Potential of a Sunflower Seed By-Product as Animal Fat Replacer in Healthier Frankfurters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Frankfurters
2.3. Composition Analysis and Energy Value
2.3.1. Proximate Composition
2.3.2. Dietary Fibre Content
2.3.3. Mineral Content
2.3.4. Polyphenol Content and Profile
2.3.5. Energy Content
2.4. Composition Analysis and Energy Value
2.4.1. Processing Loss and pH
2.4.2. Colour
2.4.3. Texture Analysis
2.5. Attenuated Total Reflectance (ATR)-FTIR Spectroscopy Analysis
2.6. Preliminary Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
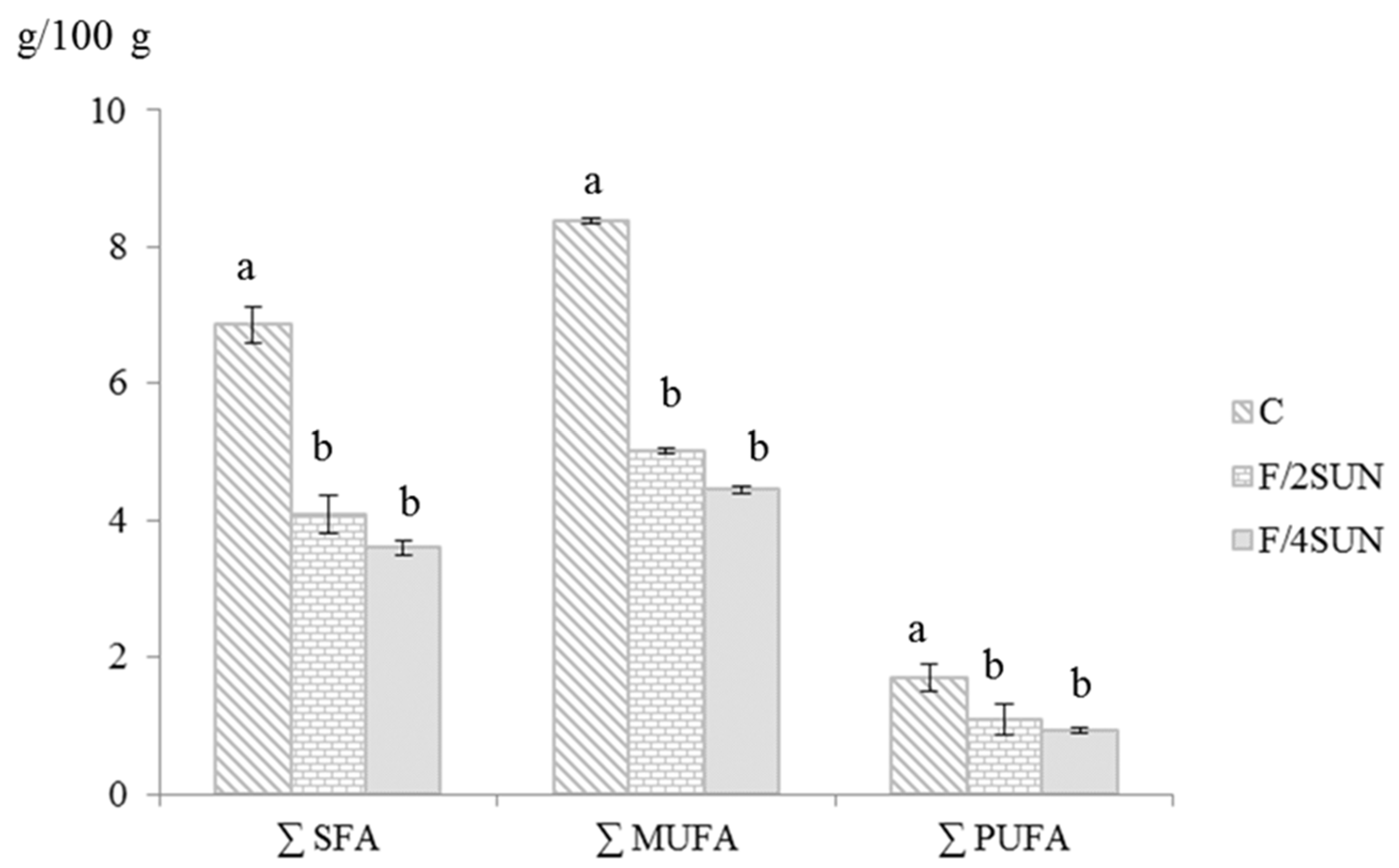
3.1. Composition and Energy Value
3.2. Polyphenol Content and Profile
3.3. Overall Nutritional Value: Nutrition and Health Claims
3.4. Processing Loss, pH and Colour
3.5. Texture Profile Analysis
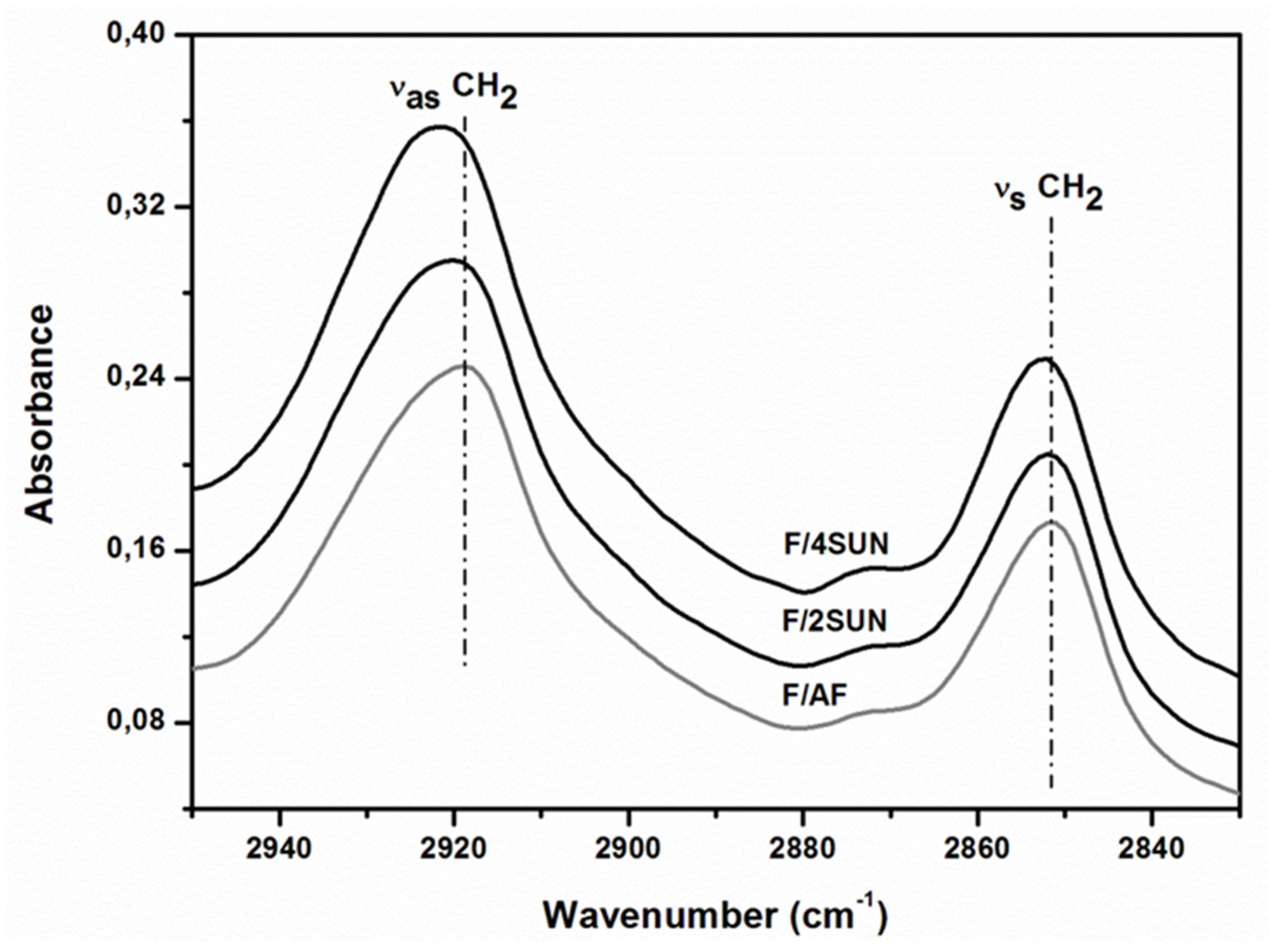
3.6. Attenuated Total Reflectance (ATR)-FTIR Spectroscopy Analysis
3.7. Preliminary Sensory Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. FAOSTAT Statistics Database—Agriculture; FAO: Rome, Italy, 2018; Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 6 April 2020).
