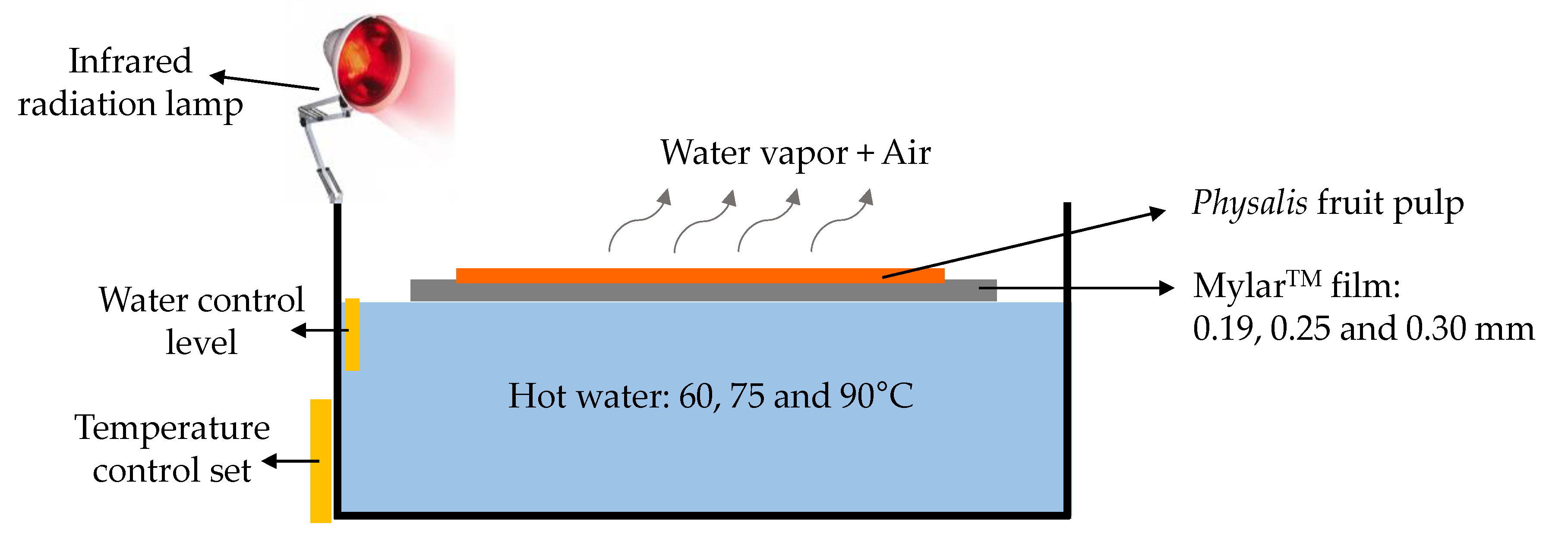
3.1. Drying Experiments
The initial moisture content, sugar content and water activity of
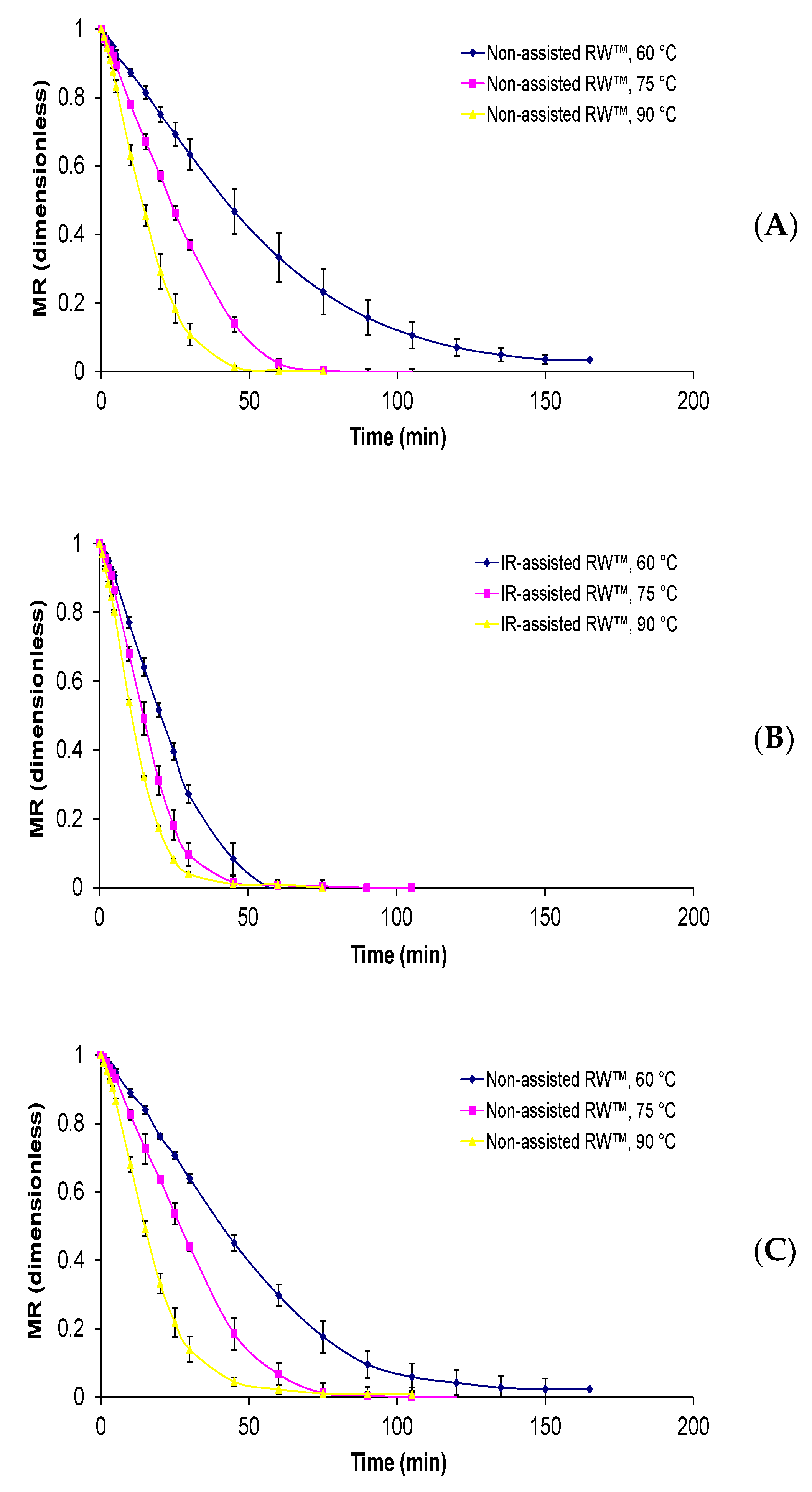
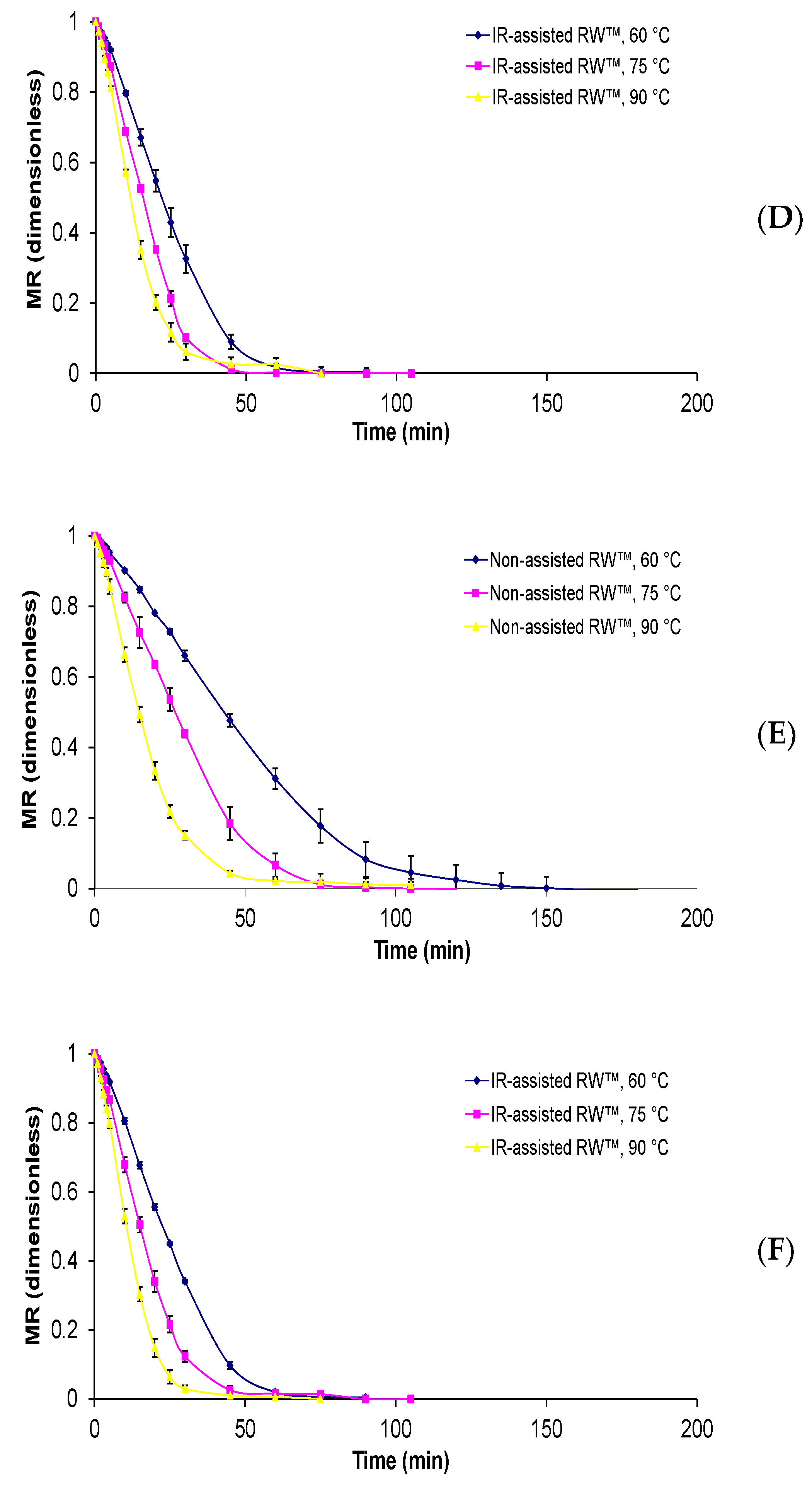
Physalis purée were of 4.155 ± 0.012 g water/g d.m, 15.48 ± 1.25 °Brix, 0.975 ± 0.001, respectively. Regarding drying kinetics, all the curves presented an exponential decay behavior and the influence of drying temperature is again remarkable (
Figure 2). The same tendency has been presented in other reports [
10,
11,
12,
13], where a decrease in process time and an increase in drying rate occurred with an increase in the drying temperature. While the drying times to reach equilibrium moisture content around 10% varied from 75 to 180 min. From the experimental drying curves of the non-assisted RW™ method, the shorter drying times were obtained at a temperature of 90 °C and a Mylar™ thickness of 0.19 mm and the longer times using a Mylar™ thickness of 0.29 mm and a drying temperature of 60 °C. Whereas when IR-assisted RW™ was used, the drying curves were more pronounced compared with just non-assisted RW™; a similar behavior was also observed in infrared-assisted convective drying of murta berry [
7].
