Determination and Comparison of Physical Meat Quality Parameters of Percidae and Salmonidae in Aquaculture
Abstract
1. Introduction
2. Material and Methods
2.1. Fish Rearing and Experimental Design
2.2. Sampling and Morphometric Data Collection
2.3. Physical Meat Quality
2.4. Statistics
3. Results
3.1. Animals
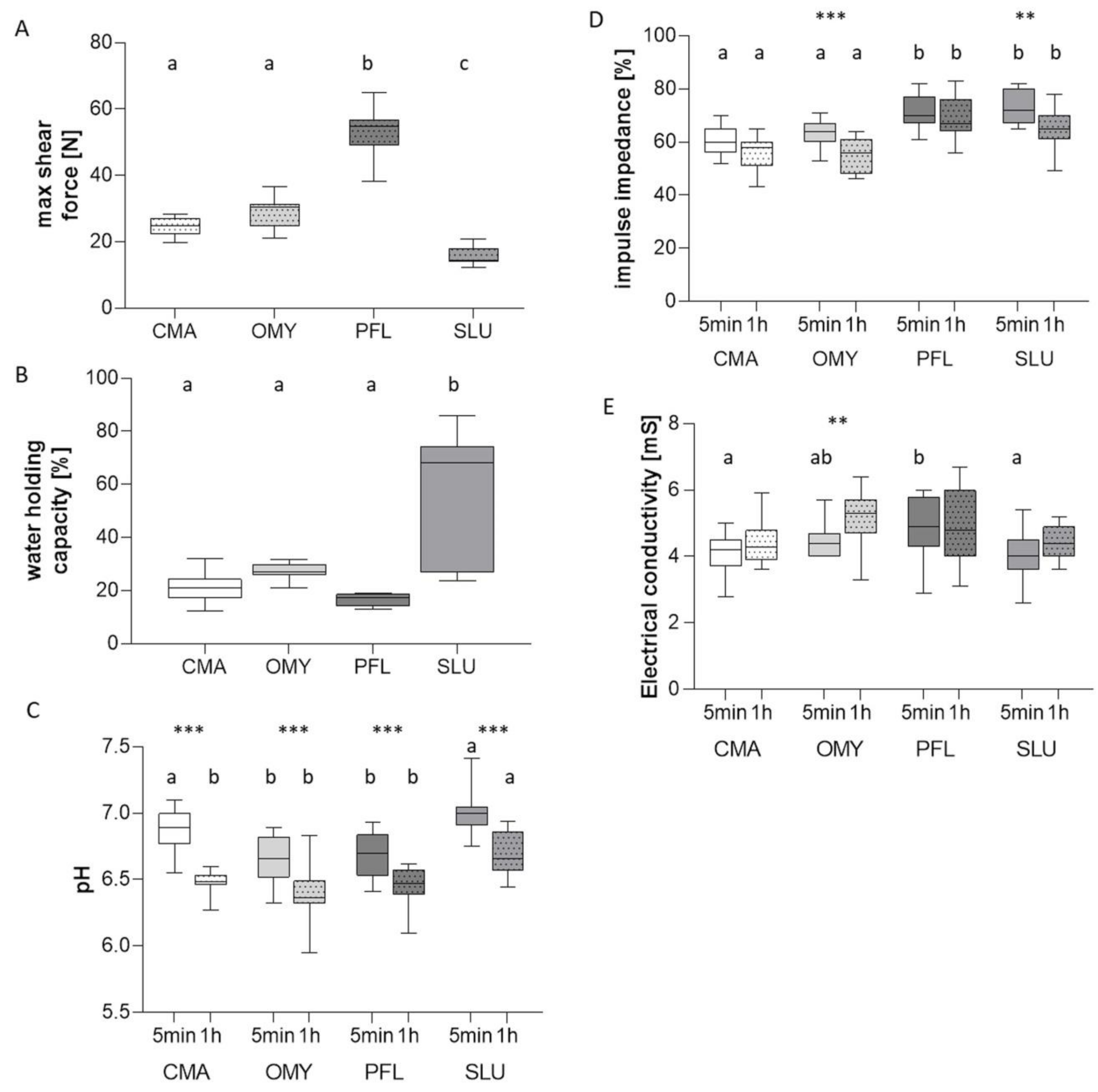
3.2. Flesh Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nathanailides, C.; Panopoulos, S.; Kakali, F.; Karipoglou, C.; Lenas, D. Antemortem and postmortem biochemistry, drip loss and lipid oxidation of European sea bass muscle tissue. Procedia Food Sci. 2011, 1, 1099–1104. [Google Scholar] [CrossRef]
- Picard, B.; Lefèvre, F.; Lebret, B. Meat and fish flesh quality improvement with proteomic applications. Anim. Front. 2012, 2, 18–25. [Google Scholar] [CrossRef]
- Hoffmann, K. Was ist Fleischqualität? Fleischwirtschaft 1973, 53, 485. [Google Scholar]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Sigurgisladottir, S.; Torrissen, O.; Lie, Ø.; Thomassen, M.; Hafsteinsson, H. Salmon quality: Methods to determine the quality parameters. Rev. Fish. Sci. 1997, 5, 223–252. [Google Scholar] [CrossRef]
- Lie, Ø. Flesh quality—The role of nutrition. Aquac. Res. 2001, 32, 341–348. [Google Scholar] [CrossRef]
- Sigurgisladottir, S.; Hafsteinsson, H.; Jonsson, A.; Lie, O.; Nortvedt, R.; Thomassen, M.; Torrissen, O. Textural Properties of Raw Salmon Fillets as Related to Sampling Method. J. Food Sci. 1999, 64, 99–104. [Google Scholar] [CrossRef]
- Bahuaud, D.; Mørkøre, T.; Østbye, T.-K.; Veiseth-Kent, E.; Thomassen, M.S.; Ofstad, R. Muscle structure responses and lysosomal cathepsins B and L in farmed Atlantic salmon (Salmo salar L.) pre- and post-rigor fillets exposed to short and long-term crowding stress. Food Chem. 2010, 118, 602–615. [Google Scholar] [CrossRef]
- Concollato, A.; Olsen, R.E.; Vargas, S.C.; Bonelli, A.; Cullere, M.; Parisi, G. Effects of stunning/slaughtering methods in rainbow trout (Oncorhynchus mykiss) from death until rigor mortis resolution. Aquaculture 2016, 464, 74–79. [Google Scholar] [CrossRef]
- Dang, H.T.T.; Gudjonsdóttir, M.; Karlsdóttir, M.G.; Van Nguyen, M.; Tómasson, T.; Arason, S. Stability of Golden redfish (Sebastes marinus) during frozen storage as affected by raw material freshness and season of capture. Food Sci. Nut. 2018, 6, 1065–1076. [Google Scholar] [CrossRef]
