Abstract
Bifidobacteria are known to inhibit, compete with and displace the adhesion of pathogens to human intestinal cells. Previously, we demonstrated that goat milk oligosaccharides (GMO) increased the attachment of Bifidobacterium longum subsp. infantis ATCC 15697 to intestinal cells in vitro. In this study, we aimed to exploit this effect as a mechanism for inhibiting pathogen association with intestinal cells. We examined the synergistic effect of GMO-treated B. infantis on preventing the attachment of a highly invasive strain of Campylobacter jejuni to intestinal HT-29 cells. The combination decreased the adherence of C. jejuni to the HT-29 cells by an average of 42% compared to the control (non-GMO treated B. infantis). Increasing the incubation time of the GMO with the Bifidobacterium strain resulted in the strain metabolizing the GMO, correlating with a subsequent 104% increase in growth over a 24 h period when compared to the control. Metabolite analysis in the 24 h period also revealed increased production of acetate, lactate, formate and ethanol by GMO-treated B. infantis. Statistically significant changes in the GMO profile were also demonstrated over the 24 h period, indicating that the strain was digesting certain structures within the pool such as lactose, lacto-N-neotetraose, lacto-N-neohexaose 3′-sialyllactose, 6′-sialyllactose, sialyllacto-N-neotetraose c and disialyllactose. It may be that early exposure to GMO modulates the adhesion of B. infantis while carbohydrate utilisation becomes more important after the bacteria have transiently colonised the host cells in adequate numbers. This study builds a strong case for the use of synbiotics that incorporate oligosaccharides sourced from goat′s milk and probiotic bifidobacteria in functional foods, particularly considering the growing popularity of formulas based on goat milk.
1. Introduction
Bifidobacteria are considered one of the first colonisers of the human gastrointestinal (GI) tract and are suggested to confer positive health outcomes to the host, explaining their prevalence as probiotics [1]. These bacteria are particularly effective at protecting against infectious diseases, regulating immune responses and exerting effects against conditions ranging from irritable bowel syndrome, allergic diseases, ulcerative colitis, and immunoglobulin E associated diseases, to atopic dermatitis [2]. In terms of protecting against infection, bifidobacteria can operate through strain-specific antagonistic means for the competitive exclusion of pathogens [3]. A strain of B. breve was found to inhibit the growth of enterotoxigenic (ETEC) and enteropathogenic (EPEC) Escherichia coli, while other species were found to inhibit intestinal colonisation of pathogens such as Salmonella, Shigella, Listeria monocytogenes, and Clostridium difficile [4,5,6]. Microbe-associated molecular patterns (MAMPs) are recognized by the host′s intestinal pattern recognition receptors (PRRs), and these interactions play key roles in the association of pathogens with the intestinal epithelia. Probiotics also express molecular patterns which can recognize the same trans-membrane receptors as the pathogens, thus blocking the sites for pathogenic contact by competitive exclusion and, in some cases, displacing already-attached pathogens [7]. However, the health benefits associated with bifidobacteria are reliant on such strains colonising the host in sufficient numbers [8]. The important step in microbial colonisation of the intestinal epithelium is the attachment of bacterial surface lectins to intestinal sugar structures. Recent studies have suggested that milk oligosaccharides may enhance the specific ability of bifidobacteria to attach to the GI epithelium [9,10,11]. Indeed, our group investigated the ability of goat milk oligosaccharides (GMO) to increase the attachment of Bifidobacterium longum subsp. infantis ATCC 15697 to HT-29 cells. Exposure of the strain to the GMO resulted in an 8.3-fold increase in its adhesion to the intestinal cells [11].
As well as aiding the colonisation of bifidobacteria, GMO can also act as a prebiotic. The prebiotic potential of GMO recovered from whey has been identified in vitro where significant growth of Bifidobacterium spp. was observed on isolated GMO [12]. Oligosaccharide-enriched fractions prepared from both stage one and stage two goats′ milk-based infant formula were also recently shown to significantly enhance the growth of bifidobacteria in vitro and reduce the adhesion of E. coli NCTC 10418 and Salmonella enterica subsp. enterica serovar Typhimurium to Caco-2 cells [13], possibly by acting as analogues of epithelial receptors on the gut cells [14]. In vivo studies have also indicated that ingestion of GMO by mice during gestation and lactation increased the relative abundance of bifidobacteria in the colon of their pups at weaning [15]. These studies suggest that a combination of both a probiotic and a prebiotic (GMO) could provide a synergistic effect and may be an effective strategy to enhance the persistence and metabolic activity of specific beneficial bifidobacterial strains. Therefore, the aim of the current study is to examine such a synbiotic combination in vitro and determine if GMO have the potential to increase the competitiveness and metabolic activity of B. infantis in the intestinal tract. The ability of the synbiotic to competitively exclude an invasive strain of Campylobacter jejuni to intestinal cells is first examined. Fermentation of the GMO by the B. infantis strain is then investigated through growth studies, metabolite analysis and oligosaccharide depletion assays.
2. Materials and Methods
2.1. Oligosaccharides Standards
The oligosaccharide standards; 2′-Fucosyllactose (2′FL), Lacto-N-tetraose (LNT), 3′-Sialyllactose (3′SL), 6′-Sialyllactose (6′SL), Disialyllactose (DSL), Lacto-N-hexaose (LNH), N-Acetylneuraminic acid (Sialic Acid), LS-tetrasaccharide c (LSTc), Lacto-N-neotetraose (LNnT) and Lacto-N-neohexaose (LNnH) were purchased from Carbosynth Ltd. (Berkshire, UK) and lactose was obtained from VWR (Dublin, Ireland).
2.2. Isolation of Goat Milk Oligosaccharides
Goat milk oligosaccharides (GMO) were isolated and characterized as previously described [11]. In brief, mature milk from goats was kindly donated by Ardsallagh Goat Farm (Carrigtwohill, Co. Cork) and stored at −80 °C on arrival. To generate low molecular weight fractions, the milk was initially defatted and de-caseinated as per Quinn et al. [11]. Large peptides and whey proteins were removed by ultrafiltration and the permeates were freeze-dried as previously described [11]. To separate lactose from the oligosaccharides, a BioGel P2 size exclusion column (Bio-rad, Deeside, UK) was employed and the fractions collected were analysed for lactose, 3-SL and 6-SL using high pH anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) as detailed below, and the protein/peptide concentration was determined by the Bradford assay [16]. Peptide-free and low-trace lactose (<80 mg/L) fractions were pooled and freeze-dried to give an oligosaccharide-enriched fraction.
2.3. Milk Oligosaccharide Analysis
Oligosaccharide analysis of the pooled GMO was performed as previously described [11]. Oligosaccharide-enriched fractions were diluted in water and analysed in order to quantify levels of lactose, 2′FL, LNT, 3′SL, 6′SL, DSL, LNH, Sialic Acid, LSTc, LNnT and LNnH using a Dionex ICS-3000 Series system (Dionex Corporation, Sunnyvale, CA, USA) equipped with an electrochemical detector.
2.4. Bifidobacterium longum subsp. infantis Culture Conditions
Bifidobacterium longum subsp. infantis ATCC® 15697™ (B. infantis) was obtained from the American Type Culture Collection (ATCC, Middlesex, UK). Bacterial cultures were maintained as previously described [10,11] The strain was stored in deMan Rogosa Sharpe (MRS) (Difco, Sparks, MD,, USA) broth containing 50% glycerol at −80 °C. The strain was cultured twice in MRS media supplemented with L-cysteine (0.05% w/v) (Merck, Dannstadt, Germany) prior to use, and was routinely grown overnight at 37 °C under anaerobic conditions generated using an Anaerocult A system (Merck).