Finally, the difference between drying temperatures and applied methods shows a significant decrease in drying time, with a difference of up to 100 min, that is the case of non-assisted RW™: 160 min at 60 °C and IR-assisted RW™: 60 min at 90 °C, both using Mylar thickness of 0.19 mm. Therefore, the temperature difference caused a decreasing drying time of around 20% to 30% in non-assisted RW™ and IR-assisted RW™, while the addition of the IR radiation led to a decreased RW™ drying process time of up to 62%, approximately. Against that background, Vega-Gálvez et al. [
14] presented drying time between 300 and 800 min for air-convective drying of
Physalis fruit in the 60 to 90 °C temperature range. Furthermore, Cabrera Ordoñez et al. [
15] evaluated the influence of two temperatures (50 and 60 °C) and two air velocity (2.0 and 3.0 m
2/s) on
Physalis drying, where drying times between 17 and 44 h were reached. Junqueira et al. [
16] studied the effect of diverse pretreatments and convective drying on kinetics and quality parameters of
Physalis fruit. The authors showed the drying times from 450 to 650 min, for each of the experiments.
RW™ drying has shown to be a promising drying technology, mainly in time reduction as compared with conventional dehydration methods. Furthermore, when using both combined drying technologies, IR and RW™, for industrial-scale dehydration of fruit and vegetable purée and pulp, as well as other food material, these could be considered as a synergy emerging technology, which allows reducing the drying time and thereby energy consumption.
3.2. Moisture Effective Diffusivities
