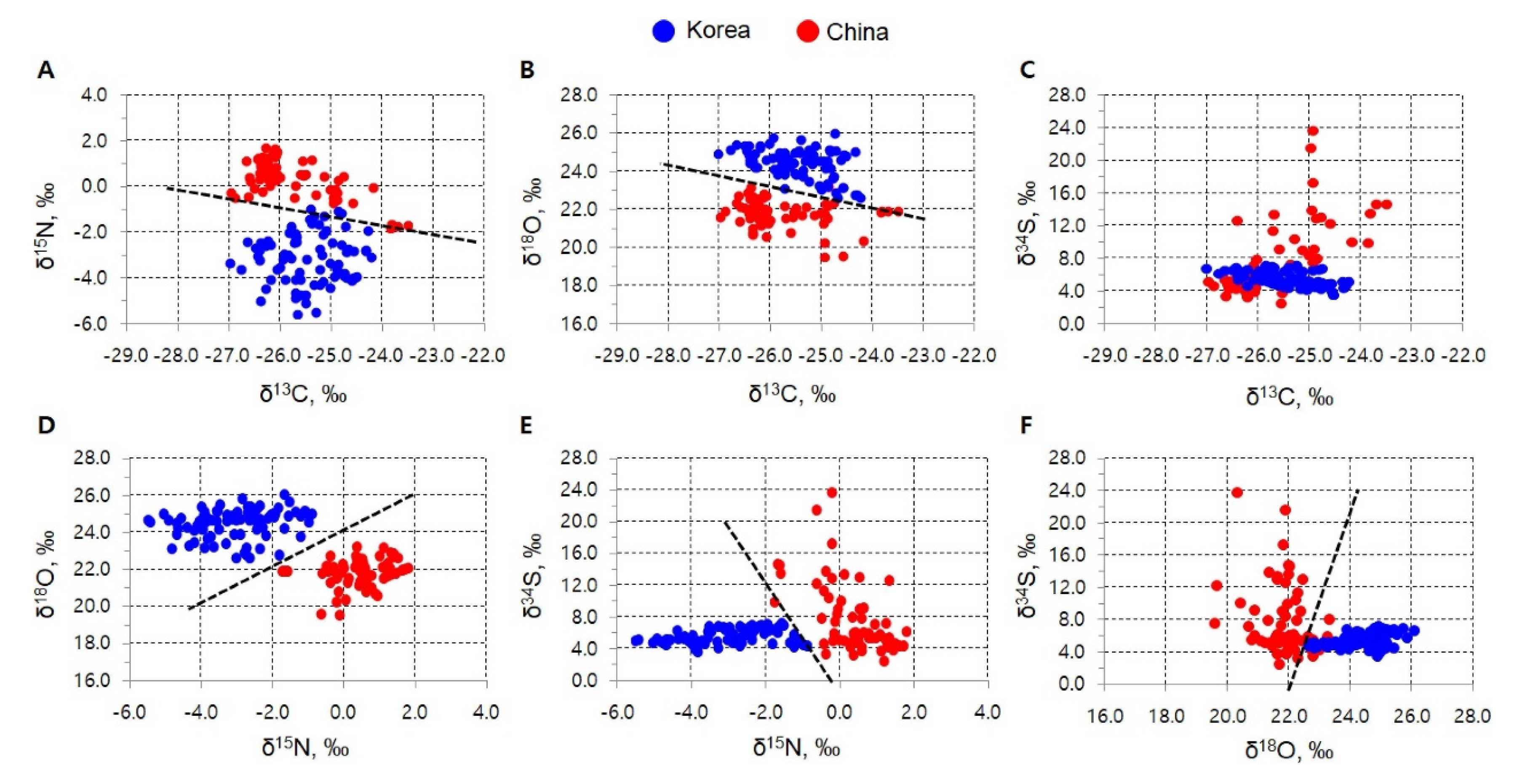
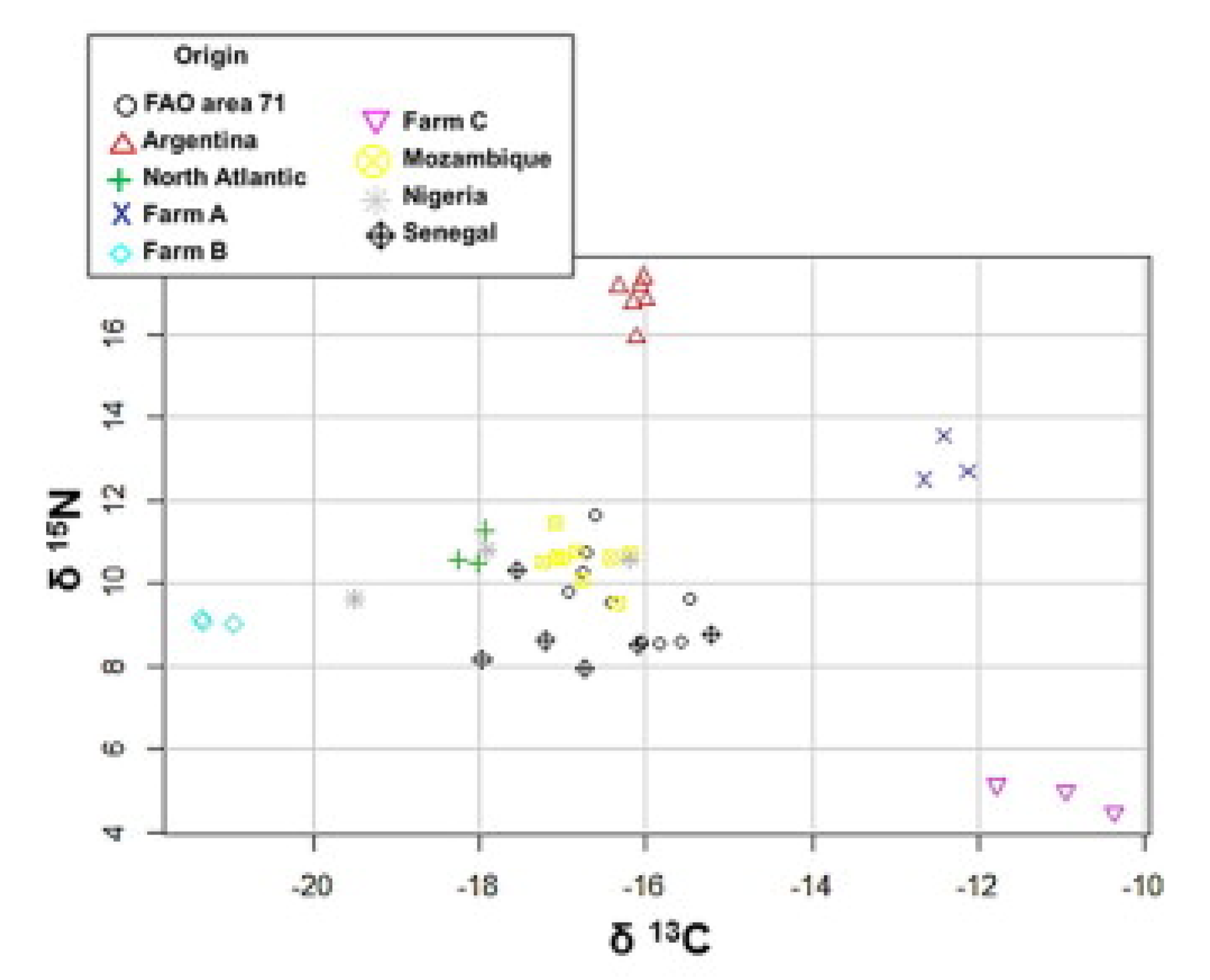
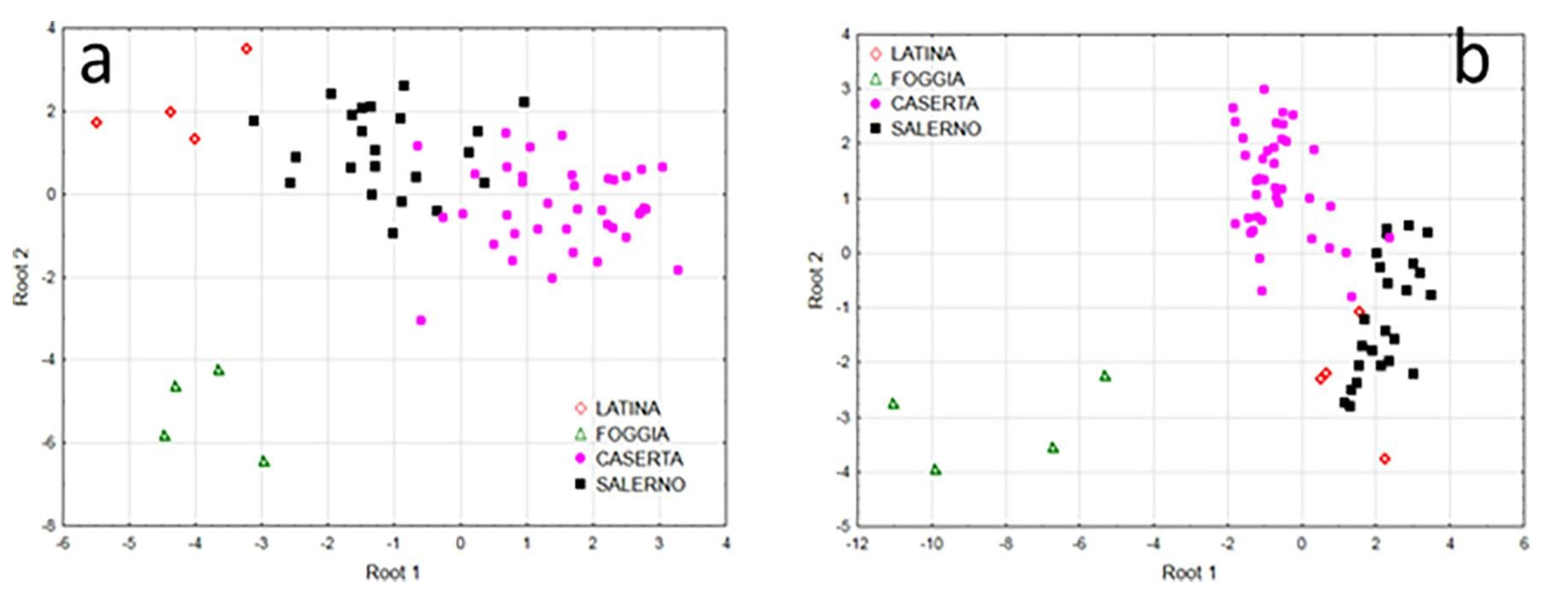
4.3. Published Research for Geographical Origin Authentication from 2015 to 2019
5. Conclusions
Author Contributions
Funding

Conflicts of Interest
References
- Stamatis, C.; Sarri, C.A.; Moutou, K.A.; Argyrakoulis, N.; Galara, I.; Godosopoulos, V.; Kolovos, M.; Liakou, C.; Stasinou, V.; Mamuris, Z. What do we think we eat? Single tracing method across foodstuff of animal origin found in Greek market. Food Res. Int. 2015, 69, 151–155. [Google Scholar] [CrossRef]
- Cubero-Leon, E.; Peñalver, R.; Maquet, A. Review on metabolomics for food authentication. Food Res. Int. 2014, 60, 95–107. [Google Scholar] [CrossRef]
- Abbas, O.; Zadravec, M.; Baeten, V.; Mikuš, T.; Lešić, T.; Vulić, A.; Prpić, J.; Jemeršić, L.; Pleadin, J. Analytical methods used for the authentication of food of animal origin. Food Chem. 2018, 246, 6–17. [Google Scholar] [CrossRef]
- Okoli, C.; Schabram, K. A Guide to Conducting a Systematic Literature Review of Information Systems Research. SSRN Electron. J. 2012, 1–3. [Google Scholar] [CrossRef]
- Albliwi, S.A.; Antony, J.; Lim, S.A.H. A systematic review of Lean Six Sigma for the manufacturing industry. Bus. Process Manag. J. 2015, 21, 665–691. [Google Scholar] [CrossRef]
- Tranfield, D.; Denyer, D.; Smart, P. Towards a Methodology for Developing Evidence-Informed Management Knowledge by Means of Systematic Review* Introduction: The need for an evidence- informed approach. Br. J. Manag. 2003, 14, 207–222. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food authentication: Techniques, trends & emerging approaches. TrAC Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar]
- Harmon, R.S.; Lawley, C.J.M.; Watts, J.; Harraden, C.L.; Somers, A.M.; Hark, R.R. Laser-induced breakdown spectroscopy-An emerging analytical tool for mineral exploration. Minerals 2019, 9, 718. [Google Scholar] [CrossRef]
- Gonzalvez, A.; Armenta, S.; de la Guardia, M. Trace-element composition and stable-isotope ratio for discrimination of foods with Protected Designation of Origin. TrAC Trends Anal. Chem. 2009, 28, 1295–1311. [Google Scholar] [CrossRef]
- Palade, L.; Popa, M. Polyphenol Fingerprinting Approaches in Wine Traceability and Authenticity: Assessment and Implications of Red Wines. Beverages 2018, 4, 75. [Google Scholar] [CrossRef]
