Abstract
Shiga toxin-producing Escherichia coli O157:H7 is a food-borne pathogen and the major cause of hemorrhagic colitis. Pseudomonas is the genus most frequent psychrotrophic spoilage microorganisms present in milk. Two-species bacterial systems with E. coli O157:H7, non-pathogenic E. coli, and P. fluorescens in skimmed milk at 7, 13, 19, or 25 °C were studied. Bacterial interactions were modelled after applying a Bayesian approach. No direct correlation between P. fluorescens’s growth rate and its effect on the maximum population densities of E. coli species was found. The results show the complexity of the interactions between two species in a food model. The use of natural microbiota members to control foodborne pathogens could be useful to improve food safety during the processing and storage of refrigerated foods.
1. Introduction
Shiga toxin-producing Escherichia coli O157:H7 strains are foodborne pathogens causing hemorrhagic colitis or the hemolytic uremic syndrome [1]. These microorganisms can be transmitted through consumption of undercooked meat, vegetables, contaminated water, unpasteurized dairy products, and raw milk [2,3,4,5]. The survival capacity of E. coli O157:H7 can go as far as several days or weeks in milk and dairy products [6,7,8] showing the importance of post processing contamination and the associated health risks.
Soil, water and vegetation are the main sources of psychrotrophic spoilage microorganisms, such as Pseudomonas, to milk [9,10,11]. During the pre-processing period (3–4 days) prior to pasteurization, psychrotrophic bacteria can grow and cause significant chemical changes [10,12]. Pseudomonas also appears in pasteurized dairy products as a post-processing contaminant [9,13].
Different bacterial species interact without physical barriers in many natural environments, and foods are one such example where co-culture experiments have shown the prevailing genotypes in mixed cultures [14,15,16,17]. Spoilage microorganisms are able to enhance, limit or be neutral on the growth of pathogenic species [18]. P. fluorescens produces extracellular materials resulting in a competitive advantage over other species [17] such that the competitor is physically displaced [19] or the nutrients access impeded [20]. In early research on the topic [21,22], enhancement of Staphylococcus aureus’s growth in the presence of Pseudomonas spp was reported. Marshall and Schmidt [22,23] and Farrag and Marth [12] found similar results when Listeria monocytogenes was co-cultured in the presence of P. fluorescens. Other studies reported conversely that P. fluorescens can inhibit the growth of L. monocytogenes [18,24,25,26,27,28,29,30]. The specific addition of glucose to TSB broth stimulated the inhibition of E. coli O157:H7 by P. fluorescens [31]. Liao [32] and Liao et al. [33] reported that P. fluorescens and Bacillus spp. were able to act as biocontrol agents of Salmonella Saintpaul on Jalapeno pepper or of E. coli O157:H7 on TSA agar and bell pepper disks. On the surface of spinach leaves, Olaya et al. [34] reported that P. fluorescens moderately suppressed the growth of E. coli O157:H7. The same research group also showed P. fluorescens inhibition of E. coli O157:H7 in poor environments such as distilled water or buffered peptone water [35].
The aim of our work is to study the interaction between co-culturing bacterial species using Bayesian inference. The Bayesian approach provides a consistent framework for estimating parameters from a model using prior knowledge about the system to improve the estimations [36], with the advantage of including uncertainties within the model [37]. Two-species systems were co-cultured with E. coli O157:H7 or non-pathogenic E. coli and P. fluorescens in skimmed milk over a range of temperatures and times that are both typical and atypical for milk storage and distribution.
2. Materials and Methods
2.1. Bacterial Cultures and Inoculation
Three strains of Escherichia coli: O157:H7 LCDC 86-51 (EcO1; Shiga toxin-producing strain isolated from hemorrhagic colitis, Ottawa, ON, Canada), O157:H7 ATCC 35150 (EcO2; Shiga toxin-producing strain isolated from hemorrhagic colitis, Oregon and Michigan, USA), and the non-pathogenic strain E. coli ATCC 8739 (Ec) were used. All strains were cultured overnight in BHI broth (Difco, BD Diagnostics, Franklin Lakes, NJ, USA) at 37 °C. Pseudomonas fluorescens ATCC 13525 (Pf; isolated from pre-filter tanks and town water works, Reading, UK) was cultured in BHI at 25 °C for 24 h.
