Abstract
So far, only a few cases of immunoglobulin E (IgE)-mediated coconut allergies have been described in the literature. Due to a growing consumption of coconut-containing foods in occidental countries, the number of coconut allergies may also increase. As there is no causative immunotherapy in clinical routine, appropriate food labelling is particularly important, also with regard to cross-contamination, to prevent serious health consequences. The purpose of this study was to develop a DNA-based detection method for coconut (Cocos nucifera). Initially, three sets of coconut-specific primers were designed and tested. A TaqMan™ probe was then developed to identify and quantify coconut by real-time PCR assay. With 27 other plant and animal species, the specificity of the primer/probe system was tested and cross reactivity was excluded. In a dilution series, a limit of detection of 1 pg/µL was determined. Thus, the developed real-time PCR assay is a suitable method to detect coconut in food.
1. Introduction
The coconut palm (Cocos nucifera) belongs to the family Palmaceae (Arecaceae) in the genus Cocos. The fruits of the coconut palm are not nuts, but drupes. The outer layer consists of a green to yellow, leathery exocarp. Below is a fibrous mesocarp, followed by a woody endocarp that surrounds the seed. This unusual fruit structure is the reason why coconuts have the ability to float thousands of kilometers between tropical islands, so that they are now spread along tropical coasts all over the world and nobody can clearly determine their center of origin. Humans have used coconuts as food, medicine, cosmetics, and as material for ropes and mats for thousands of years. In plantations, it has been cultivated since the middle of the 19th century [1]. Today, it is one of the economically most important palm species in the world, which is mainly cultivated in Indonesia, the Philippines, and India, but used all over the world [2].
In view of the growing consumption of coconut-containing products in occidental countries, the number of coconut food allergies may increase. So far, only a few cases of coconut allergies are known. But even after years of symptom-free exposure to coconuts, either through the skin or taken orally, it is still possible to develop allergenic reactions. However, not only skin rashes, but also anaphylaxis can occur, which poses a fatal danger to the affected persons. The allergic reaction is currently attributed to a 7S and an 11S globulin protein called Coc n2 and Coco n4. 7S globulins have already been described as allergens in walnuts and hazelnuts, so that some patients who are allergic to tree nuts show cross-reactions to coconuts [3,4,5,6]. As there is no causative immunotherapy in the clinical routine for food allergies so far, the only way to prevent an allergic reaction for people who are affected is to strictly avoid the allergenic food.
Consequently, appropriate food labelling is important. The EU Food Information Regulation No. 1169/2011 contains a list of 14 allergenic foods and regulates their labelling. However, this list does not yet contain coconut as an allergenic food [7]. Due to the high prevalence of peanut and tree nut allergies and concerns about possible cross-reactivity, coconut must be labelled as an ingredient in food products in the US [8]. However, special risk challenges are provided by so-called “hidden” traces of allergens, which can get into food through cross-contamination in certain food production processes. A shared concern of food allergy patients is the question, whether they can safely consume certain food products. Therefore, sensitive and specific detection methods are essential.
So far, the detection of coconut in food is routinely done using enzyme-linked immunosorbent assays (ELISA) test kits from different manufacturers. The aim of the present study was to develop a DNA-based detection method for Cocos nucifera using a TaqMan™ real-time PCR. Previous DNA-based detection methods have particularly focused on the detection of the 14 allergens regulated in the EU including soy, lupines, peanuts, and tree nuts [9,10,11,12]. Coconut, as a potential allergenic food, has not received much attention in the development of DNA-based detection methods yet.
For this purpose, different coconut-specific primers were designed and tested for their specificity. Subsequently, a TaqMan™ probe for real-time PCR assays was developed. In addition to its specificity, the primer/probe system was also tested for its sensitivity.
2. Materials and Methods
2.1. Sample Material
One sample of authentic coconut palm (Cocos nucifera) leaves were provided by Indonesian farmers, represented by Elevate Social Businesses Ltd., New York City, USA, a non-profit organization that improves the livelihoods of farmers and farming communities in Indonesia. One leaf sample each of Chilean wine palm (Jubaea chilensis), oil palm (Elaeis guineensis), and jelly palm (Butia capitata) were obtained from the Palmengarten Frankfurt/Main, Germany. All leaf samples were stored at −80 °C until DNA extraction. One coconut was purchased in a local German grocery store. One sample of coconut chips from Sri Lanka and one sample of Indonesian coconut blossom sugar were provided by a trading company (Voicevale Ltd., London, UK).
