Study of Xoconostle (Opuntia spp.) Powder as Source of Dietary Fiber and Antioxidants
Abstract
1. Introduction
2. Materials and Methods
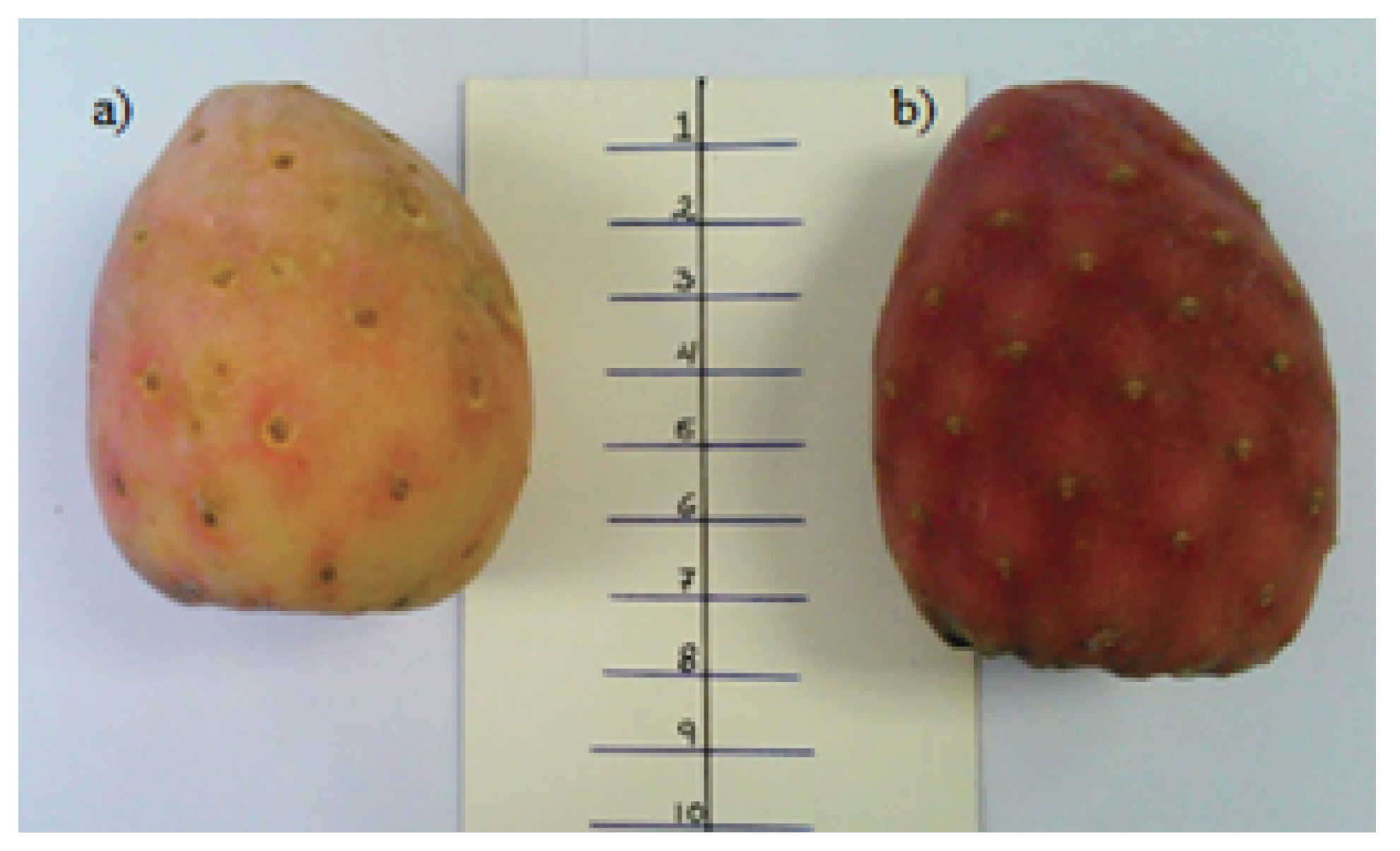
2.1. Plant Materials and Sample Preparation
2.2. Nutritional Composition
2.2.1. Proximate Analysis
2.2.2. Total Soluble Sugars
2.2.3. Total Dietary Fiber
2.2.4. Organic Acids and Vitamin C
2.3. Antioxidant Properties
2.3.1. Extraction of Antioxidants
2.3.2. Total Phenolic Compounds (TPC)
Folin–Ciocalteu
Fast Blue BB (FBBB)
Quencher Fast Blue BB (Q-FBBB)
2.3.3. Antioxidant Capacity
ABTS·+ Methodology
DPPH· Methodology
2.4. Physicochemical Characteristics
2.4.1. Color Measurement
2.4.2. Hydration Properties
2.4.3. Glucose Retention Index
2.5. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Composition
3.2. Antioxidant Properties
3.3. Color
3.4. Functional Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pimienta–Barrios, E.; Méndez–Morán, L.; Ramírez–Hernández, B.C.; García de Alba–García, J.E.; Domínguez–Arias, R.M. Efecto de la ingestión del fruto de xoconostle (Opuntia joconostle Web.) sobre la glucosa y lípidos séricos. Agrociencia 2008, 42, 645–653. [Google Scholar] [CrossRef]
- Osorio-Esquivel, O.; Ortiz-Moreno, A.; Garduño-Siciliano, L.; Álvarez, V.B.; Hernández-Navarro, M.D. Antihyperlipidemic Effect of Methanolic Extract from Opuntia joconostle Seeds in Mice Fed a Hypercholesterolemic Diet. Plant. Foods Hum. Nutr. 2012, 67, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fuentes, A.D.; Trapala-Islas, A.; Gallegos-Vásquez, C.; Campos-Montiel, R.G.; Pinedo-Espinoza, J.M.; Guzmán-Maldonado, S.H. Physicochemical variability and nutritional and functional characteristics of xoconostles (Opuntia spp.) accessions from Mexico. Fruits 2015, 70, 109–116. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; Gómez-García, M.D.R.; Valverde, M.E.; Paredes-López, O. Nopal (Opuntia spp.) and its Effects on Metabolic Syndrome: New Insights for the Use of a Millenary Plant. Curr. Pharm Des. 2019, 25, 3457–3477. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Cactus stems (Opuntia spp.): A review on their chemistry, technology, and uses. Mol. Nutr. Food Res. 2005, 49, 175–194. [Google Scholar] [CrossRef]
- Guevera-Figueroa, T.; Jimenez-Islas, H.; Reyes-Escobido, M.L.; Mortensen, A.G.; Laursen, B.B.; Lin, L.W.; De Léon-Rodriguez, A.; Fomsgaard, I.S.; Barba de la Rosa, A.P. Proximate composition, phenolic acids, and flavonoids characterization of commercial and wild nopal (Opuntia spp.). J. Food Compost. Anal. 2010, 23, 525–532. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Perez-Torrero, E.; Hernández Urbiola, M.I.; Hernández-Quevedo, G.; Del Real, A.; Rivera Muñoz, E.M.; Rodriguez-García, M.E. Evaluation of oxalates and calcium in nopal pads (Opuntia ficus-indica var. redonda) at different maturity stages. J. Food Compost. Anal. 2011, 24, 38–43. [Google Scholar] [CrossRef]