Bacterial cultures were grown at 37 °C until a population of 109 CFU/mL was reached as previously described [38]. Briefly, serial dilutions were prepared in 0.1% sterile peptone water (Difco) and 1 mL aliquots from adequate dilutions were added to 250 mL blue capped bottles containing 100 mL of 10% reconstituted sterile skimmed milk until populations of ca. 104 CFU/mL were achieved. These populations were evaluated by spreading onto TSA (Difco, BD Diagnostics, Franklin Lakes, NJ, USA) plates and incubating at 37 °C for 48 h.
2.2. Co-Cultures and Enumeration
A first 4-level factor (EcO1, EcO2, Ec, and Pf strains) and a second 4-level factor (7, 13, 19 or 25 °C) were used for a 4 × 4 full factorial experiment. Starting concentrations of ca. 104 CFU/mL were selected and co-cultures of EcO1, EcO2, Ec, and Pf were prepared and stored at 7, 13, 19 or 25 °C. Actual co-culture starting populations of 3.9–4.1, 4.0–4.1, 4.0–4.1, and 3.9–4.1 log CFU/mL were obtained for EcO1, EcO2, Ec, and Pf, respectively. Single cultures of EcO1, EcO2, Ec, and Pf were cultivated with the same initial populations. Control cultures of un-inoculated skimmed milk were prepared and stored under the same conditions. Assays were carried out in triplicate.
Cultures were at 0, 2, 4, 6, 12, or 24 h, and 2, 4, 8, 12, 16, 20, 28 days. Aliquots (0.1 mL) were surface-plated onto MacConkey Sorbitol Agar (Difco, BD Diagnostics, Franklin Lakes, NJ, USA) and Fluorocult VRB-Agar (Merk, Darmstadt, Germany) or onto Pseudomonas Agar F or Flo Agar (Difco, BD Diagnostics, Franklin Lakes, NJ, USA). For Escherichia spp. counting, plates were incubated at 37 °C for 18–24 h, and random colonies were serologically confirmed using the E. coli O157 Latex Test Kit (Oxoid, Thermo Fisher Scientific, Basingstoke, UK). P. fluorescens colonies were counted after an incubation at 35 °C for 24–48 h.
2.3. Bayesian Modeling of Microbial Interactions
Plate counts of E. coli and P. fluorescens were transformed to decimal logarithmic values. The lag time (λ), the maximum population density (Nmax), and the time to reach (ttr) populations of 6 or 8 log CFU/mL were estimated for each culture using the DMFit, ComBase [39]. Then, a modified generic primary growth model [40] was selected:
where (dNt/dt)/Nt is the relative or instantaneous growth rate of the microorganism, Nt is the cell concentration in a bacterial culture at time t, and µmax is the maximum growth rate. The term αt is an adjustment function, and ft is a logistic inhibition function for two-species mixed cultures [41]:
where λ is the lag time, Nat and Nbt are the cell concentration of the microorganisms a or b in co-culture at time t, and Nmax is the total carrying capacity (both species). For EcO1 cultures the model can be re-defined:
where µEcO1 (3a) and µEcO1(Pf) (3b) are the maximal growth rates of EcO1 cultured alone or in the presence of P. fluorescens, respectively. Similar approaches to the equations (3a–b) were done for the cultures of EcO2 (µEcO2, µEcO2(Pf)), Ec (µEc, µEc(Pf)), and P. fluorescens (µPf, µPf(EcO1), µPf(EcO2), and µPf(Ec)). When the cultures reached their maximal values, a decline period was observed. The decline phase was modeled alone with a modification of equations (3a–b), i.e., µ was replaced by the negative-sign parameter k in order to reflect the negative slope of that survival growth section.