2.2. Sample Preparation and DNA Extraction
Palm leaves were washed with 70% ethanol, frozen in dry ice, and ground in a knife mill (Grindomix GM 200, Retsch GmbH, Haan, Germany). Coconuts were cracked and cut into small pieces with a knife. Coconut chips and coconut sugar were used directly for DNA extraction.
DNA was extracted using a cetyltrimethylammonium bromid (CTAB) method according to the German official methodology described in the German food law (LFGB), § 64 LFGB L 00.00-119:2014-02 based on DIN EN ISO 21571:2013-08 [13]. In brief, about 2 to 5 g sample were mixed with 5 to 10 mL of CTAB-containing lysis buffer and 30 µL of 20 µg/µL proteinase K. The mixture was incubated at 65 °C overnight. DNA purification was performed by a washing step with 2 mL trichloromethane, followed by DNA precipitation with CTAB precipitation buffer. The supernatant was removed and the DNA pellet dissolved in sodium chloride solution (1.2 M). Further purification with trichloromethane/3-methyl-1-butanol (24:1) and precipitation with 2-propanol and glycogen followed. The pellet was finally purified with 70% ethanol, dried, and dissolved in tris-(hydroxymethyl)-aminomethane-ethylenediaminetetraacetic-acid (TE) buffer.
2.3. Determination of DNA Concentration and Purity
Estimation of DNA concentration and purity was performed using a spectrophotometer (BioSpectrometer® basic, Eppendorf AG, Hamburg, Germany) at 260 and 280 nm. For a quantitative determination of DNA, the fluorescence signal was measured after addition of the DNA intercalation dye PicoGreen (Quant-iT™ PicoGreen® dsDNA Reagent, Thermo Fisher Scientific Inc., Waltham, MA, USA) against a λ-DNA (Thermo Fisher Scientific Inc., Waltham, MA, USA) standard curve using a microtiter plate reader (GENios, TECAN Group Ltd., Männedorf, Switzerland).
2.4. PCR Primers and Probes
In the present study, different sets of primers and one TaqMan™ probe were designed.
DNA sequences and descriptions of the primers and probes are listed in Table 1.

Table 1.
DNA sequences and description of the forward (= for.) and reverse (= rev.) primers and probes listed alphabetically.
For a verification of the botanical origin of the palm leaves, universal Palmae-specific gene primers were designed using the DNA sequence of the nuclear gene phosphoribulokinase (prk). It encodes a chloroplast enzyme essential for the photosynthetic process of CO2 assimilation of the Calvin cycle [14] and has already been widely used in the phylogenetic analysis of Palmae species [15,16]. The sequences of the prk gene of the coconut palm (Cocos nucifera), Chilean wine palm (Jubaea chilensis), oil palm (Elaeis guineensis), and jelly palm (Butia capitata) were downloaded from the NCBI database and aligned with BioEdit 7.0.5 (Ibis Therapeutics Inc., Carlsbad, CA, USA) for selecting primer regions that are universal to the Palmae family. For the forward primer palmPRK-for, the sequence CTA GCA AAG AAT CTG ATC GAT AAG T with a length of 25 bp, a GC-content of 36%, and a melting temperature of 62 °C was selected. The sequence CAT ATT GCT TCT GTG GGT CTG with a length of 21 bp, a GC-content of 48%, and a melting temperature of 63 °C was used as reverse primer palmPRK-rev. With the web tool OligoAnalyzer (Integrated DNA Technologies Inc., Coraville, IA, USA), the formation of hairpins, self-dimers, and hetero-dimers was checked.
For the design of primer sets that could only specifically amplify Cocos nucifera, two different genes were used: the prk gene and the internal transcribed spacer 2 (ITS2) gene. ITS2 is located between the coding regions of the 5.8S and the 28S rRNA of the eukaryotic nuclear ribosomal desoxyribonucleic acid (rDNA). Due to its high divergence between species, it is a commonly used marker for phylogenetic analysis across plants, also distinguishing closely related species [17,18,19].
The sequence of the ITS2 gene of coconut palm (Cocos nucifera) was downloaded from the NCBI database and analyzed via PrimerBlast for appropriate primer sets. Out of 10 primer set suggestions, two were selected and tested for specificity. The primer set made up of cocosITS109-for (GGC CTC CTG AGG TAT ATC CG; length: 20 bp; GC-content: 60%; melting temperature: 67 °C) and cocosITS109-rev (CAT CCA CCA TCC ACC GTG TC; length: 20 bp; GC-content: 60%; melting temperature: 68 °C) amplifies a 109 bp long product. CocosITS197-for (TAT CCG GAT GTG GAT GCT GG; length: 20 bp; GC-content: 55%; melting temperature: 67 °C) and cocosITS197-rev (CAT CCG ATG GCT GGG GTG; length: 18 bp; GC-content: 67%; melting temperature: 69 °C) amplify a product of 197 bp length.