- Hernández-Urbiola, M.I.; Perez-Torrero, E.; Rodriguez-García, M.E. Chemical analysis of nutritional content of prickly pads (Opuntia ficus indica) at varied ages in an organic harvest. Int. J. Environ. Res. Public Health 2011, 8, 1287–1295. [Google Scholar] [CrossRef]
- Morales, P.; Ramírez-Moreno, E.; Sanchez-Mata, M.d.C.; Carvalho, A.M.; Ferreira, I.C.F.R. Nutritional and antioxidant properties of pulp and seeds of two xoconostle cultivars (Opuntia joconostle F.A.C. Weber ex Diguet and Opuntia matudae Scheinvar) of high consumption in Mexico. Food Res. Int. 2012, 46, 279–285. [Google Scholar] [CrossRef]
- Morales, P.; Barros, L.; Ramírez-Moreno, E.; Santos-Buelga, C.; Ferreira, I.C.F.R. Exploring xoconostle by products as sources of bioactive compounds. Food Res. Int. 2014, 65, 437–444. [Google Scholar] [CrossRef]
- Morales, P.; Barros, L.; Ramírez-Moreno, E.; Santos-Buelga, C.; Ferreira, I.C.F.R. Xoconostle fruit (Opuntia matudae Scheinvar cv. Rosa) by-products as potential functional ingredients. Food Chem. 2015, 185, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Maldonado, S.H.; Morales-Montelongo, A.L.; Mondragón-Jacobo, C.; Herrera-Hernández, G.; Guevara-Lara, F.; Reynoso-Camacho, R. Physicochemical, Nutritional, and Functional Characterization of Fruits Xoconostle (Opuntia matudae) Pears from Central-México Region. J. Food Sci. 2010, 75, C485–C492. [Google Scholar] [CrossRef] [PubMed]
- Contreras, L.E.; Jaimez, O.J.; Villanueva, R.S. Sensory profile and chemical composition of Opuntia joconostle from Hidalgo, Mexico. J. Stored Prod. Postharvest Res. 2011, 2, 37–39. [Google Scholar]
- Gallegos-Vazquez, C.; García, R. ‘Borrego’: A new variety of xoconostle with nutritional and functional value for the Central Mesa región of México. Rev. Mexicana Cienc. Agric. 2018, 9, 259–265. [Google Scholar] [CrossRef][Green Version]
- Sanchez-Gonzalez, N.; Jaime-Fonseca, M.R.; San Martin-Martinez, E.; Zepeda, L.G. Extraction, Stability, and Separation of Betalains from Opuntia joconostle cv. Using Response Surface Methodology. J. Agric. Food Chem. 2013, 61, 1995–12004. [Google Scholar] [CrossRef]
- Inglese, P.; Mondragon, C.; Nefzaoui, A.; Saenz, C.; Taguchi, M.; Makkar, H.; Louhaiichi, M. Ecologia del Cultivo, Manejo y Usos del Nopal; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2019; Available online: https://repo.mel.cgiar.org/handle/20.500.11766/9380. (accessed on 10 November 2019).