- Cortés-Herrera, C.; Artavia, G.; Leiva, A.; Granados-Chinchilla, F. Liquid chromatography analysis of common nutritional components, in feed and food. Foods 2019, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Drivelos, S.A.; Georgiou, C.A. Multi-element and multi-isotope-ratio analysis to determine the geographical origin of foods in the European Union. TrAC Trends Anal. Chem. 2012, 40, 38–51. [Google Scholar] [CrossRef]
- Camin, F.; Bontempo, L.; Perini, M.; Piasentier, E. Stable Isotope Ratio Analysis for Assessing the Authenticity of Food of Animal Origin. Compr. Rev. Food Sci. Food Saf. 2016, 15, 868–877. [Google Scholar] [CrossRef]
- Mahne Opatić, A.; Nečemer, M.; Lojen, S.; Masten, J.; Zlatić, E.; Šircelj, H.; Stopar, D.; Vidrih, R. Determination of geographical origin of commercial tomato through analysis of stable isotopes, elemental composition and chemical markers. Food Control 2018, 89, 133–141. [Google Scholar] [CrossRef]
- Mahne Opatić, A.; Nečemer, M.; Lojen, S.; Vidrih, R. Stable isotope ratio and elemental composition parameters in combination with discriminant analysis classification model to assign country of origin to commercial vegetables—A preliminary study. Food Control 2017, 80, 252–258. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, S.-H.; Bong, Y.-S. Geographical origin authentication of onions using stable isotope ratio and compositions of C, H, O, N, and S. Food Control 2019, 101, 121–125. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, D.; Dong, H.; Wan, J.; Luo, H.; Xian, Y.; Guo, X.; Qin, F.; Han, W.; Wang, L.; et al. Geographical origin of cereal grains based on element analyser-stable isotope ratio mass spectrometry (EA-SIRMS). Food Chem. 2015, 174, 553–557. [Google Scholar] [CrossRef]
- Centonze, V.; Lippolis, V.; Cervellieri, S.; Damascelli, A.; Casiello, G.; Pascale, M.; Logrieco, A.F.; Longobardi, F. Discrimination of geographical origin of oranges (Citrus sinensis L. Osbeck) by mass spectrometry-based electronic nose and characterization of volatile compounds. Food Chem. 2019, 277, 25–30. [Google Scholar] [CrossRef]
- Xu, L.; Shi, Q.; Yan, S.-M.; Fu, H.-Y.; Xie, S.; Lu, D. Chemometric Analysis of Elemental Fingerprints for GE Authentication of Multiple Geographical Origins. J. Anal. Methods Chem. 2019, 2019, 2796502. [Google Scholar] [CrossRef]