- Yegorov, B.; Turpurova, T.; Sharabaeva, E.; Bondar, Y. Prospects of using by-products of sunflower oil production in compound feed industry. J. Food Sci. Technol. 2019, 13, 106–113. [Google Scholar] [CrossRef]
- Dorrell, D.G.; Vick, B.A. Properties and processing of oilseed sunflower. Sunflower Technol. Prod. 1997, 35, 709–745. [Google Scholar]
- Wanjari, N.; Waghmare, J. Phenolic and antioxidant potential of sunflower meal. Adv. Appl. Sci. Res. 2015, 6, 221–229. [Google Scholar]
- Anal, A.K. Food Processing By-Products and Their Utilization; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Salgado, P.R.; Molina Ortiz, S.E.; Petruccelli, S.; Mauri, A.N. Functional food ingredients based on sunflower protein concentrates naturally enriched with antioxidant phenolic compounds. J. Am. Oil Chem. Soc. 2012, 89, 825–836. [Google Scholar] [CrossRef]
- Manchuliantsau, A.; Tkacheva, A. Upcycling Solid Food Wastes and By-Products into Human Consumption Products. U.S. Patent 16,370,896, 25 July 2019. [Google Scholar]
- Grasso, S.; Omoarukhe, E.; Wen, X.; Papoutsis, K.; Methven, L. The use of upcycled defatted sunflower seed flour as a functional ingredient in biscuits. Foods 2019, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Grasso, S.; Liu, S.; Methven, L. Quality of muffins enriched with upcycled defatted sunflower seed flour. LWT 2020, 119, 108893. [Google Scholar] [CrossRef]
- Jimenez-Colmenero, F.; Salcedo-Sandoval, L.; Bou, R.; Cofrades, S.; Herrero, A.M.; Ruiz-Capillas, C. Novel applications of oil-structuring methods as a strategy to improve the fat content of meat products. Trends Food Sci. Technol. 2015, 44, 177–188. [Google Scholar] [CrossRef]
- Weiss, J.; Gibis, M.; Schuh, V.; Salminen, H. Advances in ingredient and processing systems for meat and meat products. Meat Sci. 2010, 86, 196–213. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F. Healthier lipid formulation approaches in meat-based functional foods. Technological options for replacement of meat fats by non-meat fats. Trends Food Sci. Technol. 2007, 18, 567–578. [Google Scholar]
- Hjelm, L.; Mielby, L.A.; Gregersen, S.; Eggers, N.; Bertram, H.C. Partial substitution of fat with rye bran fibre in Frankfurter sausages—Bridging technological and sensory attributes through inclusion of collagenous protein. LWT 2019, 101, 607–617. [Google Scholar] [CrossRef]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Strategies for incorporation of chia (Salvia hispanica L.) in frankfurters as a health-promoting ingredient. Meat Sci. 2016, 114, 75–84. [Google Scholar] [CrossRef]
- Henning, S.S.C.; Tshalibe, P.; Hoffman, L.C. Physico-chemical properties of reduced-fat beef species sausage with pork back fat replaced by pineapple dietary fibres and water. LWT 2016, 74, 92–98. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Ren, L.; Guo, H.; Li, Y. Production of pork sausages using pleaurotus eryngii with different treatments as replacements for pork back fat. J. Food Sci. 2019, 84, 3091–3098. [Google Scholar] [CrossRef]