- Latimer, G.W. Official Methods of Analysis of the Association of Official Analytical Chemists (AOAC), 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Sanchez-Mata, M.C.; Peñuela-Teruel, M.J.; Cámara-Hurtado, M.; Díez-Marqués, C.; Torija-Isasa, M.E. Determination of Mono-, Di-, and Oligosaccharides in Legumes by High-Performance Liquid Chromatography Using an Amino-Bonded Silica Column. J. Agric. Food Chem. 1998, 46, 3648–3652. [Google Scholar] [CrossRef]
- Sánchez-Mata, M.C.; Cabrera Loera, R.D.; Morales, P.; Fernández-Ruiz, V.; Cámara, M.; Díez Marqués, C.; Pardo-de-Santayana, M.; Tardío, J. Wild vegetables of the Mediterranean area as valuable sources of bioactive compounds. Genet. Resour. Crop. Evol. 2012, 59, 431–443. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz- Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticul. 1965, 16, 144–158. [Google Scholar]
- Maieves, H.A.; López-Froilán, R.; Morales, P.; Pérez-Rodríguez, M.; HoffmannRibani, R.; Cámara, M.; Sánchez-Mata, M. Antioxidant phytochemicals of HoveniadulcisThunb. peduncles in different maturity stages. J. Funct Foods. 2015, 18, 1117–1124. [Google Scholar] [CrossRef]
- Gökmen, V.; Serpen, A.; Fogliano, V. Direct measurement of the total antioxidant capacity of foods: The “QUENCHER” approach. Trends Food Sci. Technol. 2009, 20, 278–288. [Google Scholar] [CrossRef]
- Palombini, S.V.; Claus, T.; Maruyama, S.A.; Carbonera, F.; Montanher, P.F.; Visentainer, J.V.; Gomes, S.T.M.; Matsushita, M. Optimization of a New Methodology for Determination of Total Phenolic Content in Rice Employing Fast Blue BB and QUENCHER Procedure. J. Braz. Chem. Soc. 2016, 27, 1188–1194. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Ciênc. Tecnol. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Morales, F.J.; Jiménez-Pérez, S. Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chem. 2001, 72, 119–125. [Google Scholar] [CrossRef]
- Francis, F.J. Color quality evaluation of horticultural crops. Hort. Sci. 1980, 15, 58–59. [Google Scholar]
- Valencia, G.F.E.; Román, M.M.O. Caracterización fisicoquímica y funcional de tres concentrados comerciales de fibra dietaria. Vitae 2006, 13, 54–60. [Google Scholar]
- Ou, S.; Kwok, K.; Li, Y.; Fu, L. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J Agric. Food Chem. 2001, 49, 1026–1029. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- González-Ramos, I.; Ortega-Figueroa, M.J.; Mendoza-González, S.; Martínez-Peniche, A.R. Caracterización Física y Química del Fruto de Xoconostle en la Región Suroeste del estado de Guanajuato: Guanajuato, Mexico. 2011. Available online: https://quimica.uaq.mx/docs/MCTA_2014/articulos/uaq_mcta_29_articulo_SOMECH_20091.pdf. (accessed on 10 November 2019).