- Gaarder, M.Ø.; Bahuaud, D.; Veiseth-Kent, E.; Mørkøre, T.; Thomassen, M.S. Relevance of calpain and calpastatin activity for texture in super-chilled and ice-stored Atlantic salmon (Salmo salar L.) fillets. Food Chem. 2012, 132, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lerfall, J.; Roth, B.; Skare, E.F.; Henriksen, A.; Betten, T.; Dziatkowiak-Stefaniak, M.A.; Rotabakk, B.T. Pre-mortem stress and the subsequent effect on flesh quality of pre-rigor filleted Atlantic salmon (Salmo salar L.) during ice storage. Food Chem. 2015, 175, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Rennert, B.; Wirth, M.; Gunther, S.; Schulz, C. Effect of feeding under-year zander (Sander lucioperca) on size, body mass and body composition before and after wintering. J. Appl. Ichthyol. 2005, 21, 429–432. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, J.; Zhang, X.; He, X.; Li, L.; Tang, R.; Li, D. Diet Affects Muscle Quality and Growth Traits of Grass Carp (Ctenopharyngodon idellus): A Comparison between Grass and Artificial Feed. Front. Physiol. 2018, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Aman, M.B. Effect of cooking and preservation methods on the Water Holding Capacity (WHC) of mullet fish in relation with changes occurred in muscle proteins. Z. Lebensm. Unters. Forch. 1983, 177, 345–347. [Google Scholar] [CrossRef]
- Skipnes, D.; Østby, M.; Hendrickx, M. A method for characterising cook loss and water holding capacity in heated cod (Gadus morhua) muscle. J. Food Eng. 2007, 80, 1078–1085. [Google Scholar] [CrossRef]
- Filomena-Ambrosio, A.; Quintanilla-Carvajal, M.X.; Puig, A.; Hernando, I.; Hernández-Carrión, M.; Sotelo-Díaz, I. Changes of the water-holding capacity and microstructure of panga and tilapia surimi gels using different stabilizers and processing methods. Food Sci. Technol. Int. 2016, 22, 68–78. [Google Scholar] [CrossRef]
- Andersen, U.B.; Thomassen, M.S.; Rørå, A.M.B. Texture Properties of Farmed Rainbow Trout (Oncorhynchus mykiss): Effects of Diet, Muscle Fat Content and Time of Storage on Ice. J. Sci. Food Agric. 1997, 74, 347–353. [Google Scholar] [CrossRef]
- Hocquette, J.F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal 2010, 4, 303. [Google Scholar] [CrossRef]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef]
- van Wijk, H.J.; Arts, D.J.G.; Matthews, J.O.; Webster, M.; Ducro, B.J.; Knol, E.F. Genetic parameters for carcass composition and pork quality estimated in a commercial production chain1. J. Anim. Sci. 2005, 83, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Backström, T.; Heynen, M.; Nilsson, J.; Winberg, S.; Magnhagen, C. Anaesthesia and handling stress effects on pigmentation and monoamines in Arctic charr. Environ. Biol. Fish. 2017, 100, 471–480. [Google Scholar] [CrossRef]