Effective diffusivities values were in the range of 2.74 to 13.40 × 10
−10 m
2/s, in
Table 2 it can be observed that as the process temperature raises, there is a consequent increase in the effective diffusivity, and this behavior and order of magnitude has been reported for other RW™ drying methods, such as reported [
10] for mango flakes (
Deff: 5.43–9.33 × 10
−10 m
2/s); [
11] apple slices (
Deff: 2.5–7.14 × 10
−10 m
2/s); [
12] apple slices (
Deff: 1.25–14.3 × 10
−10 m
2/s), salmon tissue (
Deff: 1.24–3.09 × 10
−10 m
2/s) and beef slices (
Deff: 1.25–3.86 × 10
−10 m
2/s); [
17] mango pulp (
Deff: 7.0–11.0 × 10
−9 m
2/s); [
18] yam slices (
Deff: 1.94–2.54 × 10
−9 m
2/s); [
19] papaya purée (
Deff: 4.25–7.68 × 10
−10 m
2/s), and [
20] cucumber fruit slices (
Deff: 1.94–2.54 × 10
−9 m
2/s). It needs to be mentioned that
Deff values of previous researches varied because of different drying temperature, sample moisture content, application of pre-treatments and Mylar™ thickness.
Deff values studied for similar conditions of temperature and thickness (with difference in infrared assistance) indicate significant differences due to the inclusion of the IR radiation that increases the moisture diffusivity values in all the compared experiments, which implies that the addition of the IR radiation directly and significantly influences the migratory capacity of the water that is extracted in the RW™ drying process.
