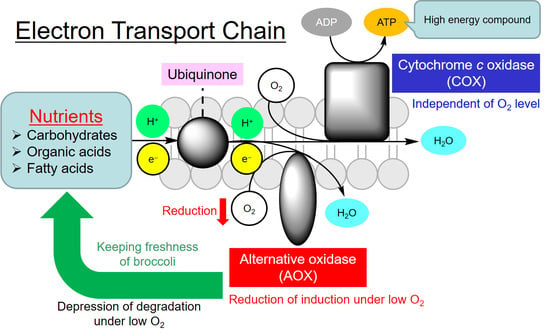
Induction of Terminal Oxidases of Electron Transport Chain in Broccoli Heads under Controlled Atmosphere Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
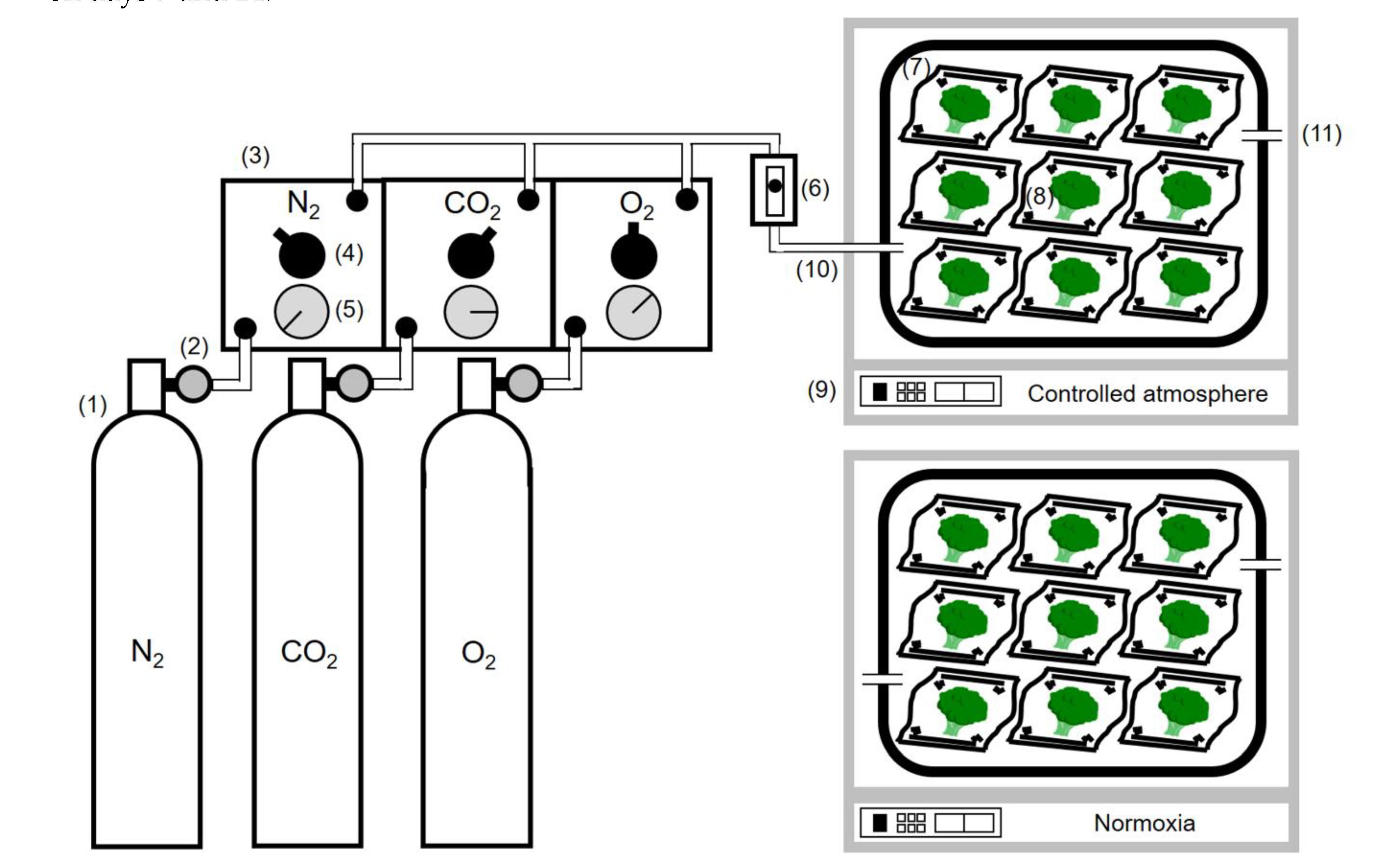
2.2. Controlled Atmosphere Storage Methods
2.3. O2 Uptake Rate Measurement
- m = mass of the broccoli head (kg)
- C = O2 partial pressure in the pouch (MPa)
- t = incubation time (h)
- T = incubator temperature (K)
- r = O2 uptake rate (mol kg−1 h−1)
- V = void volume in the pouch (L)
- R = universal gas constant (L MPa mol−1 K−1)
- Subscript 0 = initial (start) time, subscript t = incubation time
2.4. Mass Loss Measurement
2.5. L-Ascorbate Measurement
2.6. Determinations of AOX and COX Protein Amounts
2.7. Determination of O2 Isotope Discrimination
- D = discrimination value (‰);
- ρ = 18O/16O ratio of gas sample;
- ƒ = fraction of in-package O2 concentration.