- Kim, T.-K.; Yong, H.-I.; Jung, S.; Kim, Y.-B.; Choi, Y.-S. Effects of replacing pork fat with grape seed oil and gelatine/alginate for meat emulsions. Meat Sci. 2020, 163, 108079. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Herrero, A.; Pintado, T.; Solas, M.T.; Ruiz-Capillas, C. Influence of emulsified olive oil stabilizing system used for pork backfat replacement in frankfurters. Food Res. Int. 2010, 43, 2068–2076. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Cavalheiro, C.P.; Ruiz-Capillas, C. Chia and oat emulsion gels as new animal fat replacers and healthy bioactive sources in fresh sausage formulation. Meat Sci. 2018, 135, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Díaz-Rubio, M.E.; Pérez-Jiménez, J.; Saura-Calixto, F. Towards an updated methodology for measurement of dietary fiber, including associated polyphenols, in food and beverages. Food Res. Int. 2009, 42, 840–846. [Google Scholar] [CrossRef]
- Englyst, H.N.; Cummings, J. Improved method for measurement of dietary fiber as non-starch polysaccharides in plant foods. J. Assoc. Off. Anal. Chem. 1988, 71, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Pintado, T.; Herrero, A.; Ruiz-Capillas, C.; Triki, M.; Carmona, P.; Jimenez-Colmenero, F. Effects of emulsion gels containing bioactive compounds on sensorial, technological, and structural properties of frankfurters. Food Sci. Technol. Int. 2016, 22, 132–145. [Google Scholar] [CrossRef]
- Nardoia, M.; Ruiz-Capillas, C.; Casamassima, D.; Herrero, A.M.; Pintado, T.; Jiménez-Colmenero, F.; Chamorro, S.; Brenes, A. Effect of polyphenols dietary grape by-products on chicken patties. Eur. Food Res. Technol. 2018, 244, 367–377. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Section III. Polyphenols and flavonoids-14-analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1998, 1999, 152–177. [Google Scholar]
- Pérez-Ramírez, I.F.; Reynoso-Camacho, R.; Saura-Calixto, F.; Pérez-Jiménez, J. Comprehensive characterization of extractable and nonextractable phenolic compounds by high-performance liquid chromatography–electrospray ionization–quadrupole time-of-flight of a grape/pomegranate pomace dietary supplement. J. Agric. Food Chem. 2018, 66, 661–673. [Google Scholar] [CrossRef] [PubMed]
- EU Regulation. No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Eur. Comm. Off. J. Eur. Union 2011, 20, 168–213. [Google Scholar]
- Bourne, M.C. Texture profile analysis. Food Technol. 1978, 32, 62–66. [Google Scholar]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Ratcliff, R.K. Nutritional Value of Sunflower Meal for Ruminants. Ph.D. Thesis, Texas Tech University, Lubbock, TX, USA, 1977. [Google Scholar]
- Solari-Godiño, A.; Pérez-Jiménez, J.; Saura-Calixto, F.; Borderías, A.J.; Moreno, H. Anchovy mince (Engraulis ringens) enriched with polyphenol-rich grape pomace dietary fibre: In vitro polyphenols bioaccessibility, antioxidant and physico-chemical properties. Food Res. Int. 2017, 102, 639–646. [Google Scholar] [CrossRef]
- Weisz, G.M.; Kammerer, D.R.; Carle, R. Identification and quantification of phenolic compounds from sunflower (Helianthus annuus L.) kernels and shells by HPLC-DAD/ESI-MSn. Food Chem. 2009, 115, 758–765. [Google Scholar] [CrossRef]
- Laguna, O.; Barakat, A.; Alhamada, H.; Durand, E.; Baréa, B.; Fine, F.; Villeneuve, P.; Citeau, M.; Dauguet, S.; Lecomte, J. Production of proteins and phenolic compounds enriched fractions from rapeseed and sunflower meals by dry fractionation processes. Ind. Crop. Prod. 2018, 118, 160–172. [Google Scholar] [CrossRef]