In both treatments (non-assisted RW™ and IR-assisted RW™), the effective diffusivity values varies significantly with the used temperature, for it, an ANOVA is necessary to realize. Thus, the ANOVA performed on
Deff values showed a significant influence of drying temperature on this physical parameter (
p-value < 0.05). Moreover, a MRT to determine the significant means among the
Deff was delivered, showing three homogeneous groups for each Mylar™ thickness used. This temperature-dependence effect has been described using Arrhenius-type relationship to get the activation energy (
Ea) from the effective diffusivity data. Then, the same ANOVA carried out on
Deff values presented a significant influence of Mylar™ film thickness on the diffusivity values, keeping constant the RW™ method (
p-value < 0.05). MRT analysis didn’t show a greater difference among the
Deff values vs Mylar™ thickness, showing two homogeneous groups for each drying temperature used (non-assisted RW™ drying → 60 °C: 0.19 mm and 0.25–0.29 mm/75 °C: 0.19–0.25 mm and 0.29 mm/90 °C: 0.19–0.25 mm and 0.19–0.29 mm; IR-assisted RW™ drying → 60 °C: 0.19–0.25 mm and 0.29 mm/75 °C: 0.19 mm and 0.25–0.29 mm/90 °C: 0.19–0.25 mm and 0.29 mm). This showed a clear effect of drying temperature but only a weak effect of Mylar™ film thickness. In this way, it would be possible to select only one appropriate Mylar™ film thickness with respect to the kind of dried food to be developed. Furthermore, this study confirmed again that temperature is one of the main drying operational variables for any drying method, along with IR-assistance also affecting significantly the drying process. Thus, information obtained also can be important for the next hybrid dryer performance taking into account food quality as well as economic costs [
12].
Furthermore, a third ANOVA assessment was established, in this case, the
Deff values were evaluated considering the same Mylar™ thickness but different RW™ drying method. From this analysis obtained, it was demonstrated a significant influence of assistance of the infrared system on the increased
Deff (
p-value < 0.05), in all cases evaluated (x,y). This showed a clear influence of IR radiation as an assistance system for any drying method. Likewise, diverse studies have reported that moisture diffusivities under infrared-assisted drying operations were higher compared to those of non-assisted drying process [
21,
22,
23]. This could be because, during IR-assisted RW™ heating of the moist
Physalis purée, IR radiation impinged on the exposed spread purée and penetrated it. This, radiation energy is readily converted into heat and the purée exposed to IR radiation is intensely heated, reaching a temperature gradient almost to zero within a short period [
21].
3.3. Kinetic Parameters
The kinetic parameters of empirical models can be observed in both RW™ method without IR and IR-assisted RW™ drying method in
Table 3 and
Table 4, respectively. The parameter
k,
ko, and
k1 values increase as drying temperature increases, this behavior is observed for most of the models and for each RW™ drying process. In addition, it can be seen as the inclusion of the IR radiation on RW
TM drying method caused that the parameters
k,
ko, and
k1 were also directly dependent on the heat source (IR radiation), which indicates that the linear increase of parameters
k would be related to the physical parameter,
Deff [
24]. The other empirical parameters (
n,
a–c) did not present significant variations despite increasing the temperature, this could occur due to the food matrix structure that is dried. Furthermore, some authors have found the n parameter not to vary with the temperature, as well as showed an air velocity linear dependence of this parameter
n [
25,
26]. The authors [
27] proposed that particularly for parameter
n, this empirical parameter could be affected in the case of drying fruit slices with or without skin, increasing accordingly the shell thickness. Since this study presented a fruit purée drying by RW™ method, these empirical parameters would not be affected by the presence of the shell, despite the temperature being increased, as well as IR radiation included. On this basis, the Mylar™ film thickness could have been an RW™ drying process operational parameter, which could have affected to empirical parameters, but this behavior did not happen.
Statistical evaluation for each mathematical model showed a good fit because low 0.002 ≤
RMSE ≤ 0.116 and 0.001 ≤
χ2 ≤ 0.024 values were obtained. Furthermore, taking account the criterion of
R2 ≥ 0.85, most of the models displayed a good fit quality to all experimental data. However, regarding the statistical values obtained, Midilli–Kucuk (0.005 ≤
RMSE ≤ 0.012; 0.001 ≤
χ2 ≤ 0.004 and 0.997 ≤
R2 ≤ 0.999) and Page (0.002 ≤
RMSE ≤ 0.021; 0.001 ≤
χ2 ≤ 0.003 and 0.991 ≤
R2 ≤ 0.999) models showed the best-fit quality on experimental data, for non-assisted RW™ and Infrared-assisted RW™ drying, respectively. Some researchers have attained good outcome when applying both Midilli–Kucuk and Page models on the drying kinetics of different food subjected to different hybrid drying method [
9,
28,
29] as well as the RW™ drying method [
12,
13]. Always bearing in mind the use of the experimental data or any of the developed models to calculate drying time to reach equilibrium moisture content gives results that differ slightly in magnitude.