2.8. Statistics
3. Results and Discussion
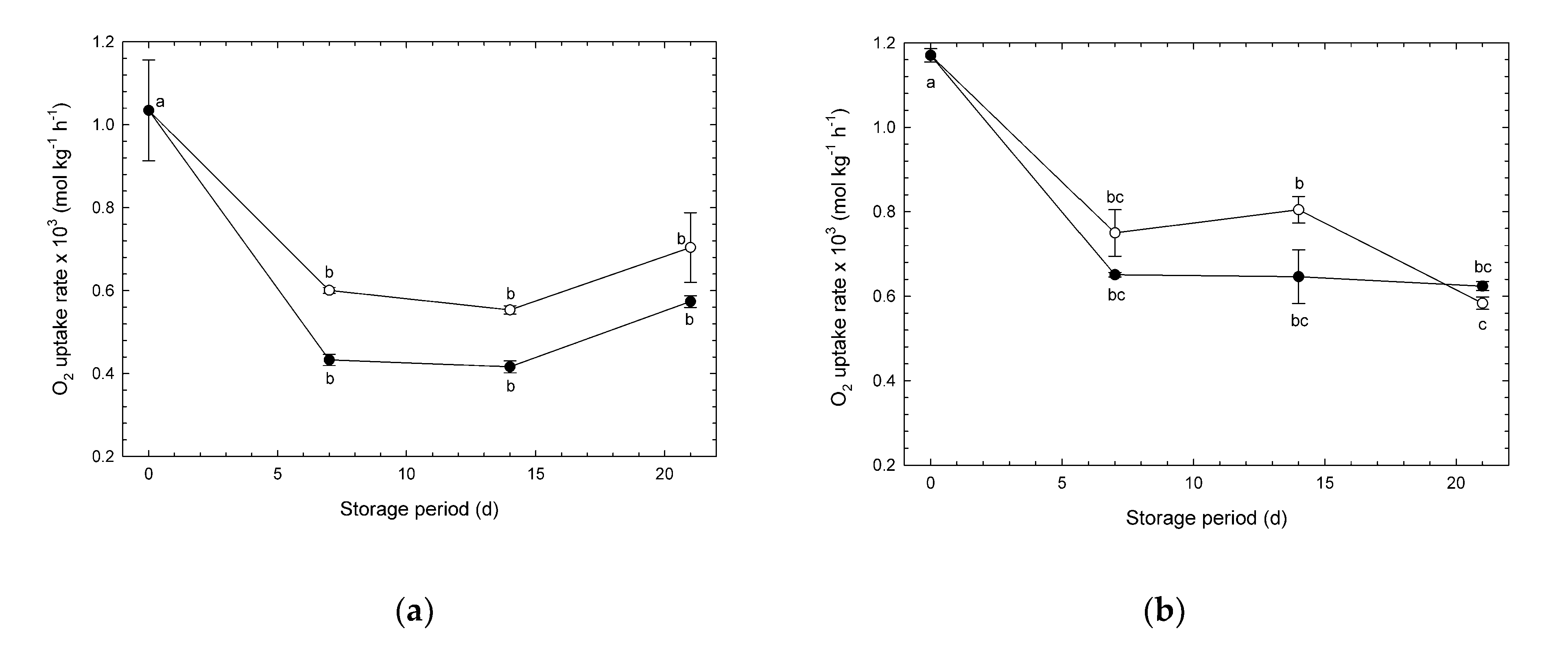
3.1. Changes in O2 Uptake Rate
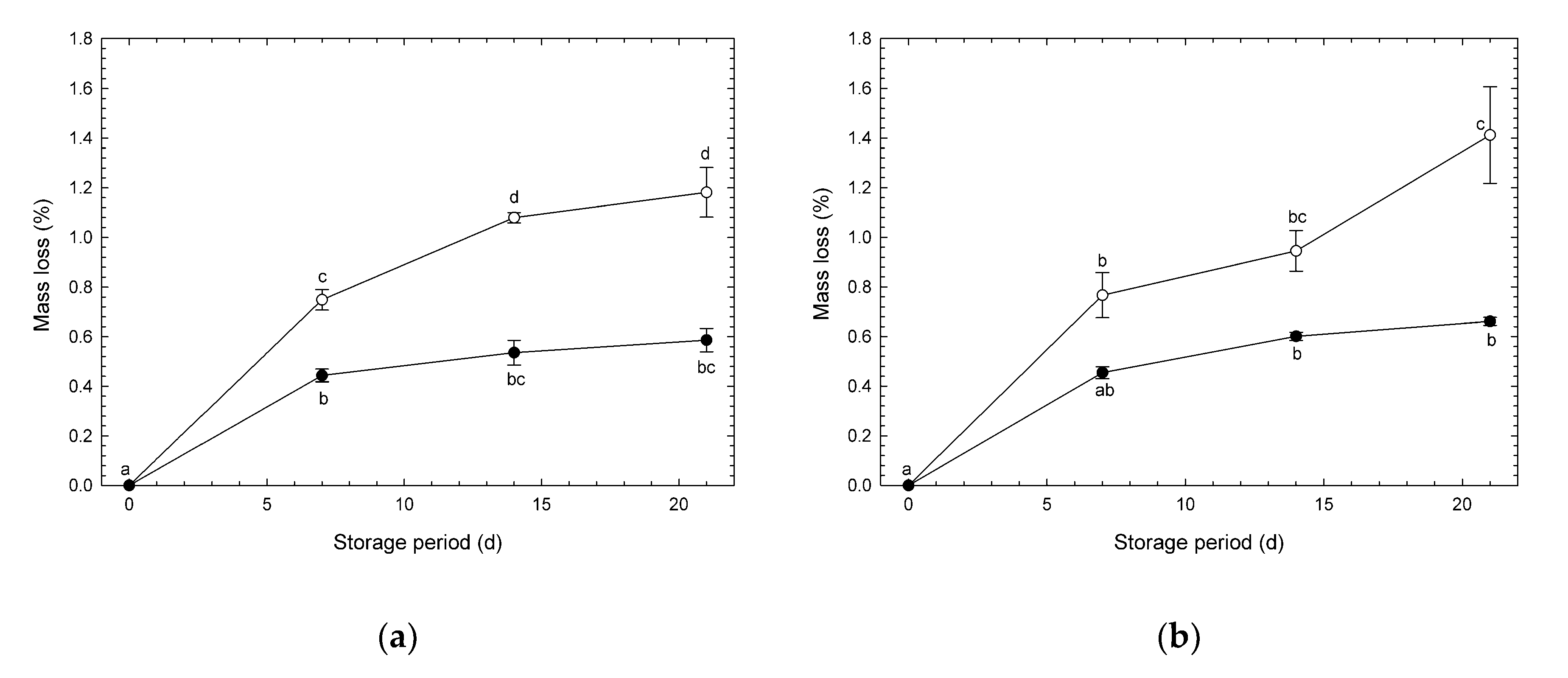
3.2. Mass Loss
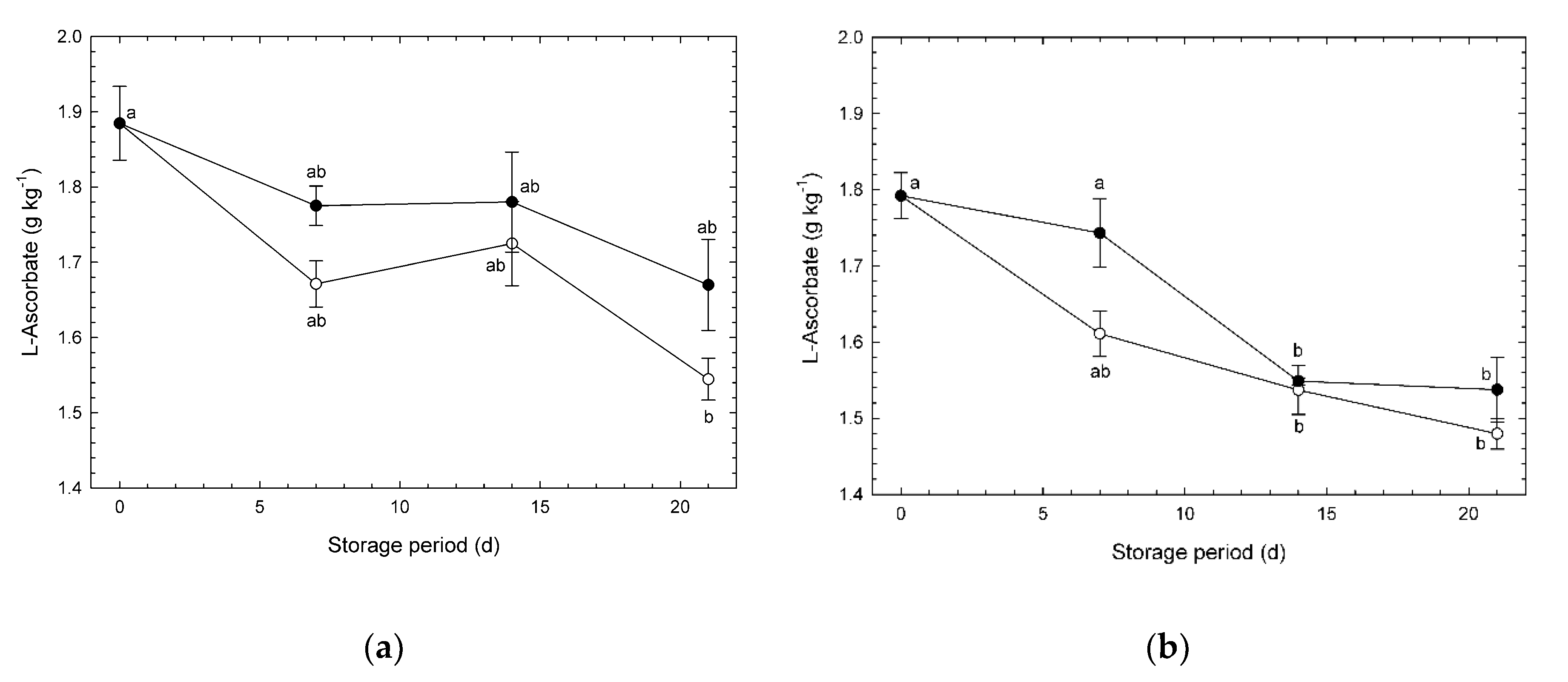