- Fu, H.; Shi, Q.; Wei, L.; Xu, L.; Guo, X.; Hu, O.; Lan, W.; Xie, S.; Yang, T. Rapid recognition of geoherbalism and authenticity of a Chinese herb by data fusion of near-infrared spectroscopy (NIR) and mid-infrared (MIR) spectroscopy combined with chemometrics. J. Spectrosc. 2019, 2019, 2467185. [Google Scholar] [CrossRef]
- Kumar, V.; Roy, B.K. Population authentication of the traditional medicinal plant Cassia tora L. based on ISSR markers and FTIR analysis. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ün, İ.; Ok, S. Analysis of olive oil for authentication and shelf life determination. J. Food Sci. Technol. 2018, 55, 2476–2487. [Google Scholar] [CrossRef] [PubMed]
- Kritioti, A.; Menexes, G.; Drouza, C. Chemometric characterization of virgin olive oils of the two major Cypriot cultivars based on their fatty acid composition. Food Res. Int. 2018, 103, 426–437. [Google Scholar] [CrossRef]
- Gumus, Z.P.; Celenk, V.U.; Tekin, S.; Yurdakul, O.; Ertas, H. Determination of trace elements and stable carbon isotope ratios in virgin olive oils from Western Turkey to authenticate geographical origin with a chemometric approach. Eur. Food Res. Technol. 2017, 243, 1719–1727. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Zhao, X.; Xu, J.; Lin, C.; Jing, P.; Hu, L.; Zhao, S.; Wang, X.; Li, B. Discrimination of dried sea cucumber (Apostichopus japonicus) products from different geographical origins by sequential windowed acquisition of all theoretical fragment ion mass spectra (SWATH-MS)-based proteomic analysis and chemometrics. Food Chem. 2019, 274, 592–602. [Google Scholar] [CrossRef]
- Vasconi, M.; Lopez, A.; Galimberti, C.; Moreno Rojas, J.M.; Muñoz Redondo, J.M.; Bellagamba, F.; Moretti, V.M. Authentication of farmed and wild european eel (Anguilla anguilla) by fatty acid profile and carbon and nitrogen isotopic analyses. Food Control 2019, 102, 112–121. [Google Scholar] [CrossRef]
- Varrà, M.O.; Ghidini, S.; Zanardi, E.; Badiani, A.; Ianieri, A. Authentication of European sea bass according to production method and geographical origin by light stable isotope ratio and rare earth elements analyses combined with chemometrics. Ital. J. Food Saf. 2019, 8, 21–25. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, S. Identification of Jiaozhou Bay Clams (Ruditapes philippinarum) by Multi-element Fingerprinting Technique. Food Anal. Methods 2016, 9, 2691–2699. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Louppis, A.P.; Karabournioti, S.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and classification of commercial thyme honeys produced in specific Mediterranean countries according to geographical origin, using physicochemical parameter values and mineral content in combination with chemometrics. Eur. Food Res. Technol. 2017, 243, 889–900. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Louppis, A.P.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and geographical discrimination of Greek pine and thyme honeys based on their mineral content, using chemometrics. Eur. Food Res. Technol. 2017, 243, 101–113. [Google Scholar] [CrossRef]
- Dong, H.; Xiao, K.; Xian, Y.; Wu, Y. Authenticity determination of honeys with non-extractable proteins by means of elemental analyzer (EA) and liquid chromatography (LC) coupled to isotope ratio mass spectroscopy (IRMS). Food Chem. 2018, 240, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Louppis, A.P.; Karabagias, I.K.; Kontakos, S.; Kontominas, M.G.; Papastephanou, C. Botanical discrimination of Greek unifloral honeys based on mineral content in combination with physicochemical parameter analysis, using a validated chemometric approach. Microchem. J. 2017, 135, 180–189. [Google Scholar] [CrossRef]
- Ahmed, N.; Sangale, D.; Tiknaik, A.; Prakash, B.; Hange, R.; Sanil, R.; Khan, S.; Khedkar, G. Authentication of origin of meat species processed under various Indian culinary procedures using DNA barcoding. Food Control 2018, 90, 259–265. [Google Scholar] [CrossRef]
- Erasmus, S.W.; Muller, M.; Van Der Rijst, M.; Hoffman, L.C. Stable isotope ratio analysis: A potential analytical tool for the authentication of South African lamb meat. Food Chem. 2016, 192, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Bontempo, L.; Barbero, A.; Bertoldi, D.; Camin, F.; Larcher, R.; Perini, M.; Sepulcri, A.; Zicarelli, L.; Piasentier, E. Isotopic and elemental profiles of Mediterranean buffalo milk and cheese and authentication of Mozzarella di Bufala Campana PDO: An initial exploratory study. Food Chem. 2019, 285, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Caredda, M.; Addis, M.; Ibba, I.; Leardi, R.; Scintu, M.F.; Piredda, G.; Sanna, G. Building of prediction models by using Mid-Infrared spectroscopy and fatty acid profile to discriminate the geographical origin of sheep milk. LWT Food Sci. Technol. 