- Prieto-García, F.; Prieto Méndez, J.; Acevedo-Sandoval, O.A.; Canales Flores, R.A.; MarmolejoSantillán, Y. Chemical and physical characterization of Opuntia spp. seeds grown in Hidalgo State, Mexico. Cienc. Investig. Agrar. 2016, 43, 143–150. [Google Scholar] [CrossRef]
- European Commission, Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims. Off. J. Eur. Union 2006, L404, 9–25.
- Marlett, J.A.; McBurney, M.; Slavin, J.L. Position of the American Dietetic Association. J. Am. Diet. Assoc. 2002, 102, 993–1000. [Google Scholar] [CrossRef]
- FAO; World Health Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Chong, C.H.; Law, C.L.; Figiel, A.; Woydylo, A.; Oziemblowski, M. Colour, phenolic content and antioxidant capacity of some fruits dehydrated by a combination of different methods. Food Chem. 2013, 141, 3889–3896. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.B. Simple and Rapid Method for the Analysis of Phenolic Compounds in Beverages and Grains. J. Agric. Food Chem. 2011, 59, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- López-Froilán, R.; Hernández-Ledesma, B.; Cámara, M.; Pérez-Rodríguez, M.L. Evaluation of the Antioxidant Potential of Mixed Fruit-Based Beverages: A New Insight on the Folin-Ciocalteu Method. Food Anal. Methods 2018, 11, 2897–2906. [Google Scholar] [CrossRef]
- Del Pino-García, R.; García-Lomillo, J.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. Adaptation and Validation of QUick, Easy, New, CHEap, and Reproducible (QUENCHER) Antioxidant Capacity Assays in Model Products Obtained from Residual Wine Pomace. J. Agric. Food Chem. 2015, 63, 6922–6931. [Google Scholar] [CrossRef]
- Medina, M.B. Determination of the total phenolics in juices and superfruits by a novel chemical method. J. Funct Foods 2011, 3, 79–87. [Google Scholar] [CrossRef]
- Petrovic, M.; Suznievic, D.; Pastor, F.; Veliovic, M.; Pezo, L.; Antic, M.; Gorianovic, S. Antioxidant Capacity Determination of Complex Samples and Individual Phenolics - Multilateral Approach. Comb. Chem. High. Throughput Screen 2016, 19, 58–65. [Google Scholar] [CrossRef]
- Cortes-García, R.M.; Ortiz-Moreno, A.; Zepeda-Vallejo, L.G.; Necoechea-Mondragón, H. Effects of cooking methods on phenolic compounds in Xoconostle (Opuntia joconostle). Plant. Food Hum. Nutr. 2015, 70, 85–90. [Google Scholar] [CrossRef]
- Monter-Arciniega, A.; Hernández-Falcón, T.A.; de Socorro Cruz-Cansino, N.; Ramírez-Moreno, E.; Alanís-García, E.; Arias-Rico, J.; Ariza-Ortega, J.A. Functional Properties, Total Phenolic Content and Antioxidant Activity of Purple Cactus Pear (Opuntia ficus-indica) Waste: Comparison with Commercial Fibers. Waste Biomass Valoriza. 2019, 10, 2897–2906. [Google Scholar] [CrossRef]
- Osorio-Esquivel, O.; Álvarez, V.B.; Dorantes-Álvarez, L.; Giusti, M.M. Phenolics, betacyanins and antioxidant activity in Opuntia joconostle fruits. Food Res. Int. 2011, 44, 2160–2168. [Google Scholar] [CrossRef]