3.3. L-Ascorbate
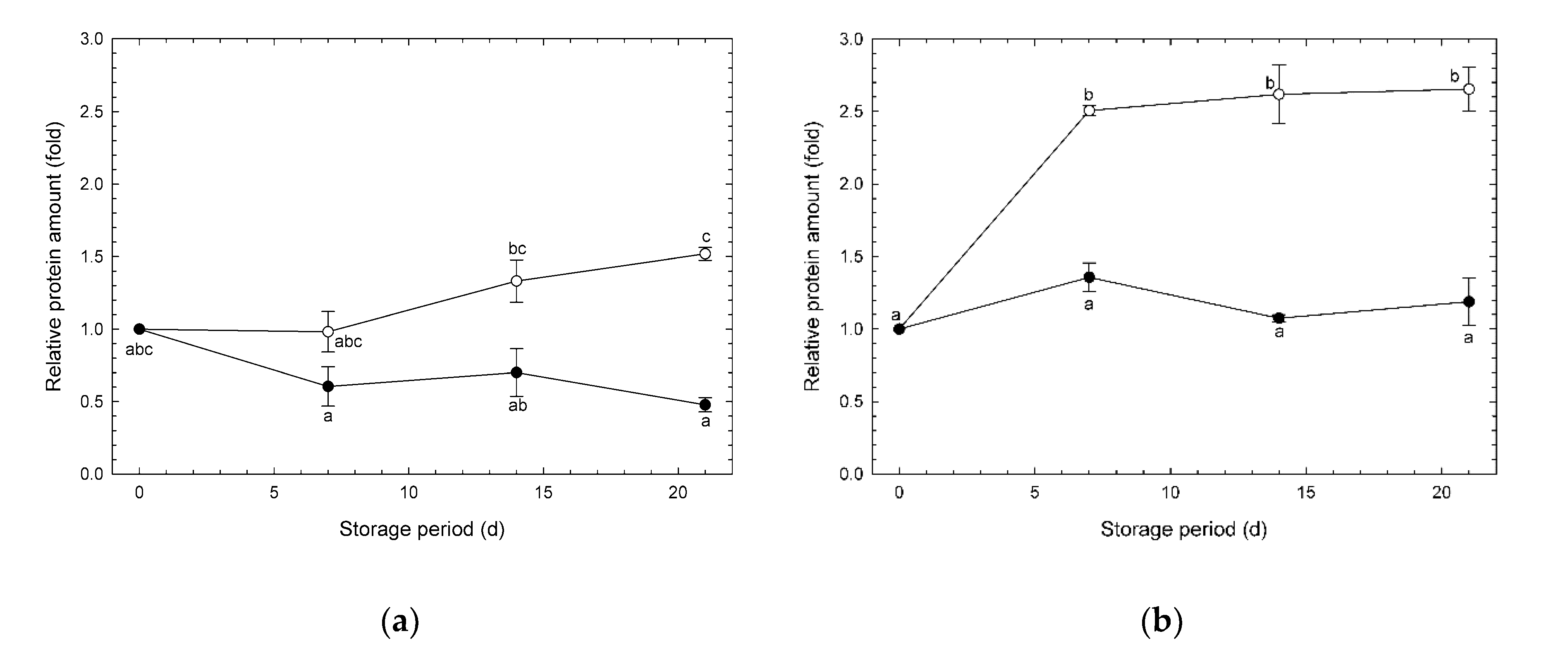
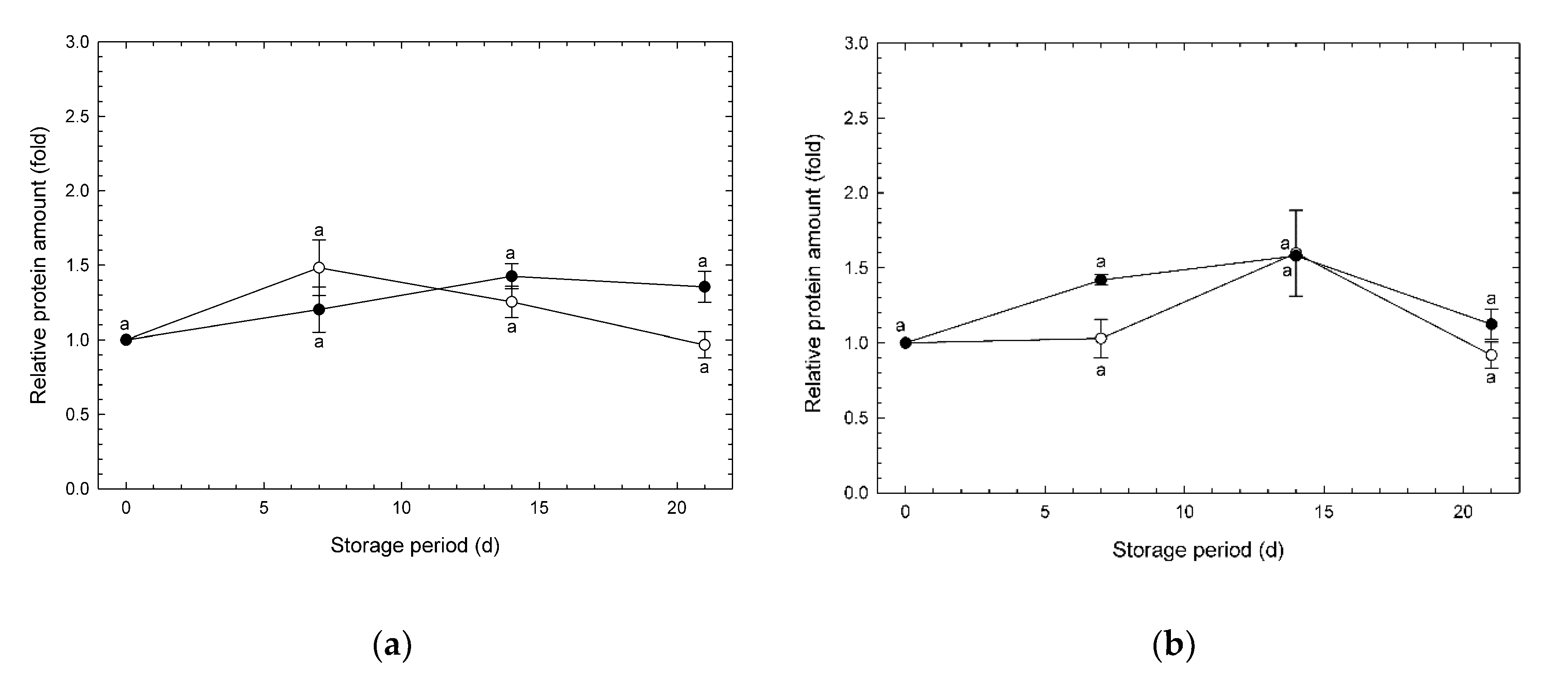
3.4. Changes in Amounts of AOX and COX Enzymes during Storage