2017, 75, 131–136. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Karabagias, I.K.; Louppis, A.; Badeka, A.; Kontominas, M.G.; Papastephanou, C. Valorization of prickly pear juice geographical origin based on mineral and volatile compound contents using LDA. Foods 2019, 8, 123. [Google Scholar] [CrossRef]
- Mahne Opatić, A.; Nečemer, M.; Budič, B.; Lojen, S. Stable isotope analysis of major bioelements, multi-element profiling, and discriminant analysis for geographical origins of organically grown potato. J. Food Compos. Anal. 2018, 71, 17–24. [Google Scholar] [CrossRef]
- Chung, I.-M.; Kim, J.-K.; Han, J.-G.; Kong, W.-S.; Kim, S.-Y.; Yang, Y.-J.; An, Y.-J.; Kwon, C.; Chi, H.-Y.; Yhung Jung, M.; et al. Potential geo-discriminative tools to trace the origins of the dried slices of shiitake (Lentinula edodes)using stable isotope ratios and OPLS-DA. Food Chem. 2019, 295, 505–513. [Google Scholar] [CrossRef]
- Achten, E.; Schütz, D.; Fischer, M.; Fauhl-Hassek, C.; Riedl, J.; Horn, B. Classification of Grain Maize (Zea mays L.) from Different Geographical Origins with FTIR Spectroscopy—A Suitable Analytical Tool for Feed Authentication? Food Anal. Methods 2019, 12, 2172–2184. [Google Scholar] [CrossRef]
- Firmani, P.; Bucci, R.; Marini, F.; Biancolillo, A. Authentication of “Avola almonds” by near infrared (NIR) spectroscopy and chemometrics. J. Food Compos. Anal. 2019, 82, 103235. [Google Scholar] [CrossRef]
- Firmani, P.; De Luca, S.; Bucci, R.; Marini, F.; Biancolillo, A. Near infrared (NIR) spectroscopy-based classification for the authentication of Darjeeling black tea. Food Control 2019, 100, 292–299. [Google Scholar] [CrossRef]
- Bittner, M.; Krähmer, A.; Schenk, R.; Springer, A.; Gudi, G.; Melzig, M.F. NIR Spectroscopy of Actaea racemosa L rhizome—En Route to Fast and Low-Cost Quality Assessment. Planta Med. 2017, 83, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, S.-C.; Guo, Q.-S.; Zheng, K.-Y.; Wang, P.-L.; Meng, Z.-G. Geographical traceability of Marsdenia tenacissima by Fourier transform infrared spectroscopy and chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 391–396. [Google Scholar] [CrossRef]