- Berg, T.; Erikson, U.; Nordtvedt, T.S. Rigor mortis assessment of Atlantic salmon (Salmo salar) and effects of stress. J. Food Sci. 1997, 62, 439–446. [Google Scholar] [CrossRef]
- Erikson, U.; Sigholt, T.; Rustad, T.; Einarsdottir, I.E.; Jørgensen, L. Contribution of Bleeding to Total Handling Stress during Slaughter of Atlantic Salmon. Aquac. Int. 1999, 7, 101–115. [Google Scholar] [CrossRef]
- Mørkøre, T.; Mazo, P.; Tahirovic, V.; Einen, O. Impact of starvation and handling stress on rigor development and quality of Atlantic salmon (Salmo salar L). Aquaculture 2008, 277, 231–238. [Google Scholar] [CrossRef]
- Diouf, B.; Rioux, P. Use of the Rigor Mortis Process as a Tool for Better Understanding of Skeletal Muscle Physiology: Effect of Ante-Mortem Stress on the Progression of Rigor Mortis in Brook Charr (Salvelinus fontinalis). Am. Biol. Teach. 1999, 61, 376–379. [Google Scholar]
- Roth, B.; Moeller, D.; Veland, J.O.; Imsland, A.; Slinde, E. The Effect of Stunning Methods on Rigor Mortis and Texture Properties of Atlantic Salmon (Salmo Salar). J. Food Sci. 2006, 67, 1462–1466. [Google Scholar] [CrossRef]
- Sigholt, T.; Erikson, U.; Rustad, T.; Johansen, S.; Nordtvedt, T.; Seland, A. Handling Stress and Storage Temperature Affect Meat Quality of Farmed-raised Atlantic Salmon (Salmo Salar). J. Food Sci. 2006, 62, 898–905. [Google Scholar] [CrossRef]
- Skjervold, P.; Fjæra, S.; Østby, P. Rigor in Atlantic salmon as affected by crowding stress prior to chilling before slaughter. Aquaculture 1999, 175, 93–101. [Google Scholar] [CrossRef]
- Thomas, P.; Pankhurst, N.; Bremner, A. The effect of stress and exercise on post-mortem biochemistry of Atlantic salmon and rainbow trout. J. Fish. Biol. 2005, 54, 1177–1196. [Google Scholar] [CrossRef]
- Poli, B.M.; Parisi, G.; Scappini, F.; Zampacavallo, G. Fish welfare and quality as affected by pre-slaughter and slaughter management. Aquac. Int. 2005, 13, 29–49. [Google Scholar] [CrossRef]
- Mantilla, D.; Kristinsson, H.G.; Balaban, M.O.; Otwell, W.S.; Chapman, F.A.; Raghavan, S. Carbonmonoxide treatments to impart and retainmuscle color in tilapia fillets. J. Food Sci. 2008, 73, C390–C399. [Google Scholar] [CrossRef] [PubMed]
- Manthey-Karl, M.; Lehmann, I.; Ostermeyer, U.; Schröder, U. Natural Chemical Composition of Commercial Fish Species: Characterisation of Pangasius, Wild and Farmed Turbot and Barramundi. Foods 2016, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Stubhaug, I.; Menoyo, D.; Esatbeyoglu, T.; Wagner, A.E.; Struksnæs, G.; Rimbach, G. Dietary alpha-tocopherol affects tissue vitamin e and malondialdehyde levels but does not change antioxidant enzymes and fatty acid composition in farmed Atlantic salmon (Salmo salar L.). Int. J. Vitam. Nut. Res. 2013, 83, 238–245. [Google Scholar] [CrossRef]