2.5. Campylobacter jejuni Culture Conditions
Campylobacter jejuni 81–176 (C. jejuni) is a well-characterized, mobile flagellated invasive strain which has been used in many previous studies [17,18]. The pathogen was stored in Mueller–Hinton broth (Oxoid, Ireland c/o Fannin Healthcare, Dublin, Ireland) containing 50% glycerol at −80 °C and cultured directly from storage onto Mueller–Hinton agar plates. The pathogen was grown under microaerophilic conditions generated using CampyGen gas packs (Oxoid), for 48 h at 37 °C. Prior to pathogen inhibition assays, C. jejuni 81–176 was grown on Mueller–Hinton agar and then transferred to biphasic media in 25 cm2 tissue culture flasks (Corning, NY, USA) consisting of Mueller–Hinton agar supplemented with Campylobacter selective supplement (Skirrow), (Oxoid) and 6 mL of McCoy′s 5A media (Merck) supplemented with 2% FBS. The flask was then incubated for 24 h under microaerophilic conditions at 37 °C.
2.6. Exposure of B. infantis to Goat Milk Oligosaccharides
Exposure of the bacteria to GMO was performed as previously described [11]. A final concentration of 5 mg/mL of GMO was used reflecting the concentration of oligosaccharides present in mature human milk [11,19,20,21,22,23,24,25,26,27]. Bacterial suspensions were then incubated for 1 h at 37 °C under anaerobic conditions. Following this, bacteria were harvested by centrifugation (3850× g, 5 min), the supernatants removed, and pellets were washed three times in phosphate-buffered saline (PBS) and then re-suspended in non-supplemented McCoy′s media prior to use in the adhesion assays.
2.7. Mammalian Cell Culture Conditions
HT-29 cells were grown as previously described [11] and maintained in McCoy′s 5A modified medium (Merck) supplemented with 10% fetal bovine serum (FBS) using 75 cm2 tissue culture flasks incubated at 37 °C in 5% CO2 in a humidified atmosphere. Once the cells were nearing confluency (approximately 80%–90%), they were passaged into 48 well tissue culture plates (Sarstedt Ltd., Wexford, Ireland) at a density of 1 × 105 cells/mL between passages 15–21. The cells were then used once fully confluent (approximately 2 × 106 cells/well). The media was changed every other day and supplemented with 2% FBS 24 h prior to use.
2.8. Adhesion Assays with B. infantis
Adhesion assays with B. infantis were performed as previously described [11]. HT-29 cells were washed twice with PBS, and 250 µL of the bacteria and media suspensions were added to the wells, corresponding to approximately 40 bacterial cells per human cell. Bacterial cells were incubated with the HT-29 cells after which they were washed with PBS to remove non-adherent bacteria. HT-29 cells were then lysed and the lysates were serially diluted and enumerated by spot-plating on MRS plates to enumerate bacterial colony forming units (CFU). The adhesion of the bacteria was determined as the percentage of original inoculum that attached, thus accounting for variations in the starting inoculum. Percentage adhesion = (CFU/mL of recovered adherent bacteria/CFU/mL of inoculum) × 100. Experiments were performed in triplicate on three separate occasions.
2.9. Anti-Infective Assays and Exclusion Assay
Anti-infective assays were performed as previously described [18] with minor modifications. In brief, C. jejuni was incubated in the absence and presence of GMO (5 mg/mL) at a final OD600nm of 0.3 (approximately 5.14 × 108 CFU/mL) in McCoys′s media and incubated under microaerophilic conditions for 1 h at 37 °C; 250 µL of the mix was then applied to three wells containing HT-29 cells, and allowed to incubate for 3 h, after which the eukaryotic cells were washed five times with PBS, lysed with 250 µL 0.1% Triton X-100 (Merck) in PBS and spread plated onto Mueller–Hinton agar plates and incubated under microaerophilic conditions for 72 h at 37 °C to enumerate CFU. For exclusion assays, exposure of B. infantis to 5 mg/mL GMO was performed as described above, and this suspension was subsequently incubated with the HT-29 cells for 2 h. A non-supplemented control was also included. Non-adherent bacteria were removed from the cells as described above, after which the cell line was challenged with C. jejuni. To do this, the pathogen was harvested from the biphasic medium, washed twice in non-supplemented McCoy′s, and diluted to an OD600nm of 0.3. From this suspension, 250 μL was then added to each well, and cells were incubated under anaerobic conditions for 3 h at 37 °C. Cells were then washed five times with PBS, lysed with 0.1% Triton X-100 (Merck) in PBS and plated onto supplemented Mueller–Hinton agar. Mueller–Hinton plates were incubated under microaerophilic conditions for 72 h at 37 °C, after which bacterial CFU were enumerated. The assays were performed in triplicate on three separate occasions. The exclusion of C. jejuni was determined as the average CFU/mL of recovered adherent bacteria. The percentage decrease in C. jejuni adhesion was calculated as the difference in C. jejuni CFU/mL between non-supplemented and GMO-supplemented bifidobacteria.
2.10. Effect of GMO on the Growth of B. infantis
B. infantis was grown in the absence and presence of 5 mg/mL GMO over a 72 h period under adhesion assay conditions. Aliquots of 150 μL of the bacterial suspensions were added to the individual wells of a 96 well microtiter plate. Other controls included a control containing no bacteria, and bacteria grown in deMan Rogosa Sharpe (MRS) (Difco, Sparks, MD, USA) broth supplemented with L-cysteine (0.05% w/v) (Merck). These experiments were performed in a concept 400 anaerobic chamber (Baker, ME, USA) and bacterial growth was monitored by determining OD600nm using a Synergy-HT multidetector microplate reader driven by Gen5 reader control and data analysis software (BioTek Instruments Inc. Bedfordshire UK) at 0, 12, 24, 48 and 72 h. The microtitre plate was automatically shaken for 30 s prior to each measurement to achieve a homogenous suspension. The results are represented as the average OD600nm of triplicate experiments performed on three separate occasions. The percentage increase in growth of B. infantis was calculated as the difference in OD600nm between non-supplemented and GMO-supplemented bifidobacteria.
2.11. GMO Consumption by B. infantis
B. infantis was grown overnight under the optimal conditions outlined above and then re-suspended in McCoy′s media at an OD600nm of 0.25 with a final oligosaccharide concentration of 5 mg/mL. A one milli-litre aliquot of this cell suspension was then dispensed into a sterile Eppendorf (Merck) after 0 h and 24 h of growth. A negative control containing no bacteria was also included. These solutions were then centrifuged for 5 min (3850× g) and the supernatants collected. The process was repeated a total of three times to ensure bacteria were not present in the supernatant. The sample was also treated with ultraviolet light for 30 min in a laminar flow hood to ensure no further metabolic activity occurred., 2′FL, LNT, 3′SL, 6′SL, DSL, LNH, Sialic Acid, LSTc, LNnT, and LNnH were quantified using HPAEC-PAD analyses to quantify oligosaccharide levels before and after 24 h of fermentation using a Dionex ICS-3000 Series system (Dionex Corporation). Reductions in the areas of the peaks after immediate exposure and over the 24 h period were calculated through comparative analysis as per Lane et al. [28]. These experiments were performed on three separate occasions.