- Tena, N.; Boix, A.; von Holst, C. Identification of botanical and geographical origin of distillers dried grains with solubles by near infrared microscopy. Food Control 2015, 54, 103–110. [Google Scholar] [CrossRef]
- Vermeulen, P.; Fernández Pierna, J.A.; Abbas, O.; Dardenne, P.; Baeten, V. Origin identification of dried distillers grains with solubles using attenuated total reflection Fourier transform mid-infrared spectroscopy after in situ oil extraction. Food Chem. 2015, 189, 19–26. [Google Scholar] [CrossRef]
- Teye, E.; Amuah, C.L.Y.; McGrath, T.; Elliott, C. Innovative and rapid analysis for rice authenticity using hand-held NIR spectrometry and chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 217, 147–154. [Google Scholar] [CrossRef]
- Kahmann, A.; Anzanello, M.J.; Marcelo, M.C.A.; Pozebon, D. Near infrared spectroscopy and element concentration analysis for assessing yerba mate (Ilex paraguariensis) samples according to the country of origin. Comput. Electron. Agric. 2017, 140, 348–360. [Google Scholar] [CrossRef]
- Vera, D.N.; Jiménez-Carvelo, A.M.; Cuadros-Rodríguez, L.; Ruisánchez, I.; Callao, M.P. Authentication of the geographical origin of extra-virgin olive oil of the Arbequina cultivar by chromatographic fingerprinting and chemometrics. Talanta 2019, 203, 194–202. [Google Scholar] [CrossRef]
- Merchak, N.; Silvestre, V.; Loquet, D.; Rizk, T.; Akoka, S.; Bejjani, J. A strategy for simultaneous determination of fatty acid composition, fatty acid position, and position-specific isotope contents in triacylglycerol matrices by 13C-NMR. Anal. Bioanal. Chem. 2017, 409, 307–315. [Google Scholar] [CrossRef]
- Fiorino, G.M.; Losito, I.; De Angelis, E.; Arlorio, M.; Logrieco, A.F.; Monaci, L. Assessing fish authenticity by direct analysis in real time-high resolution mass spectrometry and multivariate analysis: Discrimination between wild-type and farmed salmon. Food Res. Int. 2019, 116, 1258–1265. [Google Scholar] [CrossRef]
- Ortea, I.; Gallardo, J.M. Investigation of production method, geographical origin and species authentication in commercially relevant shrimps using stable isotope ratio and/or multi-element analyses combined with chemometrics: An exploratory analysis. Food Chem. 2015, 170, 145–153. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Li, Y.; Zhang, X.; Qi, H. Authentication of the sea cucumber (Apostichopus japonicus) using amino acids carbon stable isotope fingerprinting. Food Control 2018, 91, 128–137. [Google Scholar] [CrossRef]