For the primer set of the prk gene, the coconut-specific sequences cocosPRK-for (ACA AGA CCT ACT GGA CTG G; length: 19 bp; GC-content: 53%; melting temperature: 63 °C) and cocosPRK-rev (TCT GAT ATG TAT AAG ACT CAG CA; length: 23 bp; GC-content: 35%; melting temperature: 59 °C), which amplify a 164 bp long product, were selected based on the alignment in BioEdit.
The synthesis of the PCR primers was purchased from Integrated DNA Technologies Inc. (Coraville, IA, USA).
The designed TaqMan™ probe was purchased from TIB MOLBIOL Syntheselabor GmbH (Berlin, Germany).
2.5. Conventional PCR
Conventional polymerase chain reaction (PCR) was performed using a commercially available master mix (Q5® Hot Start High-Fidelity Master Mix (2X), New England BioLabs, Inc., Ipswich, MA, USA). The amount of reaction components and the thermocycling conditions were selected according to manufacturer’s instructions. For a total volume of 25 µL PCR reaction mixture, 1.25 µL of forward primer (10 µM), 1.25 µL of reverse primer (10 µM), 5 µL of DNA template (1–5 ng/µL), and 5 µL of distilled water were added to 12.5 µL of master mix. Non-template samples with 5 µL of distilled water instead of DNA were used as negative controls.
The PCR was run on a thermocycler (Mastercycler® nexus gradient, Eppendorf AG, Hamburg, Germany) with the following conditions: initial denaturation at 98 °C for 10 min followed by 35 cycles of denaturation at 98 °C for 10 s, annealing at a primer specific temperature (euk18S: 68 °C, palmPRK: 63 °C, cocosPRK: 60 °C, cocosITS109: 68 °C, cocosITS197: 68 °C) for 25 s, and extension at 72 °C for 15 s and 1 cycle of final extension at 72 °C for 2 min.
2.6. Gel Electrophoresis
All PCR products were separated with gel electrophoresis on a 2% agarose gel containing 5 µL of the intercalating nucleic acid stain GelRed® 10000x (Biotium, Inc., Fremont, CA, USA) in 1x tris-(hydroxymethyl)-aminomethane-borate-ethylenediaminetetraacetic-acid (TBE) buffer. Then, 4 µL PCR product was mixed with 3 µL DNA loading dye (FastDigest Green Buffer (10x), Thermo Fisher Scientific Inc., Waltham, MA, USA), loaded into each well, and electrophoresed at 90 V for 1.5 h. Electrophoresis gels were visualized and analyzed by UV transillumination. A 100 bp DNA ladder (Quick-Load® Purple 100 bp DNA Ladder, New England BioLabs, Inc., Ipswich, MA, USA) was used for comparison.
2.7. DNA Sequencing
To confirm the sequence of PCR products, the DNA was isolated from the agarose gel using a PCR clean-up and gel extraction kit (NucleoSpin® Gel and PCR Clean-up, MACHEREY-NAGEL GmbH & Co. KG, Düren, Germany) and was sent to a laboratory specialized in sequencing (Eurofins Genomics Germany GmbH, Cologne, Germany). The results were verified using a sequence comparison with BLASTn against the NCBI nucleotide database.
2.8. Real-Time PCR
Real-time PCR was run on a qTOWER3-thermocycler (Analytik Jena AG, Jena, Germany). Each reaction mixture of 20 µL contained 10 µL of innuMIX qPCR MasterMix Probe (Analytik Jena AG, Jena, Germany), 3.8 µL of distilled water, 0.5 µL of forward primer (20 µM), 0.5 µL of reverse primer (20 µM), 0.2 µL of probe (20 µM), and 5 µL of DNA template (10 ng/µL). The thermocycling conditions were set as follows: 50 °C for 2 min, 95 °C for 10 min, followed by 45 cycles of 95 °C for 15 s, and 60 °C for 1 min.