The approach above assumes deterministic behavior, but an error term may be introduced to reflect the influence of factors outside the experimental design. Thus, the observed concentration of bacteria at time t may modelled as Nt* = Nt + εt, where Nt is the population of EcO1, EcO2, Ec, or Pf cultured alone or in co-culture, and εt is a normally distributed error term with zero mean and constant variance equal to σt: Nt* ~ Normal (Nt, σt).
A Bayesian estimation of the parameters for computing the posterior distribution of parameters of the model was carried out. The estimated parameters are shown in Figure 1 as circles: The growth rates of the microorganisms cultured alone (µEcO1, µEcO2, µEc, and µPf), the 2-species mixtures (µEcO1(Pf), µEcO2(Pf), µEc(Pf), µPf(EcO1), µPf(EcO2), and µPf(Ec)), and the standard deviation of errors (σt); the other terms are constants and are shown as squares. Decline rates and the 95% credible intervals were also estimated based on the posterior distribution of parameters from equations 3a–b. The estimation of parameters, by means of a Bayesian methodology, is undertaken by simulating the posterior distribution of the model parameters, which includes the likelihood of the experimental data (assuming lognormality) and the prior distributions of the parameters. A general introduction to this methodology in differential equations for biological systems can be seen in [42].
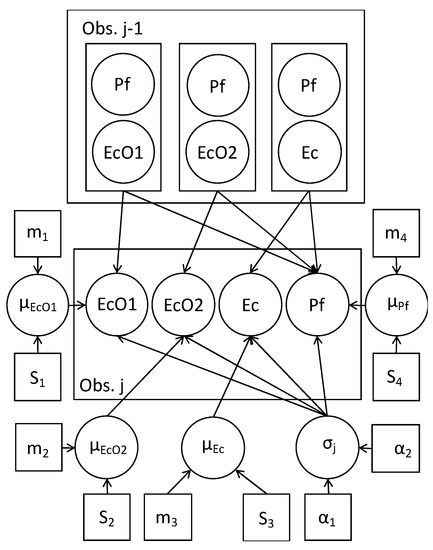
Figure 1.
Bayesian model directed acyclic graph (DAG). Circles: random variables. Squares: constants (initial parameters of the distributions of the variables). Arrows: conditional dependence. Obs. j-1: observed data of E. coli spp. (EcO1, EcO2, and Ec), and P. fluorescens (Pf). µ: microorganisms’ growth rates with Normal distribution (mean m and standard deviation S). σj: standard deviation of errors with a Gamma distribution (parameters α).
The Runge-Kutta method was used to discretize the system of differential equations [43]; then the system was included in a probabilistic model and the Hamiltonian Monte Carlo method (HMCM) was used for parameters estimation [44] generating samples from the posterior distributions of parameters μt and σt [45]. In each iteration of the HMCM sequence it is calculated a discretized version of equations (3a) and (3b) by means of the Runge-Kutta procedure for determining the likelihood of the experimental data. After the convolution with the prior distributions of parameters it is obtained a sequence of values of the posterior distribution. Then, the means and intervals based on these draws are obtained and shown as the final estimates of the parameters. R [46] via Rstan [47] was used for algorithmic programming. Codes are available from author JMM.