- Endaye, M.; Atlabachew, M.; Mehari, B.; Alemayehu, M.; Mengistu, D.A.; Kerisew, B. Combining Multi-Element Analysis with Statistical Modeling for Tracing the Origin of Green Coffee Beans from Amhara Region, Ethiopia. Biol. Trace Elem. Res. 2019. [Google Scholar] [CrossRef]
- Worku, M.; Upadhayay, H.R.; Latruwe, K.; Taylor, A.; Blake, W.; Vanhaecke, F.; Duchateau, L.; Boeckx, P. Differentiating the geographical origin of Ethiopian coffee using XRF- and ICP-based multi-element and stable isotope profiling. Food Chem. 2019, 290, 295–307. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Q. The suitability of rare earth elements for geographical traceability of tea leaves. J. Sci. Food Agric. 2019, 99, 6509–6514. [Google Scholar] [CrossRef]
- Liu, H.; Wei, Y.; Lu, H.; Wei, S.; Jiang, T.; Zhang, Y.; Guo, B. Combination of the 87Sr/86Sr ratio and light stable isotopic values (δ13C, δ15N and δd) for identifying the geographical origin of winter wheat in China. Food Chem. 2016, 212, 367–373. [Google Scholar] [CrossRef]
- De Girolamo, A.; Cortese, M.; Cervellieri, S.; Lippolis, V.; Pascale, M.; Logrieco, A.F.; Suman, M. Tracing the geographical origin of durum wheat by FT-NIR spectroscopy. Foods 2019, 8, 450. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katerinopoulou, K.; Kontogeorgos, A.; Salmas, C.E.; Patakas, A.; Ladavos, A. Geographical Origin Authentication of Agri-Food Products: A Review. Foods 2020, 9, 489. https://doi.org/10.3390/foods9040489
Katerinopoulou K, Kontogeorgos A, Salmas CE, Patakas A, Ladavos A. Geographical Origin Authentication of Agri-Food Products: A Review. Foods. 2020; 9(4):489. https://doi.org/10.3390/foods9040489
Chicago/Turabian StyleKaterinopoulou, Katerina, Achilleas Kontogeorgos, Constantinos E. Salmas, Angelos Patakas, and Athanasios Ladavos. 2020. "Geographical Origin Authentication of Agri-Food Products: A Review" Foods 9, no. 4: 489. https://doi.org/10.3390/foods9040489
APA StyleKaterinopoulou, K., Kontogeorgos, A., Salmas, C. E., Patakas, A., & Ladavos, A. (2020). Geographical Origin Authentication of Agri-Food Products: A Review. Foods, 9(4), 489. https://doi.org/10.3390/foods9040489