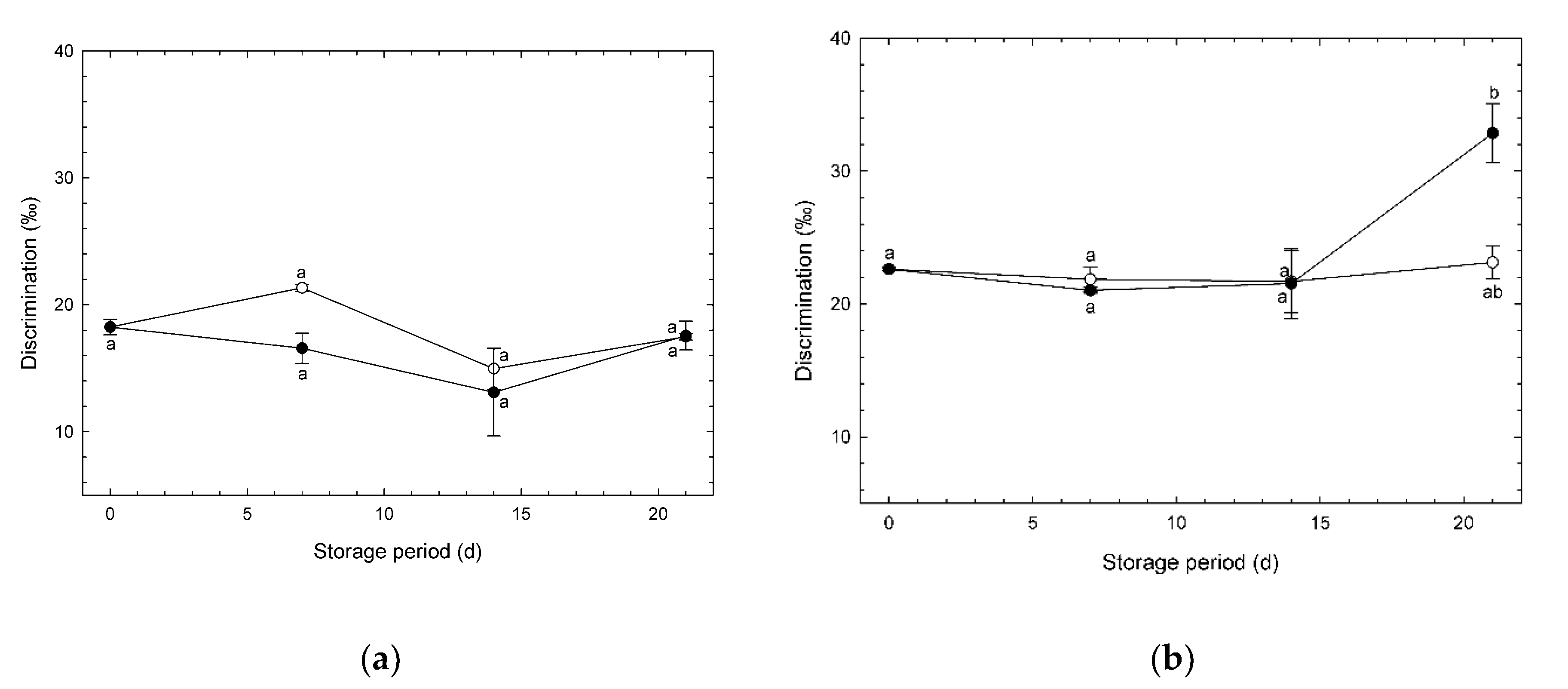
3.5. Changes in O2 Isotope Discrimination during Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thompson, A.K. Controlled Atmosphere Storage for Fruit and Vegetables; CAB Int.: Oxon, NY, USA, 1998; pp. 14–55. [Google Scholar]
- Monero, D.A.; Carvajal, M.; López-Berenguer, C.; García-Viguera, C. Chemical and biological characterisation of nutraceutical compounds of broccoli. J. Pharm. Biomed. Anal. 2006, 41, 1508–1522. [Google Scholar]
- Fao Stat. Available online: http://www.fao.org/faostat/en/#home (accessed on 3 December 2019).