3. Results
3.1. Bayesian Modelling of Microbial Interactions
Bayesian inference examples of growth and decline periods for E. coli O157:H7 LCDC 86-51 (EcO1), E. coli O157:H7 ATCC 35,150 (EcO2), and non-pathogenic E. coli (Ec) co-cultured with P. fluorescens (Pf) at 7 or 25 °C in skimmed milk are shown in Figure 2, Figures S1 and S2, and in Figure 3, Figures S3 and S4, respectively. As a comparison, growth and decline periods from EcO1 cultured alone are also shown (Figure 2; Figure 3). Figure 4 shows the µ estimates from E. coli spp. co-cultured with P. fluorescens or single-cultured at 7, 13, 19, or 25 °C. The highest µ values were detected at 19 and 25 °C. It is interesting to observe the differences on the growth rates between E. coli spp. and P. fluorescens cultured alone: a psychrotrophic bacteria such as P. fluorescens did not show the lowest growth rates at any temperature except for 25 °C; however, E. coli spp. showed it at 7 and 13 °C. Co-cultured P. fluorescens showed similar growth rates as when cultured alone. The effect of P. fluorescens on the growth rate of E. coli strains appears to be greater at low temperatures (7 and 13 °C) increasing E. coli spp. µ values. At higher temperatures (19 and 25 °C) P. fluorescens does not seems to cause the same effect. Table S1 shows posterior means of the parameters and the limits of the credible intervals of growth rates (µEcO1, µEcO2, µEc, or µPf) and decline rates (kEcO1, kEcO2, kEc, or kPf) of E. coli spp. and P. fluorescens cultured alone or in co-cultures. The E. coli spp. co-cultures show the lowest µ values at 7 and 13 °C ranging from 0.563 day−1 for the EcO1(Pf) co-cultures at 7 °C to 0.945 day−1 for the Ec(Pf) co-cultures at 13 °C. At 19 and 25 °C the µ values were similar ranging from 1.358 day−1 for the EcO1(Pf) co-cultures to 2.178 day−1 for the Ec(Pf) co-cultures at 25 °C.
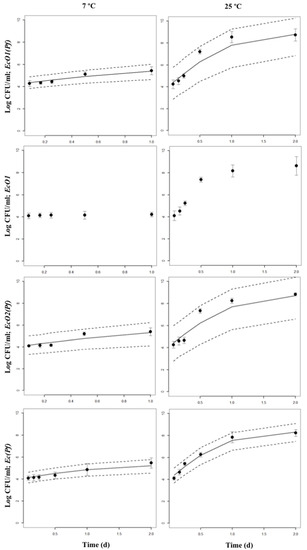
Figure 2.
Bayesian inference of growth periods of co-cultures of E. coli spp. with P. fluorescens at 7 or 25 °C in skimmed milk. The 95% Highest Posterior Density intervals (2.5 and 97.5%) are shown. Points are the original data: mean and standard deviation are shown. Growth periods from EcO1 cultured alone are also shown.
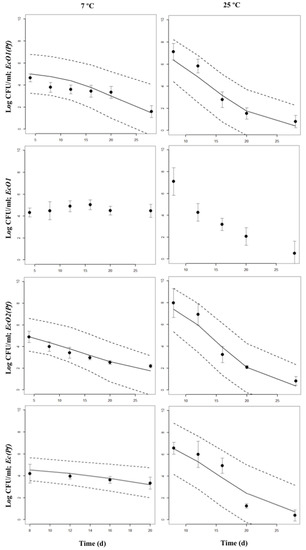
Figure 3.
Bayesian inference of decline periods of co-cultures of E. coli spp. with P. fluorescens at 7 or 25 °C in skimmed milk. The 95% Highest Posterior Density intervals (2.5 and 97.5%) are shown. Points are the original data; mean and standard deviation are shown. Decline periods from EcO1 cultured alone are also shown.
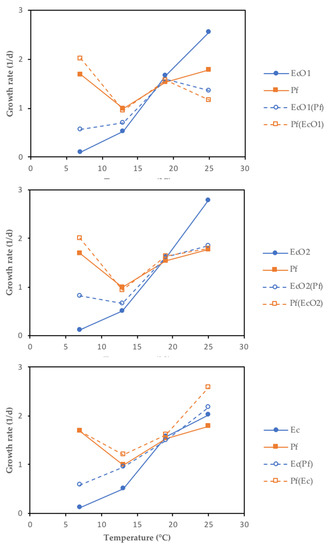
Figure 4.
Scatter plots of the growth rates (µ mean) from E. coli spp. co-cultured with P. fluorescens (EcO1(Pf), EcO2(Pf), and Ec(Pf)) or single-cultured (EcO1, EcO2, and Ec) at 7, 13, 19 or 25 °C. Growth rates from P. fluorescens co-cultured (Pf(EcO1), Pf(EcO2), and Pf(Ec)) or single-cultured (Pf) are also shown.