- Slabi, S.A.; Mathé, C.; Framboisier, X.; Defaix, C.; Mesieres, O.; Galet, O.; Kapel, R. A new SE-HPLC method for simultaneous quantification of proteins and main phenolic compounds from sunflower meal aqueous extracts. Anal. Bioanal. Chem. 2019, 411, 2089–2099. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Eur. Comm. Off. J. Eur. Union 2006, 404, 3–18. [Google Scholar]
- European Commission. Regulation (EU) No 432/2012 of the European Parliament and of the Council of 16 may 2012 establishing a list of permited health claims made on foods other than those referring to the reduction of disease risk and to children’s development and health. Eur. Comm. Off. J. Eur. Union 2012, 136, 1–40. [Google Scholar]
- Sousa, S.C.; Fragoso, S.P.; Penna, C.R.A.; Arcanjo, N.M.O.; Silva, F.A.P.; Ferreira, V.C.S.; Barreto, M.D.S.; Araújo, Í.B.S. Quality parameters of frankfurter-type sausages with partial replacement of fat by hydrolyzed collagen. LWT-Food Sci. Technol. 2017, 76, 320–325. [Google Scholar] [CrossRef]
- Herrero, A.M.; Ruiz-Capillas, C.; Pintado, T.; Carmona, P.; Jimenez-Colmenero, F. Infrared spectroscopy used to determine effects of chia and olive oil incorporation strategies on lipid structure of reduced-fat frankfurters. Food Chem. 2017, 221, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Pintado, T.; Ruiz-Capillas, C.; Jiménez-Colmenero, F.; Carmona, P.; Herrero, A.M. Oil-in-water emulsion gels stabilized with chia (Salvia hispanica L.) and cold gelling agents: Technological and infrared spectroscopic characterization. Food Chem. 2015, 185, 470–478. [Google Scholar] [CrossRef] [PubMed]
- López-López, I.; Cofrades, S.; Jiménez-Colmenero, F. Low-fat frankfurters enriched with n−3 PUFA and edible seaweed: Effects of olive oil and chilled storage on physicochemical, sensory and microbial characteristics. Meat Sci. 2009, 83, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Deliza, R.; Saldivar, S.S.; Germani, R.; Benassi, V.; Cabral, L. The effects of colored textured soybean protein (TSP) on sensory and physical attributes of ground beef patties. J. Sens. Stud. 2002, 17, 121–132. [Google Scholar] [CrossRef]
- Pereira, A.G.T.; Ramos, E.M.; Teixeira, J.T.; Cardoso, G.P.; Ramos, A.D.L.S.; Fontes, P.R. Effects of the addition of mechanically deboned poultry meat and collagen fibers on quality characteristics of frankfurter-type sausages. Meat Sci. 2011, 89, 519–525. [Google Scholar] [CrossRef]
- Guillen, M.D.; Cabo, N. Infrared spectroscopy in the study of edible oils and fats. J. Sci. Food Agric. 1997, 75, 1–11. [Google Scholar] [CrossRef]
- Fraile, M.; Patrón-Gallardo, B.; López-Rodrıguez, G.; Carmona, P. FT-IR study of multilamellar lipid dispersions containing cholesteryl linoleate and dipalmitoylphosphatidylcholine. Chem. Phys. Lipids 1999, 97, 119–128. [Google Scholar] [CrossRef]
- Herrero, A.M.; Carmona, P.; Pintado, T.; Jiménez-Colmenero, F.; Ruíz-Capillas, C. Infrared spectroscopic analysis of structural features and interactions in olive oil-in-water emulsions stabilized with soy protein. Food Res. Int. 2011, 44, 360–366. [Google Scholar] [CrossRef]