- Mock, T.S.; Francis, D.S.; Jago, M.K.; Glencross, B.D.; Smullen, R.P.; Turchini, G.M. Endogenous biosynthesis of n-3 long-chain PUFA in Atlantic salmon. Br. J. Nutr. 2019, 121, 1108–1123. [Google Scholar] [CrossRef]
- Buhrke, F.; Bochert, R.; Tielebier, A. Local research: European perch production in the Research facility Born a Darß. Mitteilungen Landesforschungsanstalt Landwirtsch. Fisch. Mecklenbg. Vorpommern 2019, 61, 79–90. [Google Scholar]
- Knaus, U.; Gallandt, G. Reproduktion, Erbrütung, Anfütterung, Nahrungsumstellung und Haltung von Zandern (Sander lucioperca, L.). Mitteilungen Landesforschungsanstalt Landwirtsch. Fisch. 2011, 45, 71–85. [Google Scholar]
- Rapp, T.; Stüeken, M. Pikeperch—A species for the diversification of aquaculture production in recirculating systems. Mitteilungen Landesforschungsanstalt Landwirtsch. Fisch. 2019, 61, 64–72. [Google Scholar]
- Rebl, A.; Verleih, M.; Nipkow, M.; Altmann, S.; Bochert, R.; Goldammer, T. Gradual and Acute Temperature Rise Induces Crossing Endocrine, Metabolic, and Immunological Pathways in Maraena Whitefish (Coregonus maraena). Front. Genet. 2018, 9, 241. [Google Scholar] [CrossRef]
- Zakęś, Z.; Demska-Zakęś, K. Controlled reproduction of pikeperch Sander lucioperca (L.): A review. Arch. Pol. Fish. 2009, 17, 153–170. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Ponzoni, R.W.; Hamzah, A.; Yee, H.Y.; Abu-Bakar, K.R.; Khaw, H.L. Genetics of Flesh Quality in Fish. In Proceedings of the 9th World Congress on Genetics Applied to Livestock Production proceedings, Leipzig, Germany, 1–6 August 2010. [Google Scholar]
- Anders, E.; Bochert, R. Meilenstein BORN-Forelle. In 50 Jahre Aquakulturforschung in Born. Mitteilungen Landesforschungsanstalt Landwirtsch. Fisch. Mecklenbg. Vorpommern 2019, 61, 53–63. [Google Scholar]
- Chen, L.; Opara, U.L. Texture measurement approaches in fresh and processed foods—A review. Food Res. Int. 2013, 51, 823–835. [Google Scholar] [CrossRef]
- Grosse, F.; Brettschneider, U.; Saß, G. Eine Methode zur direkten Bestimmung des Preßsaftes von Fleisch. Fleisch 1975, 29, 104. [Google Scholar]
- Grau, R.; Hamm, R. Über das Wasserbindevermögen im Fleisch. Fleischwirtschaft 1957, 32, 295. [Google Scholar]
- Fiala, M.; Honikel, K.O. The application of the differential scanning calorimetry (dsc). About the detection of the state of aging of beef with dsc and impulse impedance. Fleischwirtschaft 1995, 75, 920–925. [Google Scholar]