3.4. Activation Energy
The activation energy values calculated based on
Deff values for the temperature range 60 to 95 °C, taking account both RW™ method and Mylar™ thickness as independent-factors. The
Ea values for non-assisted RW
TM drying methods were of 41.3, 33.4, and 31.1 kJ/mol for each Mylar™ thickness of 0.19, 0.25, and 0.29 mm, respectively. Whereas
Ea results for IR-assisted RW™ drying technique were of 34.5, 33.0, and 26.6 kJ/mol for each Mylar
TM thickness of 0.19, 0.25, and 0.29 mm, respectively. From comparing the
Ea results obtained by different RW™ methods for the same Mylar thickness, the non-assisted RW™ drying method obtained
Ea values slightly higher than IR-assisted RW™. This may be in part because
Ea is the energy barrier to activate water diffusion; hence, the IR-assisted RW™ would need less energy to start the moisture diffusion transport. The results obtained are similar to those obtained in other report [
11] on the air drying of
Physalis fruit obtaining 38.78 kJ/mol, other results obtained are similar, 37.27 kJ/mol for figs [
27]; but significantly less than those obtained [
30] for the Berberis between 110.84–130.61 kJ/mol.
3.5. Microstructure Analysis
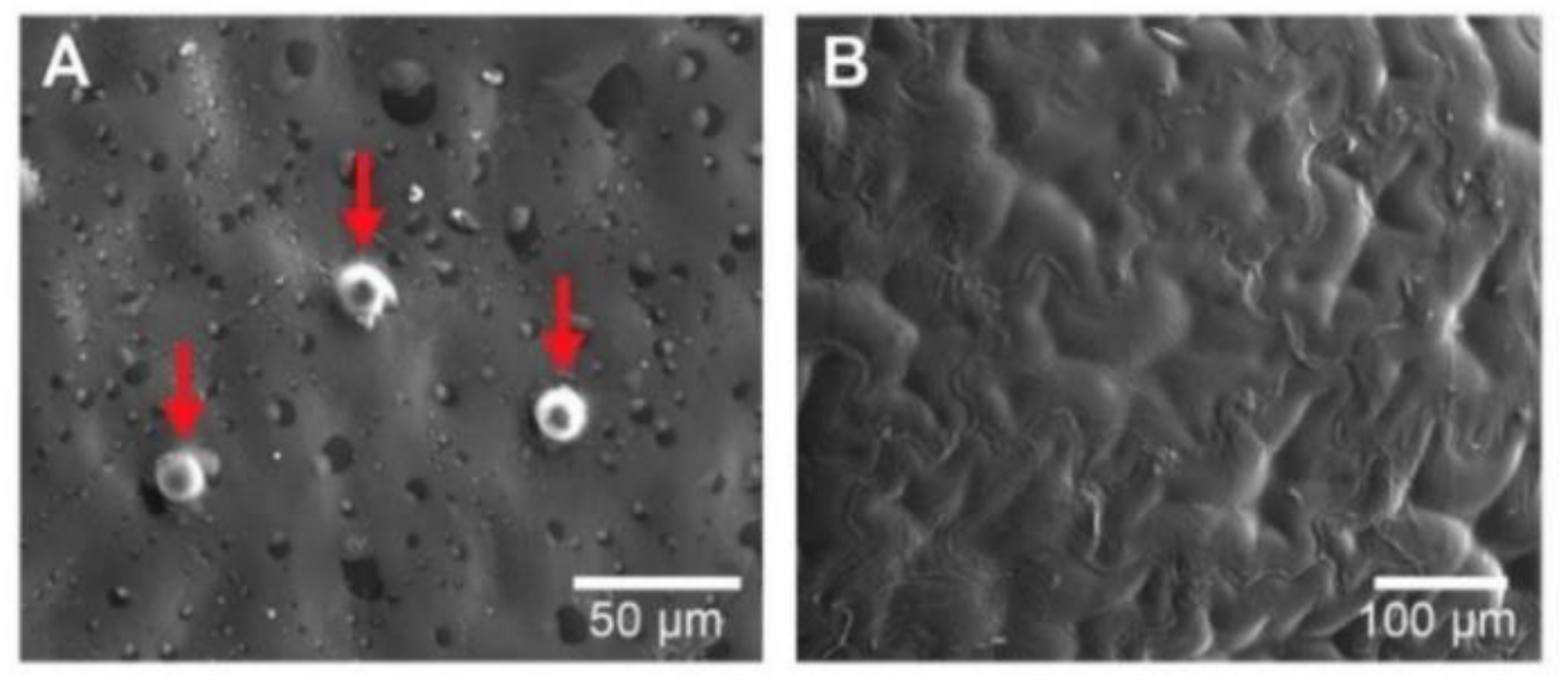
It’s worth recalling that fresh Physalis purée is conformed of flesh, peel, and seeds. Hence the microstructural observations were accomplished on these different sections of fresh Physalis purée. The microstructure analysis consists of a complex network made of peel and seeds together with the flesh from the fruit, holding water about them.