2.12. B. infantis Metabolite Analysis
Supernatants collected for HPAEC-PAD analysis at 0 and 24 h were also assessed for changes in metabolite production using high-pressure liquid chromatography. Metabolic end products, lactate, acetate, formate and ethanol were measured using an Agilent 1200 HPLC system (Agilent Technologies, Santa Clara, CA, USA) with a refractive index detector. Metabolite peaks and concentrations were identified and calculated based on known metabolite retention times and standard solutions at known concentrations. A negative control of non-supplemented media was also included. A REZEX 8 m 8%H, organic acid column (300 × 7.8 mM Phenomenex, CA, USA) was used and the elution was performed for 25 min with a 0.01 M H2SO4 solution at a constant flow rate of 0.6 mL/min and a temperature of 65 °C. The standard solutions were prepared as per Table 1. These experiments were performed in triplicate. The ratios of acetate to lactate, acetate to formate, and lactate to ethanol were also calculated.

Table 1.
Volumes of standards used for metabolite analysis.
2.13. Statistical Analysis
Graphs were drawn using Microsoft Excel. The results are presented as the mean ± standard deviations of replicate experiments, and the unpaired Student t-test was used to determine statistically significant results. For all experiments, p < 0.05 was considered significant.
3. Results and Discussion
3.1. Characterisation of the Goat Milk Oligosaccharides
The batch of goat′s milk used in this study was previously characterised in terms of its oligosaccharide content [29]. Forty-one oligosaccharide structures were identified including both neutral and acidic structures, of which 64% of the acidic fraction were Neu5Gc linked [29]. In this study, we isolated oligosaccharide from the same batch of goats milk and quantified some of the major structures, which included LNnT, LNnH, 3′-SL, 6′-SL, LSTc and DSL (Table 2). In terms of the difference between human and animal milk composition, type I oligosaccharides predominate in human milk [27], while type II oligosaccharides are either exclusive or predominate over type I structures in animal milk. Regarding human milk oligosaccharide (HMO) structures, 70% are fucosylated, with lacto-N-biose (type I) structures (Gal(b1–3)GlcNAc) predominating over structures containing the N-acetyllactosamine (type II) (Gal(b1–4)GlcNAc) [29]. In contrast, animal-derived oligosaccharides are predominantly sialylated, containing N-acetylneuraminic acid (Neu5Ac) and/or N-glycolylneuraminic acid (Neu5Gc) [30,31]. However, it is important to note that the oligosaccharide yield obtained from animal milk is much lower than that of human milk. While changes in GMO composition occur across lactation [24], previous studies have indicated that a number of oligosaccharides, such as 3′ and 6′ sialylactose, β3′-galactosyllactose, β6′-galactosyllactose, 2′-fucosyllactose, Lactose-N-hexaose, 6′-N-acetylneuraminyllactose and 3′-N-acetylneuraminyllactose are present in both human and goat milk [32,33,34,35]. In addition, a recent study by Leong et al. 2019 [13] investigated the presence of naturally occurring oligosaccharides in commercial goats′ milk-based stage one and stage two infant formulas and their prebiotic properties. Fourteen quantifiable oligosaccharides in goats′ milk-based infant formula were detectable by LC/MS, indicating that, structurally, GMO may represent an alternative to the human milk derivatives where breastfeeding is not possible. In addition, high purity and recovery of GMO consisting of 67.6% acidic and 34.4% neutral oligosaccharides have been demonstrated [36], indicating that potentially viable commercial production methods may be available in the not too distant future.

Table 2.
Levels of different oligosaccharides present in goat milk ogliosaccharides (GMO) pool.
3.2. Combined Effect of GMO and B. infantis on C. jejuni Adhesion
Probiotic bacteria, such as Lactobacillus acidophilus UO 001 and Lactobacillus gasseri UO 002, are known to inhibit the growth of Campylobacter without interfering with the normal microbiota of the gastrointestinal tract, suggesting other probiotic bacteria may have similar effects [37,38]. Murine fecal microbiota transplantation treatment was shown to alleviate intestinal and systemic immune responses in C. jejuni-infected mice harbouring a human gut microbiota. These with mice displayed higher numbers of lactobacilli and bifidobacteria, further suggesting the beneficial effects of probiotics against pathogen colonisation [39]. However, a prerequisite for survival in the intestinal tract is the ability of probiotic strains to transiently adhere efficiently to the intestinal mucosa. Probiotic and pathogenic strains have been suggested to share similarities in terms of their surface adhesins and, thus, may compete for adhesion sites [3,40]. Previously, we demonstrated an increase in B. infantis adherence to HT-29 cells after pre-exposure to GMO [11]. The HT-29 cells are extensively used as a model of the gastrointestinal tract, particularly as in vitro intestinal models of bacterial colonisation [41,42]. These cells exhibit classical characteristics that model small intestinal absorptive epithelial cells upon reaching confluence [43] and are a useful indicator of the structural landscape of the intestinal epithelium [44]. In the current study, we also employed HT-29 cells and hypothesised that the increase in B. infantis adhesion following GMO treatment may provide a protective effect against C. jejuni colonisation of HT-29 cells. We pre-treated B. infantis with GMO and observed an average increase in adhesion of 4.4 fold, demonstrating a clear increase in adhesion potential over three biological replicate experiments performed in triplicate (Figure S1). Following this, anti-infective assays were conducted to investigate if GMO alone could prevent C. jejuni colonisation. Overall, an average inoculum of 5.14 × 108 CFU/mL was applied to the HT-29 cells, of which 1.35 × 106 CFU/mL of C. jejuni were demonstrated to adhere. The percentage of C. jejuni from the original inoculum which adhered to the HT-29 cells was 0.26% ± 0.05. Notably, both GMO alone and B. infantis alone had no protective effects against C. jejuni colonisation (p-value: > 0.5) (Figure 1). Exclusion assays assessed the ability of B. infantis alone and pre-treated with GMO to prevent attachment and invasion of C. jejuni to HT-29 cells. The assays revealed a prophylactic protective effect following prior treatment of B. infantis with GMO (Figure 1) with an average significant decrease ranging from 42%–46% in C. jejuni adherence observed over triplicate experiments performed on three separate occasions. Interestingly, the non-supplemented B. infantis control demonstrated no protective effect against C. jejuni colonisation. This may suggest that a critical population level of attached bifidobacteria is required to result in the competitive exclusion of a pathogen. In contrast to the results demonstrated here, a previous study in mice with L. johnsonii indicated no reduction in lower intestinal C. jejuni colonisation; however, a suppressed intestinal and systemic pro-inflammatory and enhanced anti-inflammatory immune response were both observed, indicating other potential benefits of the use of probiotics on C. jejuni infection [45]. Notably, no statistically significant increase in C. jejuni growth in the presence of GMO was observed under anti-infective and exclusion assay conditions.
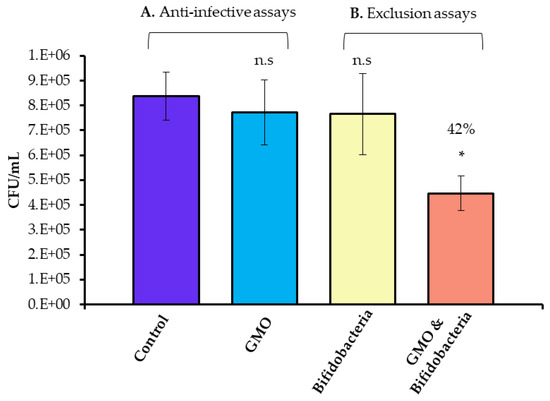
Figure 1.