2.8.1. Specificity of the Real-Time PCR Assay
In order to verify specificity, the cross-reactivity of the primer/probe system was tested with DNA isolated from the following plant and animal species: almond (Amygdalus communis), apricot (Armeniaca vulgaris), Atlantic redfish (Sebastes norvegicus), brazil nut (Bertholletia excelsa), cattle (Bos taurus), celery (Apium graveolens), chicken (Gallus gallus), Chilean wine palm (Jubaea chilensis), chili pepper (Capsicum frutescens), hazelnut (Corylus avellana), lupine (Lupine spp.), mango (Mangifera indica), oil palm (Elaeis guineensis), pangasius (Pangasianodon hypophthalmus), papaya (Carica papaya), peanut (Arachis hypogaea), pepper (Capsicum annuum), pistachio (Pistacia vera), rye (Secale cereale), sesame (Sesamum indicum), soy (Glycine max), sunflower (Helianthus annuus), turkey (Meleagris gallopavo), walnut (Juglans regia), wheat (Triticum aestivum), white mustard (Sinapis alba), jelly palm (Butia capitata).
For evaluating sensitivity of the real-time PCR assay, the isolated targeted DNA was serially diluted 10-fold, reaching a concentration range from 100 ng/µL to 0.1 pg/µL.
3. Results
3.1. Extraction of DNA from Palm Leaves
DNA was isolated from coconut leaves, Chilean wine, oil, and jelly palm in four replicates (A, B, C, D) using the standard CTAB method. The purity and concentration of the DNA was determined spectrophotometrically. Performing a conventional PCR, followed by gel electrophoresis of the PCR products, the amplifiability of the isolated DNA was verified. Therefore, the well-established primers euk18S-for and euk18S-rev were used to amplify a 137 bp long sequence of the 18S ribosomal ribonucleic acid (rRNA) gene specific for eukaryotes [20,21]. The results of DNA isolation and the conventional PCR assay are presented in Table 2. DNA was successfully isolated from all samples and of comparatively good quality.

Table 2.
Properties of the DNA extracted from the leaves of coconut palm (Cocos nucifera), Chilean wine palm (Jubaea chilensis), oil palm (Elaeis guineensis), and jelly palm (Butia capitata).
3.2. Verification of the Botanical Origin of the Palm Leaves
For a verification of the botanical origin of the palm leaves, universal Palmae-specific gene primers palmPRK-for and palmPRK-rev were designed and a conventional PCR followed by a gel electrophoretic analysis was performed. The PCR products, varying in length according to the palm species, were sequenced and verified with a Blastn search in the NCBI nucleotide database. The result of the sequence comparison clearly matched with Cocos nucifera, Jubaea chilensis, Elaeis guineensis, and Butia capitata and confirmed the botanical origin of the palm leaves.
3.3. Design and Specificity of Coconut-Specific Primers
Three different sets of primers, cocosPRK, cocosITS109, and cocosITS197, which should only specifically amplify Cocos nucifera, were designed.
In order to verify the specificity of the primer sets, a conventional PCR, followed by a gel electrophoretic analysis was performed using DNA templates of coconut palm (Cocos nucifera), Chilean wine palm (Jubaea chilensis), oil palm (Elaeis guineensis), and jelly palm (Butia capitata). The results are presented in Figure 1. While the primer set cocosPRK only showed PCR products in a length of 164 bp specific for Cocos nucifera, the primer sets cocosITS109 and cocosITS197 also amplified other species.
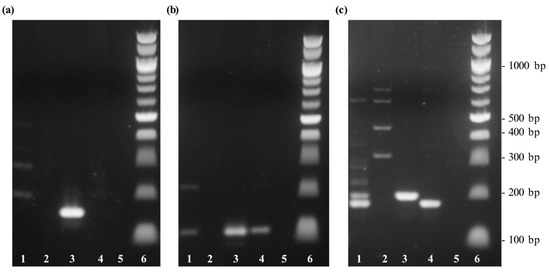
Figure 1.
Gel electrophoresis of DNA extracted from different palm species to test the specificity of different sets of primer. Lane 1: Chilean wine palm (Jubaea chilensis); lane 2: oil palm (Elaeis guineensis); lane 3: coconut palm (Cocos nucifera), lane 4: jelly palm (Butia capitata); lane 5: non-template sample with water; lane 6: 100 bp DNA ladder. (a) PCR amplification with the primer set cocosPRK. (b) PCR amplification using the primer set cocosITS109. (c) PCR amplification performed with the primer set cocosITS197.
The coconut-specific primer set of the prk gene was selected for follow-up experiments.