At 7 °C, decreasing populations of the three E. coli strains cultured alone were not detected (Table S1). Decreasing populations (k values) were not found for EcO2 and Ec strains single-cultured, and for EcO1(Pf), EcO2(Pf) and Ec(Pf) co-cultures at 13 °C. The positive k values are included within the Highest Posterior Density (HPD) intervals between a negative 2.5% interval value and a positive 97.5% interval value. The zero value is in the interval meaning that the estimated k values are not significantly different from zero, i.e., there is not growth nor decline with a 97.5% of confidence, and the populations are stable. The fastest k decline rates were observed in the EcO1(Pf) and Ec(Pf) co-cultures at 19 °C (–1.804 day−1 and –1.709 day−1, respectively). The higher k decline rates from single-cultured strains were found in EcO2 cultures at 19 °C (–0.233 day−1) and 25 °C (–0.247 day−1).
The standard deviations from the survival curves, i.e., with growth and decline periods included, are shown in Figure 5 and Table S2. Table S2 shows posterior means of the parameters and the limits of the credible intervals (2.5% and 97.5%) of the standard deviations for growth rates (σEcO1, σEcO2, σEc, or σPf) and decline rates (−σEcO1, −σEcO2, −σEc, or −σPf) of E. coli spp. and P. fluorescens cultured alone or in co-cultures. Standard deviation is read as the predicted concentrations’ random error of the microorganisms’ real observations.
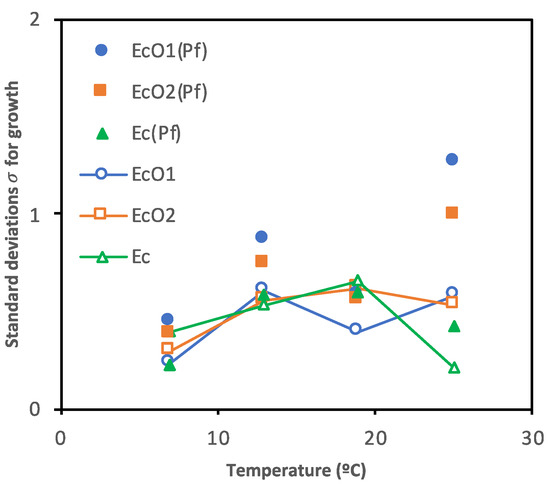
Figure 5.
Scatter plot of the standard deviations for growth (σ) from E. coli spp. co-cultured with P. fluorescens (EcO1(Pf), EcO2(Pf), and Ec(Pf)) or single-cultured (EcO1, EcO2, and Ec) at 7, 13, 19 or 25 °C.
3.2. Estimation of the Nmax and the ttr
The maximum population density (Nmax) of E. coli spp. and P. fluorescens in single cultures or co-cultured in milk are shown in Table S3. The lowest E. coli spp. Nmax values were observed at 7 °C (4.4–4.8 log CFU/mL in single cultures and 5.2–5.4 log CFU/mL in co-cultures); at 13, 19, or 25 °C, the Nmax values were similar for all co-cultures (8.0–8.5 log CFU/mL in single cultures and 7.9–8.5 log CFU/mL in co-cultures). The P. fluorescens Nmax values were similar for all co-cultures at all temperatures (8-9 log CFU/mL).