Anti-infective assays (A) demonstrating Campylobacter jejuni 81–176 adhesion in the absence and presence of GMO, and (B) competitive exclusion assays demonstrating Campylobacter jejuni 81–176 adhesion to HT-29 cells following pre-treatment of the HT-29 cells with Bifidobacterium longum subsp. infantis 15697 (yellow) and Bifidobacterium longum subsp. infantis 15697 pre-treated with GMO (orange). Results demonstrate the average colony forming units (CFU)/mL of adherent Campylobacter jejuni 81–176 of one representative triplicate experiment, with error bars representing standard deviation. The unpaired non-parametric t-test was used, *: p-value: < 0.05, n.s: not significant.
The results presented here are particularly significant as Campylobacter is one of four key global causes of diarrhoeal diseases and is considered to be the most common bacterial cause of human gastroenteritis in the world [46] C. jejuni, in particular, poses a great risk worldwide due to its associated diarrhoeal disease and the risk of development of severe secondary diseases, such as irritable bowel syndrome and Guillain–Barré syndrome post-infection [17,47]. A number of studies have suggested that probiotic bacteria may inhibit pathogens through competitive exclusion to pathogen adhesion sites and nutrients [48]. Commercial broiler chickens are a major reservoir for C. jejuni and consumption can lead to human infection [49]. However, swine and cattle can also act as zoonotic vectors in addition to ingestion of contaminated surface waters [50,51]. Notably, strains such as Lactobacillus spp., i.e., acidophilus, casei, crispatus, gasseri, helveticus, pentosus, plantarum, rhamnosus, and salivarius have been suggested to exhibit anti-Campylobacter activities in vitro and in vivo [49]. In addition, Dec et al. [52], in screening Lactobacillus isolates for anti-Campylobacter activity, selected seven Lactobacillus isolates with potential applications in reducing Campylobacter spp. in chickens, which may have potential to prevent infections in both birds and humans [52]. In vivo studies in poultry using synbiotic combinations of Bifidobacterium longum PCB 133 and galactooligosaccharides in animal feed have been shown to reduce C. jejuni infection, demonstrating the potential to reduce transmission along the food chain, which is of fundamental importance for the safety of poultry meat consumers [53]. Microencapsulated Bifidobacterium longum PCB133 and xylooligosaccharides (XOS), when combined, have also been shown to have a synbiotic effect on improving the safety of poultry meat by protecting against C. jejuni infection (p-value: < 0.01) at the beginning of life, while the microbiota is still developing [54]. Additionally, in vivo studies in chicks have demonstrated the ability of three chicken commensal isolates to induce a 1–2 log reduction in Campylobacter numbers. However, isolates were only capable of reducing Campylobacter levels in one out of three trials. Notably, follow–up experiments demonstrated Lactobacillus salivarius subsp. salicinius, in combination with 0.04% mannan oligosaccharides resulted in a 3-log decrease of cecal Campylobacter suggesting that, similar to the current study, the use of synbiotic combinations may be more effective at preventing Campylobacter colonisation when compared to a commercial strain alone [55]. The cumulative prebiotic and colonisation-promoting effect of the GMO on the B. infantis strain in the current study further supports the use of such synbiotics in foods aimed at preventing infection.
3.3. Prebiotic Effects of GMO
The ability of GMO to stimulate the growth of B. infantis was determined using growth curves (Figure 2). When GMO was incubated with B. infantis following the pre-treatment period, an average increase in growth of 104% was observed in the presence of GMO at 24 h. No significant increases in the growth of B. infantis occurred during earlier incubation periods (≤12 h). Thus, it is unlikely that this growth contributed to the observed increase in bifidobacterial adhesion to the HT-29 cells after the 1 h exposure.
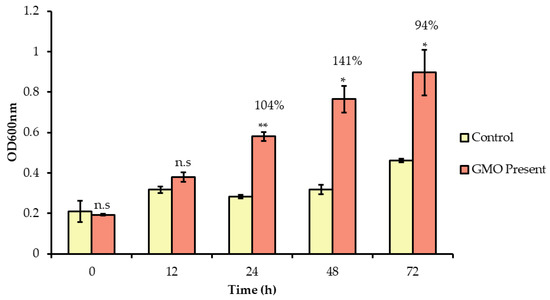
Figure 2.
Growth of B. longum subsp. infantis in McCoy′s media supplemented with GMO over 72 h. Optical density readings were taken at time 0, 12, 24, 48 and 72 h. Results are represented as the average of three biological replicates, with error bars representing standard deviation. The unpaired non-parametric t-test was used, *: p-value: < 0.05, ** p-value: < 0.005, n.s: not significant.
Previous studies have demonstrated statistically significant increases in Bifidobacterium and Bacteroides growth following incubation with GMO [12]. Similarly, GMO has also been shown to increase levels of bifidobacteria using in vitro fermentation models and in in vivo mouse trials [15], [39]. The prebiotic effect observed here is not surprising given that B. infantis ATCC 15697 is particularly adept at the utilising human milk glycans due to the presence of a 43 kb gene cluster responsible for their transport and utilization [56,57,58,59,60,61,62,63,64]. Indeed, Bifidobacterium longum subsp. infantis has been described as the “champion colonizer of the infant gut” and is unique among gut bacteria in its prodigious capacity to digest and consume milk oligosaccharide structures [58,59,60]. Moreover, a number of bifidobacterial strains have the ability to utilize milk oligosaccharides as substrates for growth [65]. In vitro fermentation of human milk oligosaccharides by different bifidobacterial strains including B. longum subsp. infantis ATCC 15697, B. longum ATCC 15707, B. breve ATCC 27539, B. adolescentis ATCC 15703 and B. bifidum ATCC 29521 has been previously demonstrated [66].
The ability of B. infantis ATCC 15697 to use multiple specific carbohydrate structures (2′FL, LNT, 3′SL, 6′SL, DSL, LNH, Sialic Acid, LSTc, LNnT and LNnH) within the pool was also investigated by generating HPAEC-PAD profiles of the oligosaccharides in the media before and after 24 h of bacterial growth (Figure 3). Notably, before the incubation period, a total of 24 peaks were detected in the GMO by the method used, of which six (3′SL, 6′SL, DSL, LSTc, LNnT and LNnH) were identified. Following 24 h incubation with B. infantis, 19 peaks were detected, of which 11 unidentified peaks were found in trace amounts that were not detected in the starting material. 2′FL, LNT, LNH, and free sialic acid were not detected at either time point. Overall, our results demonstrated that the strain was capable of utilizing multiple structures. For instance, 3′ and 6′ SL were depleted by 94% and 71% respectively, while LNnT and LNnH, were 100% utilised. LSTc was depleted by 94%, DSL was depleted by 43.1%, and lactose was depleted by 52%. This may be expected as B. longum subsp. longum has been shown to utilize free LNnT, one of the dominant components of HMOs, [66,67]. In addition, B. longum subsp. infantis BRS8-2 and TPY1201 are known to degrade 2′FL, 3FL, 3′SL and LNnT, while B. longum subsp. infantis DSM 20088 has been shown to utilise 2′FL, 3FL, and LNnT [68]. Therefore, it is likely that the prebiotic effect associated with GMOs may extend to other strains.