3.4. PCR Amplification of DNA from Coconut, Coconut Chips, and Coconut Blossom Sugar
For further verification of the cocosPRK primer set, a conventional PCR followed by gel electrophoresis was performed using DNA isolated from coconut, coconut chips, and coconut blossom sugar. As shown in Figure 2, positive results were obtained with the coconut and coconut chips samples. Negative PCR results were obtained using the DNA template isolated from coconut blossom sugar. As the concentration of DNA isolated from coconut blossom sugar determined by spectrophotometer was 210 ng/µL and the quotient of A260/A280 was 1.95, the negative amplification reaction was not the result of insufficient or poor DNA quality. Amplifiability of the DNA with the eukaryotic-specific primers euk18S confirmed that there were no PCR inhibitory contaminants. However, as coconut sap is a fast-fermenting product [22,23], there is the hypothesis that the isolated DNA was from yeasts and coconut-specific DNA was too low in concentration and quality for PCR amplification.
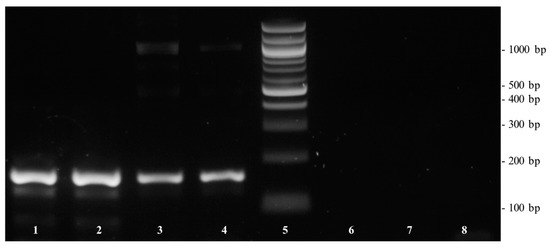
Figure 2.
Gel electrophoresis of DNA extracted from coconut, coconut chips, and coconut blossom sugar. Lane 1–2: coconut chips; lane 3–4: coconut; lane 5: 100 bp DNA ladder; lane 6–7: coconut blossom sugar; lane 8: non-template sample with water.
3.5. Development of a TaqMan™ Real-Time PCR Assay
For the development of a coconut-specific real-time PCR assay, a TaqMan™ probe was developed. TaqMan™ probes are hydrolysis probes which are oligonucleotides consisting of a reporter fluorophore attached to the 5’-end, a quencher at the 3’-end, and a 3′-extension block. They are located within the gene fragment delimited by the primer set. During amplification, the probe is detached from the DNA template and the fluorophore and quencher separate so that the fluorescence signal emitted by the fluorophore is no longer quenched [24,25].
The TaqMan™ probe was placed inside the gene fragment, which was enclosed by the cocosPRK primer set. As the gene fragment had many regions with homopolymer repeats and a very low GC-content, there were not many options to place the probe. A 30 bp long sequence (AAT TGT CTC ATT ATC TCA ATG AAC CGG GTG; GC-content: 40%; melting temperature: 68 °C) in close proximity to the 3’-end of the reverse primer was selected. Using the OligoAnalyzer web tool (Integrated DNA Technologies Inc., Coraville, IA, USA), the formation of hairpins, self-dimers, and hetero-dimers was checked. As fluorophore 6-carboxyfluorescein (6-FAM) with an absorption wavelength of 495 nm and an emission wavelength of 520 nm was used. It is a long-established and widely used fluorescent dye in life sciences [26,27]. The BlackHole™ Dark Quencher BHQ-1 from LGC Biosearch Technologies Inc. (London, UK) was used as quencher. It quenches emission at wavelengths of 480–580 nm and has the advantage that it does not release any additional fluorescence compared to the previously used tetramethylrhodamine (TAMRA) quencher [28,29]. Synthesis of the probe was purchased from TIB MOLBIOL Syntheselabor GmbH (Berlin, Germany).
3.6. Assay Specificity and Sensitivity
The specificity of the primer/probe system was tested by amplification of DNA in a concentration of 10 ng/µL from 28 different plant and animal species mentioned in Section 2.8.1. As shown in Figure 3, no amplification was detected with species other than Cocos nucifera. The result showed that the primer/probe system was specific for the identification of Cocos nucifera and showed no cross-reactivity.
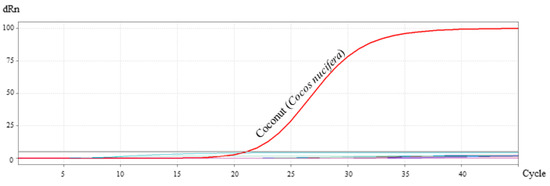
Figure 3.
Specificity test of the primer/probe system for coconut identification. No amplification was detected with species other than coconut.
For the sensitivity test of the real-time PCR assay, the DNA of Cocos nucifera was serially diluted 10-fold for reaching a concentration range from 100 ng/µL to 0.1 pg/µL. The reactions were performed in duplicates. The results presented in Figure 4a showed that samples with a DNA concentration of 0.1 pg/µL were not amplified. The lowest quantifiable level of Cocos nucifera and consequently, the limit of detection, was 1 pg/µL with a Ct value of 35. By plotting Ct values against the log10 input DNA concentration, a standard curve was constructed (Figure 4b). Ideally, a real-time PCR reaction should have an efficiency in a range of 90% to 110%, which corresponds to a slope between −3.58 and −3.10 [30]. With −3.49, it was in the optimal range in the present study.