- Robinson, J.E.; Brown, K.M.; Burton, W.G. Storage characteristics of some vegetables and soft fruits. Ann. Appl. Biol. 1975, 81, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.W.; Bhattacharjya, A.; Chakraborty, I.; Dhua, R.S. 6-Benzylaminopurine improves shelf life, organoleptic quality and health promoting compounds of fresh-cut broccoli florets. J. Sci. Ind. Res. 2011, 70, 461–465. [Google Scholar]
- Li, B.; Liu, B.; Su, T.; Fang, Y.; Xie, G.L.; Wang, G.F.; Wang, Y.L.; Sun, G.C. Effect of chitosan solution on the inhibition of pseudomonas fluorescens causing bacterial head rot of broccoli. Plant Pathol. J. 2010, 26, 189–193. [Google Scholar] [CrossRef]
- Bovi, G.G.; Caleb, O.J.; Linke, M.; Rauh, C.; Mahajan, P.V. Transpiration and moisture evolution in packaged fresh horticultural produce and the role of integrated mathematical models: A review. Biosyst. Eng. 2016, 150, 24–39. [Google Scholar] [CrossRef]
- Makino, Y. Oxygen consumption by fruits and vegetables. Food Sci. Technol. Res. 2013, 19, 523–529. [Google Scholar] [CrossRef]
- Dilley, D. Approaches to maintenance of postharvest integrity. J. Food Biochem. 1978, 2, 235–242. [Google Scholar] [CrossRef]
- Lipton, W.J.; Harris, C.M. Controlled atmosphere effects on the market quality of stored broccoli (Brassica oleracea L., italica group). J. Am. Soc. Hort. Sci. 1974, 99, 200–205. [Google Scholar]
- Deschene, A.; Paliyath, G.; Lougheed, E.C.; Dumbroff, E.B.; Thompson, E.B. Membrane deterioration during postharvest senescence of broccoli florets: Modulation by temperature and controlled atmosphere storage. Postharvest Biol. Technol. 1991, 1, 19–31. [Google Scholar] [CrossRef]
- Makhlouf, J.; Castaigne, F.; Arul, J.; Willemot, C.; Gosselin, A. Long-term storage of broccoli under controlled-atmosphere. HortScience 1989, 24, 637–639. [Google Scholar]
- Wang, H.W.; Makino, Y.; Inoue, J.; Maejima, K.; Funayama-Noguchi, S.; Yamada, T.; Noguchi, K. Influence of modified atmosphere on induction and activity of respiratory enzymes in broccoli florets during the early stage of postharvest storage. J. Agric. Food Chem. 2017, 65, 8538–8543. [Google Scholar] [CrossRef] [PubMed]
- Vanlerberghe, G.C.; McIntosh, L. Alternative oxidase: From gene to function. Annu. Rev. Plant Biol. 1997, 48, 703–734. [Google Scholar] [CrossRef] [PubMed]
- Makino, Y.; Iwasaki, K.; Hirata, T. Oxygen consumption model for fresh produce on the basis of adsorption theory. Trans. ASAE 1996, 39, 1067–1073. [Google Scholar] [CrossRef]
- Noguchi, K.; Taylor, N.L.; Millar, A.H.; Lambers, H.; Day, D.A. Response of mitochondria to light intensity in the leaves of sun and shade species. Plant Cell Environ. 2005, 28, 760–771. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Guy, R.D.; Berry, J.A.; Fogel, M.L.; Hoering, T.C. Differential fractionation of oxygen isotopes by cyanide-resistant and cyanide-sensitive respiration in plants. Planta 1989, 177, 483–491. [Google Scholar] [CrossRef]
- Makino, Y.; Ichimura, M.; Kawagoe, Y.; Oshita, S. Cytochrome c oxidase as a cause of variation in oxygen uptake rates among vegetables. J. Am. Soc. Hort. Sci. 2007, 132, 239–245. [Google Scholar] [CrossRef]
- Irving, D.E.; Baird, V.M. Heat production and respiration by broccoli florets during senescence at 20 degrees C. N. Z. J. Crop Hort. Sci. 1996, 24, 199–202. [Google Scholar] [CrossRef]