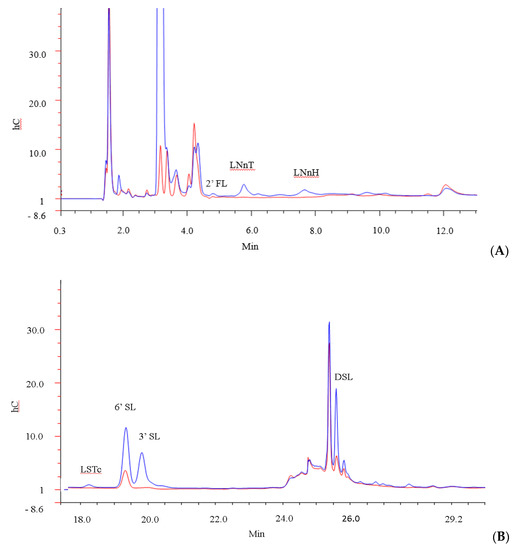
Figure 3.
GMO profile of (A) high molecular weight oligosaccharides and (B) low molecular weight oligosaccharides after 0 h (blue) and 24 h (red) incubation with B. infantis.
The presence of bifidobacteria in the gut are known to influence the production of formate, acetate, ethanol and lactate [69] and gut homeostasis is achieved through their production [70]. The inhibition of gram-negative bacteria through the production of metabolites has been shown [71]. Acetate is the most prominent short chain fatty acids (SCFA) [72] and accounts for over half the total SCFA content in stools [73,74]. Acetate has been shown to improve protection against pathogen colonisation as it modulates the gut epithelium and induces anti-inflammatory and anti-apoptotic effects [75].
In this study, HPLC was implemented to determine the production of metabolites such as acetate, lactate, formate and ethanol by B. infantis, following growth on GMO (Table 3). The results indicated that GMO resulted in a 12-fold (p-value = 0.0009) higher concentration of acetic acid at 24 h in comparison to the non-supplemented control. Indeed, the concentration of lactic acid was 15-fold higher (p-value = 0.0019), while the concentration of formic acid was 8-fold (p-value = 0.0001) higher than the non-supplemented control at 24 h. Notably, ethanol was only detected in the GMO-supplemented sample at a concentration of 8mM at 24 h (Table 3). Notably, bifidobacterial strains can catabolize 2 moles of hexose resulting in the production of 2 moles of lactic acid and 3 moles of acetic acid using the fructose-6-phosphate phosphoketolase pathway, resulting in a theoretical yield of acetate:lactate ratio of 1.5:1 in hexose [76]. However, this ratio is rarely obtained and varies depending on the growth substrate and/or culture conditions [77]. For example, growth on substrates LNT, LNnT, inulin-type fructans and 2FL has been demonstrated to result in higher ratios of acetate to lactate [76,78,79]. Similarly, in this study, the acetate to lactate ratio of acetate to lactate produced by B. infantis was 3.3:1 following 24 h growth on GMO. Increased acetate production has been reported to occur in log-phase cells and relates to the phosphorolytic splitting of pyruvate derived from carbons four, five, and six of hexose and this could account for the additional levels of acetate we observed in this study [80]. The high ratio of acetate to lactate in substrated LNT and LNnT has been suggested to be in part a result of the deacetylation of the GlcNAc [76] and this, too, may have contributed the results found here. Notably, some bifidobacteria convert pyruvic acid into formic acid and ethanol rather than into lactic acid, thereby yielding an extra ATP [77]. In this study, we detected concentrations of ethanol (8.004 mM), formic acid (8.57 mM) and lactate acid (9.04 mM) after 24 h fermentation in GMO, which also may explain the lower than expected ratio of lactate to acetate. While bifidobacteria are incapable of producing butyrate, within the host, the production of acetate and lactate can be converted to butyrate through cross-feeding pathways via indigenous colonic species, such as Faecalibacterium prausnitzii (clostridial cluster IV) and Anaerostipes, Eubacterium and Roseburia species (clostridial cluster XIVa) [70,81,82,83,84] and, thus, in vivo, may have additional beneficial effects.

Table 3.
Production of metabolites by B. longum subsp infantis American Type Culture Collection (ATCC) 15697 after 0 and 24 h incubation with GMO.
4. Conclusions
Initial exposure of bifidobacteria to GMO resulted in increased attachment to HT-29 cells and, in turn, had a prophylactic effect against C. jejuni attachment and invasion of intestinal cells in vitro. Protection against pathogen colonisation through competitive exclusion is a key benefit of the increasing bifidobacterial colonisation and could be exploited in order to deal with the rising numbers of Campylobacter infections. While other pathogens were not assessed in this study, there is potential that GMO treatment could provide overall resistance to pathogenic colonisation if used prophylactically as a synbiotic with bifidobacteria. Longer exposures of B. infantis to GMO increased the growth of the strain, highlighting its ability to adapt to environmental factors and promote its overall survival and colonisation in the GI tract, further highlighting the benefits of using synbiotic combinations. Overall, this study highlights the potential benefits in combining oligosaccharides from goat milk with select probiotic strains for promoting a healthy gut ecosystem, whilst protecting the host against pathogenic disease. Moreover, the use of such synbiotics is not limited to gastro-intestinal disorders, as low numbers of bifidobacteria have been associated with many other disorders. Such disorders include periodontal disease, rheumatoid arthritis, atherosclerosis, allergy, multi-organ failure, asthma and allergic diseases in addition to inflammatory bowel diseases, such as Crohn′s disease, irritable bowel syndrome and ulcerative colitis [85,86]. Synbiotics may, therefore, be more effective than either probiotics or prebiotics when used alone in the treatment and management of human health.
Supplementary Materials
The following are available online at https://www.mdpi.com/2304-8158/9/3/348/s1, Figure S1: Adhesion of B. longum subsp. infantis ATCC 15697 to HT-29 cells following incubation with goat milk oligosaccharides.
Author Contributions
Conceptualisation, L.J. and R.M.H.; formal analysis, H.S., D.W.; funding acquisition, R.M.H.; investigation, E.M.Q.; methodology, E.M.Q.; supervision, R.M.H.; writing—original draft, E.M.Q and R.M.H. All authors have read and agreed to the published version of the manuscript.
Funding
Erinn Quinn is in receipt of a Teagasc Walsh Fellowship.
Acknowledgments
Authors would like to thank Ardsallagh Goat Farm, Carrigtwohill, County Cork, Ireland for donating the goats’ milk.