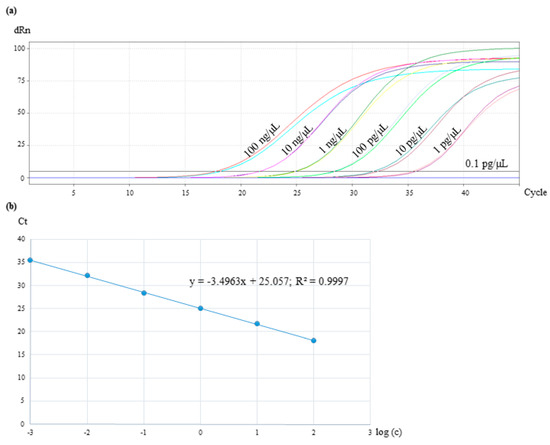
Figure 4.
Sensitivity test of the real-time PCR assay with 10-fold diluted DNA of coconut in a concentration range from 100 ng/µL to 0.1 pg/µL. The reactions were performed in duplicates. (a) The amplification plot shows Ct values for samples from 100 ng/µL to 1 pg/µL. (b) Standard curve of the coconut TagMan real-time PCR system by plotting Ct values against the log10 input DNA concentration.
4. Discussion
In the present study, three different sets of primers were designed and tested for their specificity towards Cocos nucifera. While the primer sets cocosITS107 and cocosITS197 amplify PCR products with very similar base length also for other palm species, cocosPRK amplifies a 164 bp long amplicon characteristic for Cocos nucifera. Matching this gene fragment, a TaqMan™ probe was developed. The primer/probe system showed no cross-reactivity to other plant and animal species and was therefore highly specific to Cocos nucifera. The developed real-time PCR assay was sensitive to detect DNA concentrations of 1 pg/µL and can be used to detect traces of coconut. Unfortunately, DNA isolated from coconut blossom sugar was not amplifiable with the coconut-specific primers cocosPRK. For this purpose, extraction methods other than the CTAB method should be developed and evaluated.
The developed real-time PCR assay is an alternative method to the existing ELISA test kits. The present results can contribute to detect coconut in food and to protect consumers from hidden traces and to prevent dangerous food allergic reactions. Instead of ELISA, PCR is an indirect method for the detection of food allergens. While ELISA is based on highly specific antigen–antibody reactions and detects allergenic proteins, real-time PCR detects and quantifies matrix-specific DNA sequences [31]. A detection of DNA of allergenic food does not reveal anything about the content of allergenic traces in the form of allergenic proteins. It does not consider the actual allergen content and the potential of an allergenic response. But for the comparison of the two methods, it must be taken into account that ELISA also detects only a limited number of target proteins, considering that 17 peanut allergens have been described, and while DNA often breaks down into small sequences of less than 200 bp, proteins are often denatured, which in turn can influence the allergenic response [32,33,34]. However, the mandatory need of animals for producing antibodies is one of the main disadvantages of ELISA, because the production procedure of antibodies is rather cruel for the animal and therefore getting more and more restricted in many countries, e.g., by the European legislation (directive 2010/63/EU) [35].
Regardless of the actual allergenic content, many studies showed that ELISA and real-time PCR assays achieve comparable results and that real-time PCR is therefore an important tool for the rapid detection of allergenic food traces in the food industry [12,36].
Author Contributions
Conceptualization, J.W.; methodology, J.W. and O.K.; investigation, J.W.; resources, J.K.; writing—original draft preparation, J.W.; writing—review and editing, S.R.; visualization, J.W.; supervision, J.K.; project administration, J.W.; funding acquisition, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
The project is supported by funds of the Federal Ministry of Food and Agriculture (BMEL) based on a decision of the Parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (BLE) under the innovation support program (Grant. No. FKZ 281601914).
Acknowledgments
We thank Hilke Steinecke from Palmengarten Frankfurt/Main, Germany and Eberhard Kirchner from Elevate Social Businesses Ltd., New York City, USA for providing palm leaves. Coconut blossom sugar samples were provided by Voicevale Ltd., London, UK. Furthermore, we are grateful to Anna Cankurt, Petra Knies-Platte, Carina Niemann, and Uma Reddy for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Lieberei, R.; Reisdorff, C. Nutzpflanzen; Georg Thieme Verlag: New York, NY, USA, 2012. [Google Scholar]
- FAOSTAT. Crops Statistics-Production Quantities of Coconuts by Country 2017. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 20 May 2019).