- Gil-Sánchez, L.; Martínez-Máñez, R.; Barat, J.M.; Garcia-Breijo, E. An electronic tongue for fish freshness analysis using a thick-film array of electrodes. Microchim. Acta 2016, 163, 121–129. [Google Scholar]
- FAO Fisheries and Aquaculture Fact Sheets. 2015. Available online: http://www.fao.org/3/v7180e/v7180e09.htm (accessed on 24 August 2019).
- Warner, R.D.; Greenwood, P.L.; Pethick, D.W.; Ferguson, D.M. Genetic and environmental effects on meat quality. Meat Sci. 2010, 86, 171–183. [Google Scholar] [CrossRef]
- Honikel, K.O.; Kim, C.J.; Hamm, R.; Roncales, P. Sarcomere shortening of prerigor muscles and its influence on drip loss. Meat Sci. 1986, 16, 267–282. [Google Scholar] [CrossRef]
- van Laack, R.L.; Stevens, S.G.; Stadler, K.J. The influence of ultimate pH and intramuscular fat content on pork tenderness and tenderization. J. Anim. Sci. 2001, 79, 392–397. [Google Scholar] [CrossRef]
- Verrez-Bagnis, J.N.; Sautereau, C.; Fleurence, J. Desmin Degradation in Postmortem Fish Muscle. J. Food Sci. 1999, 64, 240–242. [Google Scholar] [CrossRef]
- Sriket, C. Proteases in fish and shellfish: Role on muscle softening and prevention. Int. Food Res. J. 2014, 21, 433–445. [Google Scholar]
- Santos, A.I.; Nguyen, N.H.; Ponzoni, R.W.; Yee, H.Y.; Hamzah, A.; Ribeiro, R.P. Growth and survival rate of three genetic groups fed 28% and 34% protein diets. Aquac. Res. 2014, 45, 353–361. [Google Scholar] [CrossRef]
- Hopkins, D.L.; Toohey, E.S.; Lamb, T.A.; Kerr, M.J.; van de Ven, R.; Refshauge, G. Explaining the variation in the shear force of lamb meat using sarcomere length, the rate of rigor onset and pH. Meat Sci. 2011, 88, 794–796. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.M. Muscle and Meat Biochemistry, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Abbas, K.A.; Mohamed, A.; Jamilah, B.; Ebrahimian, M. A Review on Correlations between Fish Freshness and pH during Cold Storage. Am. J. Biochem. Biotechnol. 2008, 4, 416–421. [Google Scholar] [CrossRef]
- Daskalova, A. Farmed fish welfare: Stress, post-mortem muscle metabolism, and stress-related meat quality changes. Int. Aquat. Res. 2019, 11, 113–124. [Google Scholar] [CrossRef]
- Kyrana, V.R.; Lougovois, V.P.; Valsamis, D.S. Assessment of shelf-life of maricultured gilthead sea bream (Sparus aurata) stored in ice. Int. J. Food Sci. Technol. 1997, 32, 339–347. [Google Scholar] [CrossRef]
- Huss, H.H. Quality and quality changes in fresh fish. In FAO Fisheries Technical Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 1995. [Google Scholar]
- FAO Fisheries and Aquaculture Fact Sheets. 2015. Available online: http://www.fao.org/3/v7180e/v7180e06.htm (accessed on 24 August 2019).