Figure 3A shows the microphotography of an integral peel piece from fresh
Physalis purée. This microphotography enables exhibiting a skin piece very properly for observation. From the microphotography, a kind of bumps, called “emergencies” by the author [
31] is shown, which has been described extensively by [
32]. These emergencies behave as a secretory structure and lend the fruit a kind of semi-rough texture, in addition, these are formed by cells of the epidermis and hypodermis. Concerning
Figure 3B, this shows the seed pieces with a subtle differentiation between the test (outer layer of the seed) and the parenchymal tissue (which functions as a support in the cell network) described by [
31]. Beyond peel pieces “emergencies” size, differences in the homogeneity were observed, where seed microstructure displayed a more homogeneous microstructure.
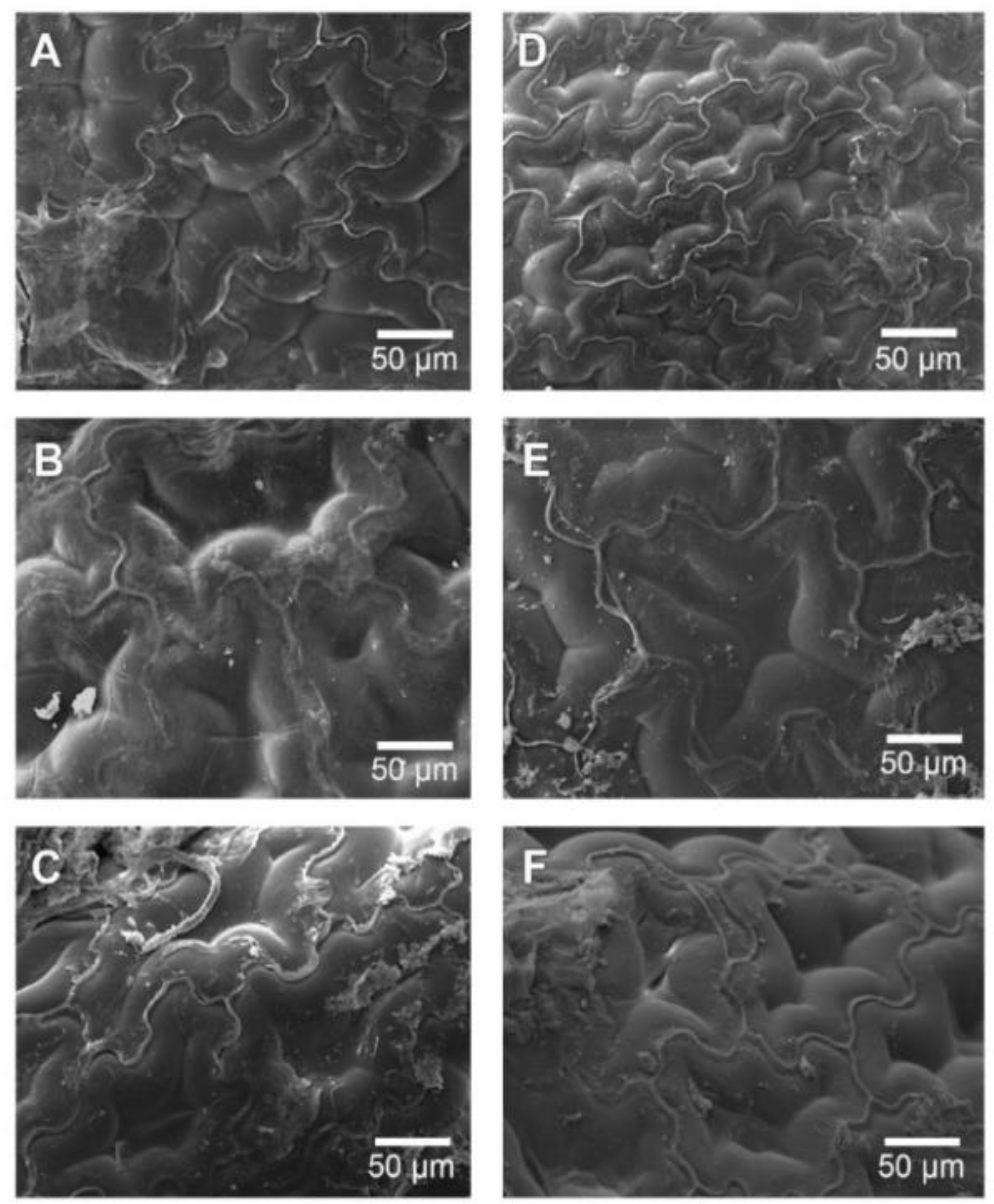
From
Figure 4, it is possible to observe the effect of both non-assisted RW™ and IR-assisted RW™ drying processes on the
Physalis purée samples.