- Carmona, P.; Ruiz-Capillas, C.; Jiménez-Colmenero, F.; Pintado, T.; Herrero, A.M. Infrared study of structural characteristics of frankfurters formulated with olive oil-in-water emulsions stabilized with casein as pork backfat replacer. J. Agric. Food Chem. 2011, 59, 12998–13003. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.M.; Carmona, P.; Cofrades, S.; Jiménez-Colmenero, F. Raman spectroscopic determination of structural changes in meat batters upon soy protein addition and heat treatment. Food Res. Int. 2008, 41, 765–772. [Google Scholar] [CrossRef]
- Kurt, A.; Gençcelep, H. Enrichment of meat emulsion with mushroom (Agaricus bisporus) powder: Impact on rheological and structural characteristics. J. Food Eng. 2018, 237, 128–136. [Google Scholar] [CrossRef]
| Samples * | Meat | Pork Back Fat | SUN ** | Water |
|---|---|---|---|---|
| F/AF | 61.0 | 15.0 | 0.0 | 21.2 |
| F/2SUN | 61.0 | 8.0 | 2.0 | 26.2 |
| F/4SUN | 61.0 | 8.0 | 4.0 | 24.2 |
| Parameters | F/AF * | F/2SUN * | F/4SUN * |
|---|---|---|---|
| Proximate composition | |||
| Moisture | 61.54 ± 0.12 c | 64.99 ± 0.19 a | 64.47 ± 0.07 b |
| Protein | 15.99 ± 0.04 c | 16.96 ± 0.10 b | 17.93 ± 0.04 a |
| Fat | 18.67 ± 0.48 a | 11.59 ± 0.26 b | 11.75 ± 0.10 b |
| Ash | 3.23 ± 0.00 a | 2.97 ± 0.18 a | 3.36 ± 0.12 a |
| Dietary fibre ** | --- | 0.56 | 1.12 |
| Mineral content | |||
| Magnesium | 36.32 ± 2.73 c | 60.72 ± 8.17 b | 85.50 ± 3.20 a |
| Potassium | 302.41 ± 10.44 c | 383.34 ± 2.98 b | 415.16 ± 20.40 a |
| Iron | 1.18 ± 0.09 b | 1.57 ± 0.16 ab | 2.02 ± 0.18 a |
| Zinc | 2.02 ± 0.04 b | 2.28 ± 0.15 b | 2.95 ± 0.16 a |
| Copper | 0.06 ± 0.01 c | 0.14 ± 0.01 b | 0.21 ± 0.01 a |
| Manganese | 0.06 ± 0.00 c | 0.18 ± 0.00 b | 0.29 ± 0.01 a |
| Polyphenols | |||
| Total extractable polyphenols (mg/100 g mf) | 51 ± 9 c | 76 ± 11 b | 93 ± 12 a |
| Energy value | 231.99 | 173.21 | 179.59 |
| ID | Class | Subclass | Proposed Compound | Experimental Mass (M-H)− | Calculated Mass (M-H)− | Error (ppm) | Molecular Formula |
|---|---|---|---|---|---|---|---|
| 1 | Phenolic acids | Hydroxybenzoic acids | Hydroxybenzoic acid | 137.023 | 137.0244 | 10.27 | C7H6O3 |
| 2 | Phenolic acids | Hydroxybenzoic acids | Vanillic acid | 167.0344 | 167.0350 | 3.47 | C8H8O4 |
| 3 | Phenolic acids | Hydroxycinnamic acids | Caffeic Acid | 179.0354 | 179.0350 | 2.32 | C9H8O4 |
| 4 | Phenolic acids | Hydroxycinnamic acids | 1-O-Caffeoylquinic Acid | 353.0864 | 353.0878 | 3.27 | C16H18O9 |
| 5 | Phenolic acids | Hydroxycinnamic acids | 3-O-Caffeoylquinic acid | 353.0915 | 353.0878 | 10.43 | C16H18O9 |
| 6 | Phenolic acids | Hydroxycinnamic acids | 4-O-Caffeoylquinic acid | 353.0849 | 353.0878 | 8.21 | C16H18O9 |
| 7 | Phenolic acids | Hydroxycinnamic acids | 5-O-Caffeoylquinic acid | 353.0899 | 353.0878 | 5.91 | C16H18O9 |
| 8 | Phenolic acids | Hydroxycinnamic acids | 3,4-di-o-caffeoylquinic acid | 515.1230 | 515.1195 | 6.78 | C25H24O12 |
| 9 | Phenolic acids | Hydroxycinnamic acids | 3,5-di-o-caffeoylquinic acid | 515.1200 | 515.1195 | 0.97 | C25H24O12 |
| 10 | Phenolic acids | Hydroxycinnamic acids | 4,5-di-o-caffeoylquinic acid | 515.1211 | 515.1195 | 3.10 | C25H24O12 |
| 11 | Phenolic acids | Hydroxycinnamic acids | Caffeoyl-1,5-quinolactone | 335.0779 | 335.0772 | 1.96 | C16H16O8 |
| 12 | Phenolic acids | Hydroxycinnamic acids | 5-O-p-Coumaroylquinic acid | 337.0949 | 337.0929 | 5.94 | C16H18O8 |
| 13 | Phenolic acids | Hydroxycinnamic acids | Feruloylquinic acid | 367.1060 | 367.1035 | 6.91 | C17H20O9 |