- Pliquett, U.; Altmann, M.; Pliquett, F.; Schöberlein, L. P(y)-a parameter for meat quality. Meat Sci. 2003, 65, 1429–1437. [Google Scholar] [CrossRef]
- Arshad, M.S. Meat Science and Nutrition. BCCAMPUS Open Educ. 2018. [Google Scholar] [CrossRef]
- Gatica, M.C.; Monti, G.E.; Knowles, T.G.; Gallo, C.B. Muscle pH, rigor mortis and blood variables in Atlantic salmon transported in two types of well-boat. Vet. Rec. 2010, 166, 45–50. [Google Scholar] [CrossRef]
- Goes, E.S.D.R.; Goes, M.D.; Castro, P.L.; de Lara, J.A.F.; de Vital, A.C.P.; Ribeiro, R.P. Imbalance of the redox system and quality of tilapia fillets subjected to pre-slaughter stress. PLoS ONE 2019, 14, e0210742. [Google Scholar] [CrossRef]
- Robb, D.H.F.; Kestin, S.C.; Warriss, P.D. Muscle activity at slaughter: I. Changes in flesh color and gaping in rainbow trout. Aquaculture 2000, 182, 261–269. [Google Scholar] [CrossRef]
| CMA | OMY | PFL | SLU | |
|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| total length [cm] | 32.95 ± 0.27 | 31.11 ± 0.24 | 37.58 ± 0.37 | 49.53 ± 0.85 |
| circumference [cm] | 16.00 ± 0.21 | 19.31 ± 0.26 | 25.17 ± 0.30 | 22.96 ± 0.56 |
| total weight [g] | 327.07 ± 7.59 | 434.93 ± 10.79 | 777.07 ± 22.00 | 994.33 ± 63.12 |
| Salmonidae | Percidae | |||
|---|---|---|---|---|
| CMA | OMY | PFL | SLU | |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| L* | 43.64 ± 0.83 a | 42.27 ± 0.74 a | 42.71 ± 0.58 a | 48.23 ± 0.51 b |
| a* | 5.21 ± 0.29 a | 4.51 ± 0.31 a | 3.37 ± 0.27 b | 0.89 ± 0.13 c |
| b* | 4.59 ± 0.47 | 4.53 ± 0.32 | 5.44 ± 0.32 | 4.58 ± 0.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komolka, K.; Bochert, R.; Franz, G.P.; Kaya, Y.; Pfuhl, R.; Grunow, B. Determination and Comparison of Physical Meat Quality Parameters of Percidae and Salmonidae in Aquaculture. Foods 2020, 9, 388. https://doi.org/10.3390/foods9040388
Komolka K, Bochert R, Franz GP, Kaya Y, Pfuhl R, Grunow B. Determination and Comparison of Physical Meat Quality Parameters of Percidae and Salmonidae in Aquaculture. Foods. 2020; 9(4):388. https://doi.org/10.3390/foods9040388
Chicago/Turabian StyleKomolka, Katrin, Ralf Bochert, George P. Franz, Yagmur Kaya, Ralf Pfuhl, and Bianka Grunow. 2020. "Determination and Comparison of Physical Meat Quality Parameters of Percidae and Salmonidae in Aquaculture" Foods 9, no. 4: 388. https://doi.org/10.3390/foods9040388
APA StyleKomolka, K., Bochert, R., Franz, G. P., Kaya, Y., Pfuhl, R., & Grunow, B. (2020). Determination and Comparison of Physical Meat Quality Parameters of Percidae and Salmonidae in Aquaculture. Foods, 9(4), 388. https://doi.org/10.3390/foods9040388