Conflicts of Interest
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- O’Callaghan, A.; Bottacini, F.; O’Connell Motherway, M.; van Sinderen, D. Pangenome Analysis of Bifidobacterium longum and Site-Directed Mutagenesis through by-Pass of Restriction-Modification Systems. BMC Genomics 2015, 16, 832. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and Their Health-Promoting Effects. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Gueimonde, M.; Margolles, A.G.; de los Reyes-Gavilán, C.; Salminen, S. Competitive Exclusion of Enteropathogens from Human Intestinal Mucus by Bifidobacterium Strains with Acquired Resistance to Bile—A Preliminary Study. Int. J. Food Microbiol. 2007, 113, 228–232. [Google Scholar] [CrossRef]
- Collado, M.C.; Gueimonde, M.; Sanz, Y.; Salminen, S. Adhesion Properties and Competitive Pathogen Exclusion Ability of Bifidobacteria with Acquired Acid Resistance. J. Food Prot. 2006, 69, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Quezada, S.; Bermudez-Brito, M.; Chenoll, E.; Genovés, S.; Gomez-Llorente, C.; Plaza-Diaz, J.; Matencio, E.; José Bernal, M.; Romero, F.; Ramón, D.; et al. Competitive Inhibition of Three Novel Bacteria Isolated from Faeces of Breast Milk-Fed Infants against Selected Enteropathogens. Br. J. Nutr. 2013, 109, S63–S69. [Google Scholar] [CrossRef] [PubMed]
- Uraipan, S.; Hongpattarakere, T. Antagonistic Characteristics Against Food-Borne Pathogenic Bacteria of Lactic Acid Bacteria and Bifidobacteria Isolated from Feces of Healthy Thai Infants. Jundishapur J. Microbiol. 2015, 8, e18264. [Google Scholar] [CrossRef]
- Madsen, K.L. Enhancement of Epithelial Barrier Function by Probiotics. J. Epithel. Biol. Pharmacol. 2012, 5, 55–59. [Google Scholar] [CrossRef]
- Westermann, C.; Gleinser, M.; Corr, S.C.; Riedel, C.U. A Critical Evaluation of Bifidobacterial Adhesion to the Host Tissue. Front. Microbiol. 2016, 7, 1220. [Google Scholar] [CrossRef]
- Chichlowski, M.; De Lartigue, G.; German, J.B.; Raybould, H.E.; Mills, D.A. Bifidobacteria Isolated From Infants and Cultured on Human Milk Oligosaccharides Affect Intestinal Epithelial Function. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 321–327. [Google Scholar] [CrossRef]
- Kavanaugh, D.W.; O’Callaghan, J.; Buttó, L.F.; Slattery, H.; Lane, J.; Clyne, M.; Kane, M.; Joshi, L.; Hickey, R.M. Exposure of Bifidobacterium longum subsp. Infantis to Milk Oligosaccharides Increases Adhesion to Epithelial Cells and Induces a Substantial Transcriptional Response. PLoS ONE 2013, 8, e67224. [Google Scholar] [CrossRef]
- Quinn, E.M.; Slattery, H.; Thompson, A.; Kilcoyne, M.; Joshi, L.; Hickey, R.M. Mining Milk for Factors Which Increase the Adherence of Bifidobacterium longum subsp. infantis to Intestinal Cells. Foods 2018, 7, 196. [Google Scholar] [CrossRef]
- Oliveira, D.L.; Costabile, A.; Wilbey, R.A.; Grandison, A.S.; Duarte, L.C.; Roseiro, L.B. In Vitro Evaluation of the Fermentation Properties and Potential Prebiotic Activity of Caprine Cheese Whey Oligosaccharides in Batch Culture Systems. BioFactors 2012, 38, 440–449. [Google Scholar] [CrossRef]
- Leong, A.; Liu, Z.; Almshawit, H.; Zisu, B.; Pillidge, C.; Rochfort, S.; Gill, H. Oligosaccharides in Goats’ Milk-Based Infant Formula and Their Prebiotic and Anti-Infection Properties. Br. J. Nutr. 2019, 122, 441–449. [Google Scholar] [CrossRef]
- Newburg, D.S.; Ruiz-Palacios, G.M.; Morrow, A.L. Human Milk Glycans Protect Infants against Enteric Pathogens. Annu. Rev. Nutr. 2005, 25, 37–58. [Google Scholar] [CrossRef]
- Thum, C.; McNabb, W.C.; Young, W.; Cookson, A.L.; Roy, N.C. Prenatal Caprine Milk Oligosaccharide Consumption Affects the Development of Mice Offspring. Mol. Nutr. Food Res. 2016, 60, 2076–2085. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Alemka, A.; Whelan, S.; Gough, R.; Clyne, M.; Gallagher, M.E.; Carrington, S.D.; Bourke, B. Purified Chicken Intestinal Mucin Attenuates Campylobacter jejuni Pathogenicity In Vitro. J. Med. Microbiol. 2010, 59, 898–903. [Google Scholar] [CrossRef]
- Lane, J.A.; Mariño, K.; Naughton, J.; Kavanaugh, D.; Clyne, M.; Carrington, S.D.; Hickey, R.M. Anti-Infective Bovine Colostrum Oligosaccharides: Campylobacter jejuni as a Case Study. Int. J. Food Microbiol. 2012, 157, 182–188. [Google Scholar] [CrossRef]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s Normal? Oligosaccharide Concentrations and Profiles in Milk Produced by Healthy Women Vary Geographically. Am. J. Clin. Nutr. 2017. [Google Scholar] [CrossRef]
- Coppa, G.V.; Pierani, P.; Zampini, L.; Carloni, I.; Carlucci, A.; Gabrielli, O. Oligosaccharides in Human Milk during Different Phases of Lactation. Acta Paediatr. 1999. [Google Scholar] [CrossRef]
- Kunz, C.; Rodriguez-Palmero, M.; Koletzko, B.; Jensen, R. Nutritional and Biochemical Properties of Human Milk, Part I: General Aspects, Proteins, and Carbohydrates. Clin. Perinatol. 1999, 26, 307–333. [Google Scholar] [CrossRef]
- Shen, Z.; Warren, C.D.; Newburg, D.S. High-Performance Capillary Electrophoresis of Sialylated Oligosaccharides of Human Milk. Anal. Biochem. 2000. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Warren, C.D.; Altaye, M.; Morrow, A.L.; Ruiz-Palacios, G.; Pickering, L.K.; Newburg, D.S. Fucosylated Human Milk Oligosaccharides Vary between Individuals and over the Course of Lactation. Glycobiology 2001, 11, 365–372. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Müller-Werner, B.; Jelinek, J.; Stahl, B. Variation of Human Milk Oligosaccharides in Relation to Milk Groups and Lactational Periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef]
- Davidson, B.; Meinzen-Derr, J.K.; Wagner, C.L.; Newburg, D.S.; Morrow, A.L. Fucolsylated Oligosaccharides in Human Milk in Relation to Gestational Age and Stage of Lactation. Adv. Ecp Med. Biol. 2004, 554, 427–430. [Google Scholar] [CrossRef]
- Bao, Y.; Zhu, L.; Newburg, D.S. Simultaneous Quantification of Sialyloligosaccharides from Human Milk by Capillary Electrophoresis. Anal. Biochem. 2007, 370, 206–214. [Google Scholar] [CrossRef]
- Urashima, T.; Asakuma, S.; Leo, F.; Fukuda, K.; Messer, M.; Oftedal, O.T. The Predominance of Type I Oligosaccharides Is a Feature Specific to Human Breast Milk. Adv. Nutr. 2012, 3, 473S–482S. [Google Scholar] [CrossRef]
- Lane, J.A.; Mariño, K.; Rudd, P.M.; Carrington, S.D.; Slattery, H.; Hickey, R.M. Methodologies for Screening of Bacteria–Carbohydrate Interactions: Anti-Adhesive Milk Oligosaccharides as a Case Study. J. Microbiol. Methods 2012, 90, 53–59. [Google Scholar] [CrossRef]