- Lutz, M.; Hernández, J.; Henríquez, C. Phenolic content and antioxidant capacity in fresh and dry fruits and vegetables grown in Chile. CyTA-Jf Food 2015, 13, 541–547. [Google Scholar] [CrossRef]
- Goñi, I.; Valdivieso, L.; Guidel-Urbano, M. Capacity of edible seaweeds to modify in vitro starch digestibility of wheat bread. Nahrung 2002, 46, 18–20. [Google Scholar] [CrossRef]
- Champ, M.; Langkilde, A.M.; Brouns, F.; Kettlitz, B.; Collet, Y.L.B. Advances in dietary fibre characterisation. 1. Definition of dietary fibre, physiological relevance, health benefits and analytical aspects. Nutr. Res. Rev. 2003, 16, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M.J.; Sánchez-Zapata, E.; PérezÁlvarez, J.A. Resistant starch as functional ingredient: A review. Food Res. Int. 2010, 43, 931–942. [Google Scholar] [CrossRef]
- López, G.; Ros, G.; Rincón, F.; Periago, M.J.; Martinez, M.C.; Ortuno, J. Relationship between physical and hydration properties of soluble and insoluble fiber of artichoke. J. Agric. Food Chem. 1996, 44, 2773–2778. [Google Scholar] [CrossRef]
- Zlatanović, S.; Ostojić, S.; Micić, D.; Rankov, S.; Dodevska, M.; Vukosavljević, P.; Gorjanović, S. Thermal behaviour and degradation kinetics of apple pomace flours. Thermochim Acta 2019, 673, 17–25. [Google Scholar] [CrossRef]
- Figuerola, F.; Hurtado, M.L.; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- Abirami, A.; Nagarani, G.; Siddhuraju, P. Measurement of functional properties and health promoting aspects-glucose retardation index of peel, pulp and peel fiber from Citrus hystrix and Citrus maxima. Bioact. Carbohydr. Diet. Fibre 2014, 4, 16–26. [Google Scholar] [CrossRef]
- Zlatanović, S.; Kalušević, A.; Micić, D.; Laličić-Petronijević, J.; Tomić, N.; Ostojić, S.; Gorjanović, S. Functionality and Storability of Cookies Fortified at the Industrial Scale with up to 75% of Apple Pomace Flour Produced by Dehydration. Foods 2019, 8, 561. [Google Scholar] [CrossRef]
- Raghavendra, S.N.; Swamy, S.R.; Rastogi, N.K.; Raghavarao, K.S.M.S.; Kumar, S.; Tharanathan, R.N. Grinding characteristics and hydration properties of coconut residue: A source of dietary fiber. J. Food Eng. 2006, 72, 281–286. [Google Scholar] [CrossRef]
| cv Cuaresmeño | cv Rosa | |
|---|---|---|
| Moisture of fresh fruit | 90.4 ± 0.7 ** | 85.9 ± 1.3 |
| Moisture | 7.2 ± 0.1 ** | 8.2 ± 0.2 |
| Protein | 4.0 ± 0.0 *** | 4.8 ± 0.1 |
| Fat | 2.5 ± 0.1 *** | 11.9 ± 0.1 |
| Ash | 11.3 ± 2.9 | 11.7 ± 1.5 |
| Total soluble sugars | 9.8 ± 0.7 *** | 29.9 ± 0.5 |
| Sucrose | 1.5 ± 0.2 *** | 6.3 ± 0.1 |
| Fructose | 1.5 ± 0.4 *** | 8.0 ± 0.3 |
| Glucose | 6.8 ± 0.2 ** | 15.6 ± 0.2 |
| Total dietary fiber | 30.8 ± 0.7 *** | 36.8 ± 0.9 |
| Soluble fiber | 8.2 ± 0.4 ** | 5.8 ± 0.4 |
| Insoluble fiber | 22.6 ± 0.4 *** | 31.0 ± 0.6 |