Figure 4A shows that the cellular network belonging to the peel was affected by the extraction of free water during the drying process, the same situation occurs in
Figure 4B, increasing the trend and observing a rough surface. As to
Figure 4C, it was not possible to clearly observe the surface described above. It was just possible to recognize peel tissue due to the “emergencies” that were described above, where the cellular damage obtained could have been caused by the application of high drying temperature, in this case at 90 °C (both RW™ drying methods); hence, obtaining a more aggressive evaporation of the water available at the cellular level, breaking the walls of the cells present in the flesh and peel. This fact may also be related to different local chemical composition (e.g., soluble solids, pH, ionic strength, specific cations) that can influence the dried
Physalis purée deformation and structure.
Figure 4D–F shows dried
Physalis purée peel by IR-assisted RW™ drying method, where these drying experiments maintained the tendency to compare it with the fresh
Physalis purée peel (
Figure 3A) obtaining a rough surface, with an evident loss of water from the cells. This could be present with a more marked tendency than in those obtained in
Figure 4A–C. In the same way this can also be observed when comparing the same drying temperatures with and without the use of infrared energy.
Figure 4A shows a dried peel with a smoother surface than
Figure 4D, indicating in both situations described above the incidence of infrared assisted RW™ drying. Likewise, the tendency towards rough surfaces is maintained in
Figure 4E, also observing the emergencies described above. Finally, in
Figure 4F, as in
Figure 4C, the cellular damage is higher than that observed in
Figure 4C, this may occur because infrared assistance, including the drying process with a higher temperature, raises the internal temperature of the extracted water more aggressively, this being an explosive process, damaging the observed tissues.
Figure 5 presents the observations performed on dried
Physalis purée seeds, where it is possible to observe as the seed structure changes slightly as the drying temperature, which can be seen in both RW™ drying methods. This change was expressed in its smooth surface towards a rougher one, obtaining a better differentiation of the parenchymal tissue, it is also possible to observe that the IR-assisted RW™ drying caused an increase in the water extraction. In addition, the quality of the end products by RW™ is higher as compared with conventional drying methods [
12].
3.6. Color Parameters and ∆E
The colorimetric values showed that all Physalis purée samples lost L* coordinate, got closer to the green color (a* coordinate) and also to the blue color, losing yellow tones (b* coordinate), regarding fresh Physalis samples. Colorimetric coordinates of fresh Physalis purée samples were 83, 99, 34, 62, and 72, 4 to L*, a* and b*, respectively.
The
L* coordinate values (
Table 5) decreased by both RW™ drying methods, this is frequent in most drying methods, however, particularly in RW™ drying, there are some similar results obtained in strawberries [
33] and also in mango flakes [
34]. There are some works presenting that the higher the degree of browning, the lower the sample
L* coordinate value. These chemical reactions are a consequence of reducing sugars and amino acids in the food being dried, where particularly in
Physalis fruit that has different amino acids (leucine, lysine, and isoleucine) and sugars (sucrose, glucose, and fructose). Thereby, dried
Physalis purée would become darker, probably because of an extensive Maillard reaction, as in this particular case of IR-assisted RW™ treated
Physalis purées.
Regarding
a* coordinate (
Table 6), diverse differences have been seen according to the food to be dried. It was found that asparagus mash drying kept the green color better than any other type of drying [
35]. On the other hand, a significant decrease of the
a* coordinate has been reported in work on the Refractance Window™ drying of strawberries [
33]. Finally, the decrease in
b* coordinate (
Table 7) could be an indication of the loss of carotenoids present in dried
Physalis purée, unlike the results that have been reported in carrot flakes drying [
36], which have improved the retention of these compounds. Furthermore, the effect of decreasing water activity in amorphous dehydrated systems can be decisive on the non-enzymatic Maillard browning reaction rate and formation of brown pigments [
13].
ANOVA analysis showed that drying temperature (a, b, c) had a significant influence on color indices of
L* and
b* value (
p-value < 0.05). Moreover, although slice Mylar™ thickness (A, B, C) influenced
L* and
b* values substantially, it failed to affect
a* values considerably. Ochoa-Martinez [
7] demonstrated that the film thickness had significant effects on
L*,
a*, and
b* coordinates for dried mango slices by Refractance Window™ drying. Moreover, a new ANOVA analysis found that RW™ drying method had a significant effect on
L*,
a*, and
b* value (
p-value < 0.05) but only at 90° (x, y, z).