- Albrecht, S.; Lane, J.A.; Mariño, K.; Al Busadah, K.A.; Carrington, S.D.; Hickey, R.M.; Rudd, P.M. A Comparative Study of Free Oligosaccharides in the Milk of Domestic Animals. Br. J. Nutr. 2014, 111, 1313–1328. [Google Scholar] [CrossRef]
- Urashima, T.; Saito, T.; Nakamura, T.; Messer, M. Oligosaccharides of Milk and Colostrum in Non-Human Mammals. Glycoconj. J. 2001, 18, 357–371. [Google Scholar] [CrossRef]
- Urashima, T.; Taueik, E.; Fukuda, K.; Asakuma, S. Recent Advances in Studies on Milk Oligosaccharides of Cows and Other Domestic Farm Animals. Biosci. Biotechnol. Biochem. 2013, 77, 455–466. [Google Scholar] [CrossRef]
- Martinez-Ferez, A.; Rudloff, S.; Guadix, A.; Henkel, C.A.; Pohlentz, G.; Boza, J.J.; Guadix, E.M.; Kunz, C. Goats’ Milk as a Natural Source of Lactose-Derived Oligosaccharides: Isolation by Membrane Technology. Int. Dairy J. 2006, 16, 173–181. [Google Scholar] [CrossRef]
- Martinez-Ferez, A.; Zapata, J.E.; Guadix, A.; Almecija, M.C.; Gomez, M.; Guadix, E.M. Obtention of Goat Milk Permeates Enriched in Lactose-Derived Oligosaccharides. Desalination 2009, 245, 730–736. [Google Scholar] [CrossRef]
- Silanikove, N.; Leitner, G.; Merin, U.; Prosser, C.G. Recent Advances in Exploiting Goat’s Milk: Quality, Safety and Production Aspects. Small Rumin. Res. 2010, 89, 110–124. [Google Scholar] [CrossRef]
- Quinn, E.M.; Hickey, R.M.; Lokesh, J. Symposium Review: Dairy-Derived Oligosacchrides-Their Influence on Host-Microbe Interactions in the Gastrointestinal Tract of of Infants. J. Dairy Sci. 2020. [Google Scholar] [CrossRef]
- Aquino, L.F.M.C.; de Moura Bell, J.M.L.N.; Cohen, J.L.; Liu, Y.; Lee, H.; de Melo Silva, V.L.; Domizio, P.; Conte Junior, C.A.; Barile, D. Purification of Caprine Oligosaccharides at Pilot-Scale. J. Food Eng. 2017, 214, 226–235. [Google Scholar] [CrossRef]
- Fernández, M.F.; Boris, S.; Barbés, C. Probiotic Properties of Human Lactobacilli Strains to Be Used in the Gastrointestinal Tract. J. Appl. Microbiol. 2003. [Google Scholar] [CrossRef]
- Servin, A.L. Antagonistic Activities of Lactobacilli and Bifidobacteria against Microbial Pathogens. FEMS Microbiol. Rev. 2004. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Mrazek, K.; Bereswill, S. Murine Fecal Microbiota Transplantation Alleviates Intestinal and Systemic Immune Responses in Campylobacter jejuni Infected Mice Harboring a Human Gut Microbiota. Front. Immunol. 2019. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Shuai, J.; Chen, J.; Zhang, Z.; Fang, W. The S-Layer Proteins of Lactobacillus crispatus Strain ZJ001 Is Responsible for Competitive Exclusion against Escherichia coli O157:H7 and Salmonella typhimurium. Int. J. Food Microbiol. 2007, 115, 307–312. [Google Scholar] [CrossRef]
- Gagnon, M.; Zihler Berner, A.; Chervet, N.; Chassard, C.; Lacroix, C. Comparison of the Caco-2, HT-29 and the Mucus-Secreting HT29-MTX Intestinal Cell Models to Investigate Salmonella Adhesion and Invasion. J. Microbiol. Methods 2013. [Google Scholar] [CrossRef] [PubMed]
- Cairns, M.T.; Gupta, A.; Naughton, J.A.; Kane, M.; Clyne, M.; Joshi, L. Glycosylation-Related Gene Expression in HT29-MTX-E12 Cells upon Infection by Helicobacter pylori. World J. Gastroenterol. 2017. [Google Scholar] [CrossRef]
- Rousset, M. The Human Colon Carcinoma Cell Lines HT-29 and Caco-2: Two In Vitro Models for the Study of Intestinal Differentiation. Biochimie 1986. [Google Scholar] [CrossRef]
- Morrin, S.T.; Owens, R.A.; Le Berre, M.; Gerlach, J.Q.; Joshi, L.; Bode, L.; Irwin, J.A.; Hickey, R.M. Interrogation of Milk-Driven Changes to the Proteome of Intestinal Epithelial Cells by Integrated Proteomics and Glycomics. J. Agric. Food Chem. 2019. [Google Scholar] [CrossRef]
- Bereswill, S.; Ekmekciu, I.; Escher, U.; Fiebiger, U.; Stingl, K.; Heimesaat, M.M. Lactobacillus johnsonii Ameliorates Intestinal, Extra-Intestinal and Systemic pro-Inflammatory Immune Responses Following Murine Campylobacter jejuni Infection. Sci. Rep. 2017. [Google Scholar] [CrossRef]
- WHO. World Health Organization; Campylobacter. Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter (accessed on 26 February 2020).
- Janssen, R.; Krogfelt, K.A.; Cawthraw, S.A.; van Pelt, W.; Wagenaar, J.A.; Owen, R.J. Host-Pathogen Interactions in Campylobacter Infections: The Host Perspective. Clin. Microbiol. Rev. 2008, 21, 505–518. [Google Scholar] [CrossRef]
- Callaway, T.R.; Edrington, T.S.; Anderson, R.C.; Harvey, R.B.; Genovese, K.J.; Kennedy, C.N.; Venn, D.W.; Nisbet, D.J. Probiotics, Prebiotics and Competitive Exclusion for Prophylaxis against Bacterial Disease. Anim. Heal. Res. Rev. 2008, 9, 217–225. [Google Scholar] [CrossRef]
- Saint-Cyr, M.J.; Guyard-Nicodème, M.; Messaoudi, S.; Chemaly, M.; Cappelier, J.-M.; Dousset, X.; Haddad, N. Recent Advances in Screening of Anti-Campylobacter Activity in Probiotics for Use in Poultry. Front. Microbiol. 2016, 7, 553. [Google Scholar] [CrossRef]
- Alter, T.; Bereswill, S.; Haag, L.M.; Heimesaat, M.M.; Glünder, G.; Rautenschlein, S.; Weber, R.M.; Hänel, I.; Lugert, R.; Zautner, A.E.; et al. Campylobacteriosis of Man: Livestock as Reservoir for Campylobacter Species. Bundesgesundheitsblatt—Gesundheitsforsch.—Gesundheitsschutz 2011. [Google Scholar] [CrossRef]
- Pielsticker, C.; Glünder, G.; Rautenschlein, S. Colonization Properties of Campylobacter jejuni in Chickens. Eur. J. Microbiol. Immunol. 2012. [Google Scholar] [CrossRef]
- Dec, M.; Nowaczek, A.; Urban-Chmiel, R.; Stępień-Pyśniak, D.; Wernicki, A. Probiotic Potential of Lactobacillus Isolates of Chicken Origin with Anti-Campylobacter Activity. J. Vet. Med. Sci. 2018, 80, 1195–1203. [Google Scholar] [CrossRef]
- Baffoni, L.; Gaggìa, F.; Di Gioia, D.; Santini, C.; Mogna, L.; Biavati, B. A Bifidobacterium-Based Synbiotic Product to Reduce the Transmission of C. jejuni along the Poultry Food Chain. Int. J. Food Microbiol. 2012, 157, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Baffoni, L.; Gaggìa, F.; Garofolo, G.; Di Serafino, G.; Buglione, E.; Di Giannatale, E.; Di Gioia, D. Evidence of Campylobacter jejuni Reduction in Broilers with Early Synbiotic Administration. Int. J. Food Microbiol. 2017, 251, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Arsi, K.; Donoghue, A.M.; Woo-Ming, A.; Blore, P.J.; Donoghue, D.J. The Efficacy of Selected Probiotic and Prebiotic Combinations in Reducing Campylobacter Colonization in Broiler Chickens. J. Appl. Poult. Res. 2015, 24, 327–334. [Google Scholar] [CrossRef]