- Anagnostou, K. Coconut allergy revisited. Children 2017, 4, 85. [Google Scholar] [CrossRef] [PubMed]
- Benito, C.; González-Mancebo, E.; de Durana, M.D.A.D.; Tolón, R.M.; Fernández-Rivas, M. Identification of a 7S globulin as a novel coconut allergen. Ann. Allergy Asthma Immunol. 2007, 98, 580–584. [Google Scholar] [CrossRef]
- Carr, H.; Plumb, G.; Parker, M.; Lambert, N. Characterisation and crystallisation of an 11S seed storage globulin from coconut (Cocos nucifera). Food Chem. 1990, 38, 11–20. [Google Scholar] [CrossRef]
- Garcia, R.N.; Arocena, R.V.; Laurena, A.C.; Tecson-Mendoza, E.M. 11S and 7S globulins of coconut (Cocos nucifera L.): Purification and characterization. J. Agric. Food. Chem. 2005, 53, 1734–1739. [Google Scholar] [CrossRef]
- EU. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004; European Union: Brussels, Belgium, 2011. [Google Scholar]
- FDA. A Food Labeling Guide; Center for Food Safety and Applied Nutrition-Office of Nutrition, Labeling, and Dietary Supplements; FDA: College Park, MD, USA, 2013.
- Galan, A.M.G.; Brohée, M.; de Andrade Silva, E.; van Hengel, A.J.; Chassaigne, H. Development of a real-time PCR method for the simultaneous detection of soya and lupin mitochondrial DNA as markers for the presence of allergens in processed food. Food Chem. 2011, 127, 834–841. [Google Scholar] [CrossRef]
- Demmel, A.; Hupfer, C.; Ilg Hampe, E.; Busch, U.; Engel, K.-H. Development of a real-time PCR for the detection of lupine DNA (Lupinus species) in foods. J. Agric. Food Chem. 2008, 56, 4328–4332. [Google Scholar] [CrossRef]
- Houhoula, D.; Koussissis, S.; Lougovois, V.; Tsaknis, J.; Kassavita, D.; Papatheodorou, S.; Akkermans, S.; Van Impe, J.; Tsakali, E. Detection of peanut allergen traces with a Real Time PCR assay-the challenge to protect food-allergic consumers. J. Food Res. 2018, 7, 32–37. [Google Scholar] [CrossRef][Green Version]
- Houhoula, D.; Papatheodorou, S.A.; Moschou, D.; Pappa, S.; Tsaatazoglou, N.; Koussissis, S.; Tsaknis, J.; Lougovois, V.; Impe, J.; Tsakali, E. Evaluation of a Real Time PCR Assay and a ELISA Method for the Detection of Walnuts and Almonds Allergen Traces in Food Products. J. Food Res. 2019, 8, 71–76. [Google Scholar] [CrossRef][Green Version]
- BVL. Amtliche Sammlung von Untersuchungsverfahren nach § 64 LFGB, Vefahren zum Nachweis von gentechnisch modifizierten Organismen und ihren Produkten in Lebensmitteln L 00.00-119:2014-02; Bundesamt für Verbraucherschutz und Lebensmittelsicherheit: Braunschweig, Germany, 2014.
- Miziorko, H.M. Phosphoribulokinase: Current perspectives on the structure/function basis for regulation and catalysis. Adv. Enzymol. Relat. Areas Mol. Biol. 2000, 74, 95–127. [Google Scholar]
- Baker, W.J.; Norup, M.V.; Clarkson, J.J.; Couvreur, T.L.; Dowe, J.L.; Lewis, C.E.; Pintaud, J.-C.; Savolainen, V.; Wilmot, T.; Chase, M.W. Phylogenetic relationships among arecoid palms (Arecaceae: Arecoideae). Ann. Bot. 2011, 108, 1417–1432. [Google Scholar] [CrossRef]
- Gunn, B.F. The phylogeny of the Cocoeae (Arecaceae) with emphasis on Cocos nucifera. Ann. Mo. Bot. Gard. 2004, 91, 505–522. [Google Scholar]
- Porter, C.H.; Collins, F.H. Species-diagnostic differences in a ribosomal DNA internal transcribed spacer from the sibling species Anopheles freeborni and Anopheles hermsi (Diptera: Culicidae). Am. J. Trop. Med. Hyg. 1991, 45, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.; Müller, T.; Achtziger, M.; Seibel, P.N.; Dandekar, T.; Wolf, M. The internal transcribed spacer 2 database—A web server for (not only) low level phylogenetic analyses. Nucleic Acids Res. 2006, 34, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Song, J.; Liu, C.; Luo, K.; Han, J.; Li, Y.; Pang, X.; Xu, H.; Zhu, Y.; Xiao, P. Use of ITS2 region as the universal DNA barcode for plants and animals. PLoS ONE 2010, 5, e13102. [Google Scholar] [CrossRef] [PubMed]