| 14 | Phenolic acids | Hydroxycinnamic acids | Feruloylquinic acid | 367.1060 | 367.1035 | 6.91 | C17H20O9 |
| 15 | Phenolic acids | Hydroxycinnamic acids | Sinapic acid | 223.0605 | 223.0612 | 3.11 | C11H12O5 |
| 16 | Phenolic acids | Hydroxycinnamic acids | Hydroxycaffeic acid | 195.0315 | 195.0299 | 8.18 | C9H8O5 |
| 17 | Phenolic acids | Hydroxycinnamic acids | 1,2-Disinapoylgentiobiose | 753.2272 | 753.2248 | 3.24 | C34H42O19 |
| 18 | Phenolic acids | Hydroxyphenylpropanoic | 3,4-dihydroxyphenyl-2-oxypropanoic acid | 179.0354 | 179.0350 | 2.32 | C9H8O4 |
| 19 | Flavonoids | Aurone flavonoids | 5-Hydroxy-4,4′,6-trimethoxyaurone | 327.1097 | 327.1085 | 0.13 | C18H16O6 |
| 20 | Flavonoids | Dihydroflavonols | Dihydroquercetin 3-O-rhamnoside | 449.1089 | 449.1089 | 0.08 | C21H22O11 |
| 21 | Flavonoids | Flavanols | (+)-Gallocatechin | 305.0695 | 305.0667 | 5.96 | C15H14O7 |
| 22 | Flavonoids | Flavonoid glycosides | Didymin | 593.1889 | 593.1876 | 2.22 | C28H34O14 |
| 23 | Flavonoids | Flavonols | Isorhamnetin hexoside | 477.1051 | 477.1038 | 2.62 | C22H22O12 |
| 24 | Other polyphenols | Hydroxycoumarins | Esculin | 339.0714 | 339.0722 | 2.22 | C15H16O9 |
| Parameters | F/AF * | F/2SUN * | F/4SUN * |
|---|---|---|---|
| Processing loss (%) | 15.56 ± 1.34 a | 16.33 ± 1.10 a | 15.46 ± 1.72 a |
| pH | 6.39 ± 0.02 a | 6.37 ± 0.05 b | 6.34 ± 0.03 c |
| Colour parameters | |||
| L* | 76.37 ± 0.50 a | 62.33 ± 1.09 b | 57.14 ± 0.88 c |
| a* | 6.37 ± 0.27 a | 3.24 ± 0.12 b | 2.88 ± 0.14 c |
| b* | 11.32 ± 0.31 a | 10.21 ± 0.21 b | 10.29 ± 0.27 b |
| TPA parameters | |||
| Hardness (N) | 17.52 ± 0.92 b | 18.27 ± 1.49 b | 21.67 ± 2.32 a |
| Cohesiveness | 0.64 ± 0.01 b | 0.67 ± 0.01 a | 0.67 ± 0.01 a |
| Springiness (mm) | 6.11 ± 0.08 b | 6.36 ± 0.08 a | 6.41 ± 0.08 a |
| Chewiness (mm × N) | 68.03 ± 3.19 c | 77.99 ± 6.06 b | 92.35 ± 9.82 a |
| Parameters | F/AF * | F/2SUN * | F/4SUN * |
|---|---|---|---|
| Aroma intensity | 3.32 ± 2.34 a | 4.19 ± 2.53 a | 4.76 ± 2.63 a |
| Firmness | 6.10 ± 1.76 b | 6.89 ± 1.51 ab | 7.82 ± 2.00 a |
| Juiciness | 5.42 ± 2.29 a | 4.70 ± 2.43 ab | 3.31 ± 2.34 b |
| Powdery | 1.67 ± 1.51 a | 2.53 ± 2.54 a | 3.00 ± 2.39 a |
| Flavour intensity | 5.45 ± 2.21 a | 5.56 ± 2.15 a | 6.54 ± 1.98 a |
| Overall acceptability | 6.07 ± 2.08 a | 5.85 ± 2.38 a | 4.87 ± 2.60 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grasso, S.; Pintado, T.; Pérez-Jiménez, J.; Ruiz-Capillas, C.; Herrero, A.M. Potential of a Sunflower Seed By-Product as Animal Fat Replacer in Healthier Frankfurters. Foods 2020, 9, 445. https://doi.org/10.3390/foods9040445
Grasso S, Pintado T, Pérez-Jiménez J, Ruiz-Capillas C, Herrero AM. Potential of a Sunflower Seed By-Product as Animal Fat Replacer in Healthier Frankfurters. Foods. 2020; 9(4):445. https://doi.org/10.3390/foods9040445
Chicago/Turabian StyleGrasso, Simona, Tatiana Pintado, Jara Pérez-Jiménez, Claudia Ruiz-Capillas, and Ana Maria Herrero. 2020. "Potential of a Sunflower Seed By-Product as Animal Fat Replacer in Healthier Frankfurters" Foods 9, no. 4: 445. https://doi.org/10.3390/foods9040445
APA StyleGrasso, S., Pintado, T., Pérez-Jiménez, J., Ruiz-Capillas, C., & Herrero, A. M. (2020). Potential of a Sunflower Seed By-Product as Animal Fat Replacer in Healthier Frankfurters. Foods, 9(4), 445. https://doi.org/10.3390/foods9040445