| Organic acids B | 20.8 ± 0.0 *** | 17.0 ± 0.0 |
| Malic acid | 1.3 ± 0.0 *** | 2.9 ± 0.0 |
| Citric acid | 18.9 ± 0.0 *** | 13.6 ± 0.0 |
| Fumaric acid | 0.1 ± 0.0 *** | 0.1 ± 0.0 |
| Oxalic acid | 0.6 ± 0.0 *** | 0.5 ± 0.0 |
| Total vitamin C B | 723.1 ± 16.0 *** | 320.2 ± 7.5 |
| Ascorbic acid (AA) | 169.4 ± 2.7 *** | 385.5 ± 6.1 |
| Dehydroascorbic acid (DHAA) | 337.6 ± 10.8 *** | 150.8 ± 6.1 |
| cv Cuaresmeño | cv Rosa | |
|---|---|---|
| Total phenolic compounds A | ||
| Folin–Ciocalteu | 1580.3 ± 33.1 *** | 1068.5 ± 70.8 |
| FBBB | 1415.2 ± 45.6 *** | 1088.5 ± 5.3 |
| Q-FBBB | 1752.4 ± 21.5 ** | 1438.5 ± 71.9 |
| Ascorbic acid B | 169.4 ± 2.7 *** | 385.5 ± 6.1 |
| ABTS·+ C | 3261.4 ± 102.7 *** | 1348.1 ± 74.0 |
| DPPH·C | 3318.7 ± 178.8 *** | 1753.5 ± 72.8 |
| cv Cuaresmeño | cv Rosa | |
|---|---|---|
| Color | ||
| Lightness (L*) | 33.5 ± 0.1 *** | 22.4 ± 0.0 |
| Intensity of red (a*) | 5.3 ± 0.0 *** | 10.4 ± 0.0 |
| Intensity of yellow (b*) | 14.0 ± 0.0 *** | 9.6 ± 0.0 |
| ° h | 69.2 ± 0.1 *** | 42.7 ± 0.0 |
| Chroma | 15.0 ± 0.0 *** | 14.2 ± 0.0 |
| Water holding capacity (WHC) A | 6.0 ± 0.1 * | 5.5 ± 0.2 |
| Swelling (S) A | 5.2 ± 0.0 | 5.5 ± 0.0 |
| Oil holding capacity (OHC) B | 6.1 ± 0.3 * | 3.4 ± 0.2 |
| Glucose retention index (GRI) C | ||
| Glucose concentration | ||
| 50 mmol/L | 48.6 ± 0.1 * | 48.1 ± 0.0 |
| 100 mmol/L | 96.8 ± 0.1 *** | 95.1 ± 0.2 |
| 200 mmol/L | 189.2 ± 0.5 | 189.8 ± 0.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Rico, J.; Cruz-Cansino, N.d.S.; Cámara-Hurtado, M.; López-Froilán, R.; Pérez-Rodríguez, M.L.; Sánchez-Mata, M.d.C.; Jaramillo-Morales, O.A.; Barrera-Gálvez, R.; Ramírez-Moreno, E. Study of Xoconostle (Opuntia spp.) Powder as Source of Dietary Fiber and Antioxidants. Foods 2020, 9, 403. https://doi.org/10.3390/foods9040403
Arias-Rico J, Cruz-Cansino NdS, Cámara-Hurtado M, López-Froilán R, Pérez-Rodríguez ML, Sánchez-Mata MdC, Jaramillo-Morales OA, Barrera-Gálvez R, Ramírez-Moreno E. Study of Xoconostle (Opuntia spp.) Powder as Source of Dietary Fiber and Antioxidants. Foods. 2020; 9(4):403. https://doi.org/10.3390/foods9040403
Chicago/Turabian StyleArias-Rico, José, Nelly del Socorro Cruz-Cansino, Montaña Cámara-Hurtado, Rebeca López-Froilán, María Luisa Pérez-Rodríguez, María de Cortes Sánchez-Mata, Osmar Antonio Jaramillo-Morales, Rosario Barrera-Gálvez, and Esther Ramírez-Moreno. 2020. "Study of Xoconostle (Opuntia spp.) Powder as Source of Dietary Fiber and Antioxidants" Foods 9, no. 4: 403. https://doi.org/10.3390/foods9040403
APA StyleArias-Rico, J., Cruz-Cansino, N. d. S., Cámara-Hurtado, M., López-Froilán, R., Pérez-Rodríguez, M. L., Sánchez-Mata, M. d. C., Jaramillo-Morales, O. A., Barrera-Gálvez, R., & Ramírez-Moreno, E. (2020). Study of Xoconostle (Opuntia spp.) Powder as Source of Dietary Fiber and Antioxidants. Foods, 9(4), 403. https://doi.org/10.3390/foods9040403