- Kader, A.A. Transpiration or water loss, Modified atmosphere during transport and storage. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California, Agriculture and Natural Resources: Oakland, CA, USA, 2002; p. 41. [Google Scholar]
- Picha, D.H. Weight-loss in sweet-potatoes during curing and storage-contribution of transpiration and respiration. J. Am. Soc. Hort. Sci. 1986, 111, 889–892. [Google Scholar]
- Smirnof, N. Ascorbic acid: Metabolism and functions of a multifaceted molecule. Curr. Opin. Plant Biol. 2000, 3, 229–235. [Google Scholar] [CrossRef]
- Boerzhijin, S.; Makino, Y.; Hirai, M.Y.; Sotome, I.; Yoshimura, M. Effect of perforation-mediated modified atmosphere packaging on the quality and bioactive compounds of soft kale (Brassica oleracea L. convaracephala (DC) Alef. var. sabellica L.) during storage. Food Packag. Shelf Life 2020, 23, 100427. [Google Scholar] [CrossRef]
- Nishikawa, F.; Kato, M.; Kamo, T.; Wang, R.; Hyodo, H.; Ikoma, Y.; Sugiura, M.; Yano, M. Enzymatic catabolism of ascorbate in florets of harvested broccoli during senescence. J. Jpn. Soc. Hort. Sci. 2001, 70, 709–715. [Google Scholar] [CrossRef]
- Barth, M.M.; Kerbel, E.L.; Perry, A.K.; Schmidt, S.J. Modified atmosphere packaging affects ascorbic acid, enzyme activity and market quality of broccoli. J. Food Sci. 1993, 58, 140–143. [Google Scholar] [CrossRef]
- Noji, H.; Yasuda, R.; Yoshida, M.; Kinosita, K., Jr. Direct observation of the rotation of F1-ATPase. Nature 1997, 386, 299–302. [Google Scholar] [CrossRef]
- Brannd, M.; Henderson, R.; Parson, W.W.; Schatz, G.; Smith, A. The respiratory chain and ATP synthase. In Molecular Biology of the Cell, 3rd ed.; Alberts, B., Bray, D., Lewis, J., Raff, M., Roberts, K., Watson, J.D., Eds.; Garland Publishing Inc.: NY, USA, 1994; pp. 672–683. [Google Scholar]
- Kidd, F. The controlling influence of carbon dioxide in the maturation, dormancy, and germination of seeds—Part I. Proc. R. Soc. Lond. B 1914, 87, 408–421. [Google Scholar] [CrossRef]
- Kidd, F. The controlling influence of carbon dioxide in the maturation, dormancy, and germination of seeds—Part II. Proc. R. Soc. Lond. B 1914, 87, 609–625. [Google Scholar] [CrossRef]
- Kidd, F. The controlling influence of carbon dioxide. Part III.—The retarding effect of carbon dioxide on respiration. Proc. R. Soc. Lond. B 1916, 89, 136–156. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makino, Y.; Inoue, J.; Wang, H.-W.; Yoshimura, M.; Maejima, K.; Funayama-Noguchi, S.; Yamada, T.; Noguchi, K. Induction of Terminal Oxidases of Electron Transport Chain in Broccoli Heads under Controlled Atmosphere Storage. Foods 2020, 9, 380. https://doi.org/10.3390/foods9040380
Makino Y, Inoue J, Wang H-W, Yoshimura M, Maejima K, Funayama-Noguchi S, Yamada T, Noguchi K. Induction of Terminal Oxidases of Electron Transport Chain in Broccoli Heads under Controlled Atmosphere Storage. Foods. 2020; 9(4):380. https://doi.org/10.3390/foods9040380
Chicago/Turabian StyleMakino, Yoshio, Jun Inoue, Hsiao-Wen Wang, Masatoshi Yoshimura, Kensaku Maejima, Sachiko Funayama-Noguchi, Takeshi Yamada, and Ko Noguchi. 2020. "Induction of Terminal Oxidases of Electron Transport Chain in Broccoli Heads under Controlled Atmosphere Storage" Foods 9, no. 4: 380. https://doi.org/10.3390/foods9040380
APA StyleMakino, Y., Inoue, J., Wang, H.-W., Yoshimura, M., Maejima, K., Funayama-Noguchi, S., Yamada, T., & Noguchi, K. (2020). Induction of Terminal Oxidases of Electron Transport Chain in Broccoli Heads under Controlled Atmosphere Storage. Foods, 9(4), 380. https://doi.org/10.3390/foods9040380