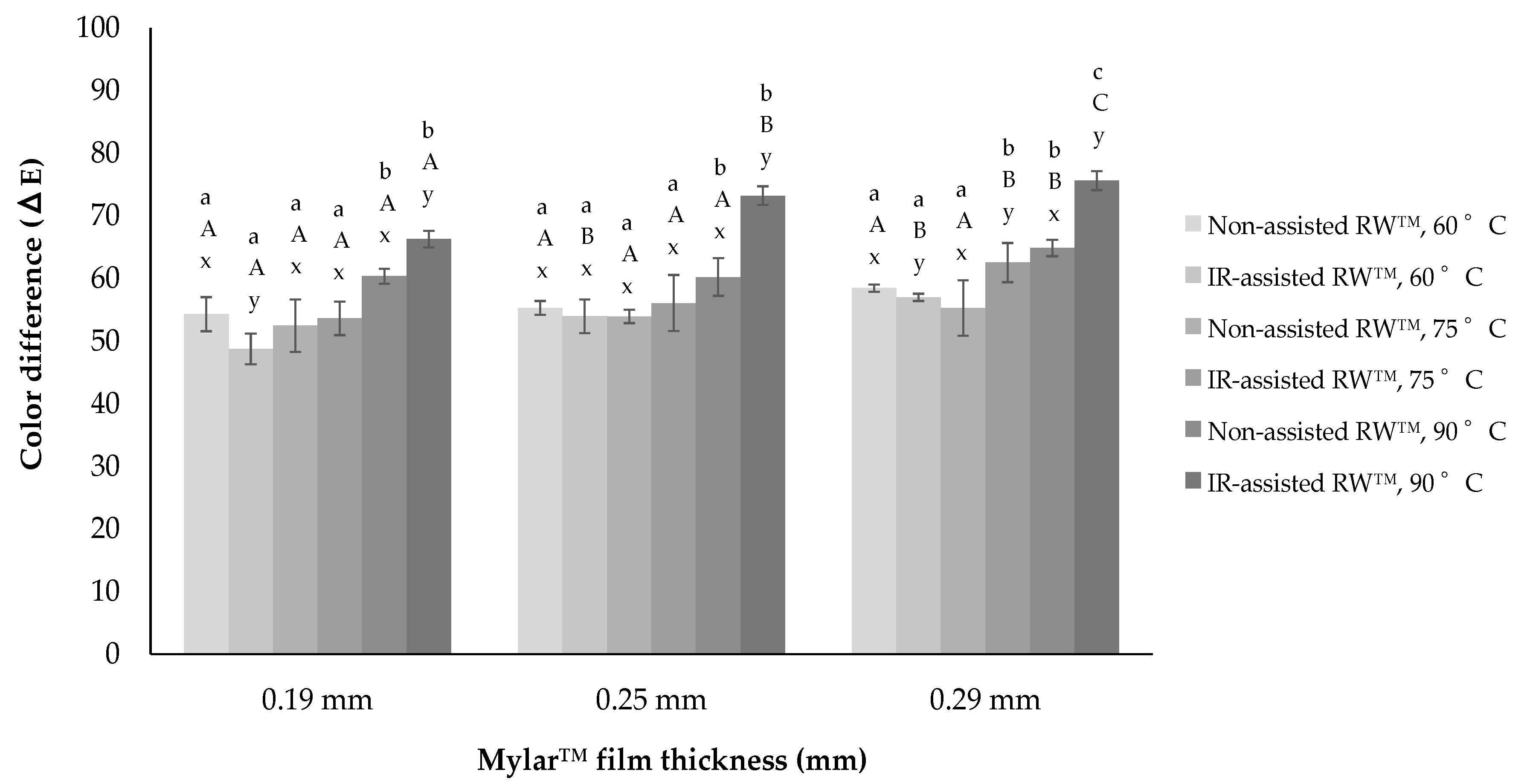
As to the total color difference or
ΔE as shown in
Figure 6, for both RW™ drying conditions, highly significant differences were observed in dried
Physalis purée (between 50 ≤
ΔE ≤ 75). An increase in the drying temperature when processing without infrared resulted in an increase in Δ
E. In general, the changes of
ΔE are mainly due to changes in the chromatic parameters
a* and
b*, where Abonyi et al. [
33] found in strawberries and carrots and Nindo et al. [
35] presented in asparagus with
ΔE values between 20 and 25. Fisher et al. [
37] and Busso et al. [
38] also found similar color changes after the thermal treatment of pomegranate juice and blackcurrant, maqui berry, and blueberry pulps, where they ascribed it to anthocyanin losses.
Thus, color changes caused by both RW™ drying process could have occurred, not only by the non-enzymatic browning reaction but also by the destruction of the pigment present in the Physalis purée, such as β-carotene. Nevertheless, the addition of the IR radiation could suppose a very invasive thermal shock for the Physalis purée drying, obtaining differences of higher color than with respect to the non-assisted RW™ drying process (e.g., IR-assisted RW™ drying at 90 °C: 65 ≤ ΔE ≤ 75). That is why, since food surface color is one of the quality parameters most evaluated by consumers, and is critical in the acceptance of the product, new strategies should also be considered for developing dried fruit purée, such as pre-treatments with additives that could better protect color compounds.
Then, an ANOVA assessment was established, where the ΔE values were evaluated considering the diverse factors under study, drying temperature, Mylar™ thickness, and RW™ drying method. This ANOVA analysis found a significant influence of drying temperature as well as RW™ drying method on the ΔE values (p-value < 0.05), in all cases evaluated. Whereas, Mylar™ thickness didn’t show a clear influence on the ΔE values (p-value > 0.05), in the same experiments evaluated.