- Zeltner, D.; Glomb, M.A.; Maede, D. Real-time PCR systems for the detection of the gluten-containing cereals wheat, spelt, kamut, rye, barley and oat. Eur. Food Res. Technol. 2009, 228, 321–330. [Google Scholar] [CrossRef]
- Allmann, M.; Candrian, U.; Höfelein, C.; Lüthy, J. Polymerase chain reaction (PCR): A possible alternative to immunochemical methods assuring safety and quality of food Detection of wheat contamination in non-wheat food products. Unters. Forsch. 1993, 196, 248–251. [Google Scholar] [CrossRef]
- Atputharajah, J.; Widanapathirana, S.; Samarajeewa, U. Microbiology and biochemistry of natural fermentation of coconut palm sap. Food Microbiol. 1986, 3, 273–280. [Google Scholar] [CrossRef]
- Hebbar, K.; Arivalagan, M.; Manikantan, M.; Mathew, A.; Thamban, C.; Thomas, G.V.; Chowdappa, P. Coconut inflorescence sap and its value addition as sugar–collection techniques, yield, properties and market perspective. Curr. Sci. 2015, 1411–1417. [Google Scholar] [CrossRef]
- Lee, L.G.; Connell, C.R.; Bloch, W. Allelic discrimination by nick-translation PCR with fluorgenic probes. Nucleic Acids Res. 1993, 21, 3761–3766. [Google Scholar] [CrossRef]
- Holland, P.M.; Abramson, R.D.; Watson, R.; Gelfand, D.H. Detection of specific polymerase chain reaction product by utilizing the 5′----3′exonuclease activity of Thermus aquaticus DNA polymerase. Proc. Natl. Acad. Sci. USA 1991, 88, 7276–7280. [Google Scholar] [CrossRef]
- Livak, K.J.; Flood, S.; Marmaro, J.; Giusti, W.; Deetz, K. Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. Genome Res. 1995, 4, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Ruan, C.; Fuller, C.W.; Glazer, A.N.; Mathies, R.A. Fluorescence energy transfer dye-labeled primers for DNA sequencing and analysis. Proc. Natl. Acad. Sci. USA 1995, 92, 4347–4351. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.K.; Cook, R.M. Intramolecular dimers: A new design strategy for fluorescence-quenched probes. Chem. Eur. J. 2003, 9, 3466–3471. [Google Scholar] [CrossRef]
- Biosearchtech. Black Hole Quencher® Dyes. Available online: https://www.biosearchtech.com/support/education/fluorophores-and-quenchers/black-hole-quencher-dyes (accessed on 16 May 2019).
- García, A.; Madrid, R.; García, T.; Martín, R.; González, I. Detection of food allergens by Taqman real-time PCR methodology. In Food Allergens: Methods and Protocols; Lin, J., Alcocer, M., Eds.; Springer: New York, NY, USA, 2017; pp. 95–108. [Google Scholar] [CrossRef]
- Busch, U. Molekularbiologische Methoden in der Lebensmittelanalytik: Grundlegende Methoden und Anwendungen; Springer: Berlin, Germany, 2010. [Google Scholar]
- WHO/IUIS. Allergen Nomenclature. World Health Organization and International Union of Immunological Societies. Available online: http://www.allergen.org/ (accessed on 17 May 2019).
- Magi, M.; Garcia, L.; Vandenbranden, M.; Palmantier, R.; Jacquet, A. Heat denaturation affects the ProDer p 1 IgE reactivity and downregulates the development of the specific allergic response. J. Allergy Clin. Immunol. 2004, 114, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Johansen, P.; Senti, G.; Martínez Gómez, J.M.; Wüthrich, B.; Bot, A.; Kündig, T.M. Heat denaturation, a simple method to improve the immunotherapeutic potential of allergens. Eur. J. Immunol. 2005, 35, 3591–3598. [Google Scholar] [CrossRef] [PubMed]
- EU. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; European Union: Brussels, Belgium, 2010 22 September. [Google Scholar]
- Stephan, O.; Vieths, S. Development of a real-time PCR and a sandwich ELISA for detection of potentially allergenic trace amounts of peanut (Arachis hypogaea) in processed foods. J. Agric. Food Chem. 2004, 52, 3754–3760. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).