- Garrido, D.; Dallas, D.C.; Mills, D.A. Consumption of Human Milk Glycoconjugates by Infant-Associated Bifidobacteria: Mechanisms and Implications. Microbiology 2013, 159, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Garrido, D.; Kim, J.H.; German, J.B.; Raybould, H.E.; Mills, D.A. Oligosaccharide Binding Proteins from Bifidobacterium longum subsp. infantis Reveal a Preference for Host Glycans. PLoS ONE 2011, 6, e17315. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; An, H.J.; Garrido, D.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Proteomic Analysis of Bifidobacterium longum subsp. infantis Reveals the Metabolic Insight on Consumption of Prebiotics and Host Glycans. PLoS ONE 2013, 8, e57535. [Google Scholar] [CrossRef]
- LoCascio, R.G.; Ninonuevo, M.R.; Freeman, S.L.; Sela, D.A.; Grimm, R.; Lebrilla, C.B.; Mills, D.A.; German, J.B. Glycoprofiling of Bifidobacterial Consumption of Human Milk Oligosaccharides Demonstrates Strain Specific, Preferential Consumption of Small Chain Glycans Secreted in Early Human Lactation. J. Agric. Food Chem. 2007, 55, 8914–8919. [Google Scholar] [CrossRef]
- LoCascio, R.G.; Desai, P.; Sela, D.A.; Weimer, B.; Mills, D.A. Broad Conservation of Milk Utilization Genes in Bifidobacterium longum subsp. infantis as Revealed by Comparative Genomic Hybridization. Appl. Environ. Microbiol. 2010, 76, 7373–7381. [Google Scholar] [CrossRef]
- Sela, D.A.; Chapman, J.; Adeuya, A.; Kim, J.H.; Chen, F.; Whitehead, T.R.; Lapidus, A.; Rokhsar, D.S.; Lebrilla, C.B.; German, J.B.; et al. The Genome Sequence of Bifidobacterium longum subsp. infantis Reveals Adaptations for Milk Utilization within the Infant Microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 18964–18969. [Google Scholar] [CrossRef]
- Sela, D.A.; Garrido, D.; Lerno, L.; Wu, S.; Tan, K.; Eom, H.-J.; Joachimiak, A.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subsp. infantis ATCC 15697 α-Fucosidases Are Active on Fucosylated Human Milk Oligosaccharides. Appl. Environ. Microbiol. 2012, 78, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Sela, D.A.; Li, Y.; Lerno, L.; Wu, S.; Marcobal, A.M.; German, J.B.; Chen, X.; Lebrilla, C.B.; Mills, D.A. An Infant-Associated Bacterial Commensal Utilizes Breast Milk Sialyloligosaccharides. J. Biol. Chem. 2011, 286, 11909–11918. [Google Scholar] [CrossRef]
- Garrido, D.; Ruiz-Moyano, S.; Mills, D.A. Release and Utilization of N-Acetyl-D-glucosamine from Human Milk Oligosaccharides by Bifidobacterium longum subsp. infantis. Anaerobe 2012, 18, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Marcobal, A.; Barboza, M.; Froehlich, J.W.; Block, D.E.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Consumption of Human Milk Oligosaccharides by Gut-Related Microbes. J. Agric. Food Chem. 2010, 58, 5334–5340. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.E.; Niñonuevo, M.; Mills, D.A.; Lebrilla, C.B.; German, J.B. In Vitro Fermentability of Human Milk Oligosaccharides by Several Strains of Bifidobacteria. Mol. Nutr. Food Res. 2007, 51, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- LoCascio, R.G.; Niñonuevo, M.R.; Kronewitter, S.R.; Freeman, S.L.; German, J.B.; Lebrilla, C.B.; Mills, D.A. A Versatile and Scalable Strategy for Glycoprofiling Bifidobacterial Consumption of Human Milk Oligosaccharides. Microb. Biotechnol. 2009, 2, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Bunesova, V.; Lacroix, C.; Schwab, C. Fucosyllactose and L-Fucose Utilization of Infant Bifidobacterium longum and Bifidobacterium kashiwanohense. BMC Microbiol. 2016, 16, 248. [Google Scholar] [CrossRef]
- Macfarlane, S.; Macfarlane, G.T. Regulation of Short-Chain Fatty Acid Production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Fooks, L.J.; Gibson, G.R. Probiotics as Modulators of the Gut Flora. Br. J. Nutr. 2002, 88, s39. [Google Scholar] [CrossRef]
- Salminen, S.; Bouley, C.; Boutron-Ruault, M.C.; Cummings, J.H.; Franck, A.; Gibson, G.R.; Isolauri, E.; Moreau, M.C.; Roberfroid, M.; Rowland, I. Functional Food Science and Gastrointestinal Physiology and Function. Br. J. Nutr. 1998, 80, S147–S171. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and Their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the Effects of Diet on Bacterial Metabolism in the Large Intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria Can Protect from Enteropathogenic Infection through Production of Acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Özcan, E.; Sela, D.A. Inefficient Metabolism of the Human Milk Oligosaccharides Lacto-N-Tetraose and Lacto-N-Neotetraose Shifts Bifidobacterium longum subsp. infantis Physiology. Front. Nutr. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Palframan, R.J.; Gibson, G.R.; Rastall, R.A. Carbohydrate Preferences of Bifidobacterium Species Isolated from the Human Gut. Curr. Issues Intest. Microbiol. 2003, 4, 71–75. [Google Scholar]
- Centanni, M.; Ferguson, S.A.; Sims, I.M.; Biswas, A.; Tannocka, G.W. Bifidobacterium bifidum ATCC 15696 and Bifidobacterium breve 24b Metabolic Interaction Based on 2′-O- Fucosyl-Lactose Studied in Steady-State Cultures in a Freter-Style Chemostat. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [PubMed]
- Perrin, S.; Warchol, M.; Grill, J.P.; Schneider, F. Fermentations of Fructo-Oligosaccharides and Their Components by Bifidobacterium infantis ATCC 15697 on Batch Culture in Semi-Synthetic Medium. J. Appl. Microbiol. 2001, 90, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Lauer, E.; Kandler, O. Mechanism of the Variation of the Acetate/Lactate/Ratio during Glucose Fermentation by Bifidobacteria. Arch. Microbiol. 1976, 110, 271–277. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Leroy, F. Cross-Feeding between Bifidobacteria and Butyrate-Producing Colon Bacteria Explains Bifdobacterial Competitiveness, Butyrate Production, and Gas Production. Int. J. Food Microbiol. 2011, 149, 73–80. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Moens, F.; Selak, M.; Rivière, A.; Leroy, F. Summer Meeting 2013: Growth and Physiology of Bifidobacteria. J. Appl. Microbiol. 2014, 116, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Gagnon, M.; Weckx, S.; Roy, D.; De Vuyst, L. Mutual Cross-Feeding Interactions between Bifidobacterium longum subsp. longum NCC2705 and Eubacterium rectale ATCC 33656 Explain the Bifidogenic and Butyrogenic Effects of Arabinoxylan Oligosaccharides. Appl. Environ. Microbiol. 2015, 81, 7767–7781. [Google Scholar] [CrossRef]
- Flint, S.W.; Hudson, J.; Lavallee, D. UK Adults’ Implicit and Explicit Attitudes towards Obesity: A Cross-Sectional Study. BMC Obes. 2015, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Lee, Y.K. Interplay between Intestinal Microbiota and Host Immune System. J. Bacteriol. Virol. 2014, 1–9. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).