The time to reach (ttr) a population of 6 or 8 log CFU/mL is shown in Figure 6 and Table S3. These populations were found just before the carrying capacities were reached, and both fall within the linear period of the exponential growth where rates show a Log-Normal distribution regardless of the environmental conditions and the initial population of microorganisms [48,49,50]. At 7 °C E. coli spp. did not reach 6 log CFU/mL whether single-cultured or co-cultured. At 13 °C all E. coli spp. cultures reached 6 log CFU/mL at 1.60–2.24 day; similar results were found at 19 or 25 °C with lower ttr 6 log values showing a faster growth: 0.56–0.64 day or 0.32–0.40 day, respectively. All P. fluorescens cultures reached ca. 6 log CFU/mL after 0.56–0.72 day at 7 °C; the ttr 6 log results at 13 °C were slightly higher (0.64–1.12 day), decreasing at 19 and 25 °C and showing faster growth: 0.56–0.64 day and 0.40–0.48 day, respectively. The ttr 6 log values of P. fluorescens were lower than those from E. coli strains at all temperatures, indicating faster growth. All E. coli spp. and P. fluorescens cultures were able to reach a population of 8 log CFU/mL, except E. coli spp. at 7 °C. At 13 °C all E. coli spp. cultures reached 8 log CFU/mL at 3.36–3.68 d; lower results were found at 19 or 25 °C indicating a faster growth: 1.04–1.12 day or 0.56–0.96 day, respectively. All P. fluorescens cultures reached at 8 log CFU/mL after 0.96–1.04 d at 7 °C; the ttr 8 log results at 13, 19, or 25 °C were lower showing a faster growth: 1.44–1.76 day, 0.96–1.04 day, or 0.56–0.88 day, respectively. The P. fluorescens’s ttr 8 log values were lower than those from E. coli strains at all temperatures, indicating overall faster growth.
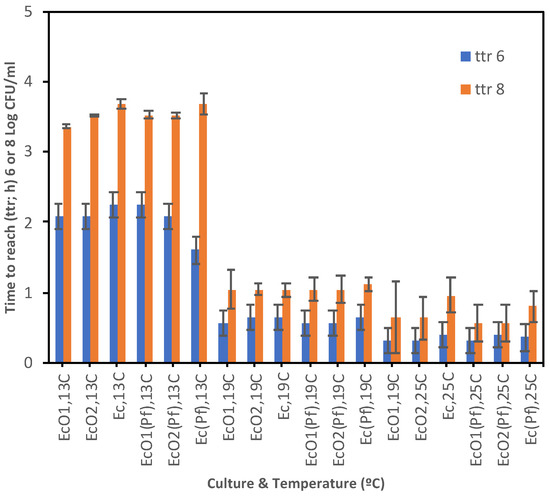
Figure 6.
Time to reach (ttr; d) a population density of 6 or 8 log CFU/mL from E. coli spp. co-cultured with P. fluorescens (EcO1(Pf), EcO2(Pf), and Ec(Pf)) or single-cultured (EcO1, EcO2, and Ec) at 7, 13, 19 or 25 °C. Standard Errors are shown.
4. Discussion
The presence of E. coli O157:H7 strains in refrigerated food such as milk depicts a health risk for the consumers. The native microbiota or the presence of protective cultures could compete with the pathogens and help in controlling E. coli O157:H7 strains during the processing and storage of refrigerated food [31,51]. Pseudomonas spp. could be important competitors in perishable refrigerated food products due to their psychrotrophic profile (able to grow at 0–15 °C) [31,52].
Antagonistic microorganisms (e.g., Pseudomonas spp.), may be useful in the control of E. coli O157:H7 growth. According to Samelis and Sofos [31], E. coli O157:H7 co-cultured with Pseudomonas sp. grew faster as the temperature increased from 10 to 15 or to 25 °C in TSB broth. These authors found that the pathogen inhibition was enhanced in co-cultures grown at 10 to 15 °C with 1% of added glucose. At 25 °C the inhibition was enhanced even without added glucose. Previously, Janisiewicz et al. [53] reported that P. syringae inoculated into apple injuries inhibited the growth of E. coli O157:H7. Similar results were found when a high level of ground beef native flora inhibited the growth of E. coli O157:H7 at 10–12 °C [54,55]. Samelis and Sofos [31] found that the maximum population density of E. coli O157:H7 was suppressed in co-culture with Pseudomonas at 10, 15, and 25 °C. These results are in agreement with the Jameson Effect [55]; indeed, the inhibition of a population not in its stationary phase by another population in it is observed [30,55], i.e., the competition in food mixed populations is restricted to the limitation of the maximum population, with no effect on the growth rate. Our work supports the Jameson-effect hypothesis as the growth rates of E. coli species seems not to be affected by P. fluorescens. Similar results were found by Buchanan and Bagi [18] when P. fluorescens suppressed Listeria monocytogenes growth by inhibiting its maximum population density at low incubation temperatures (4 °C); the inhibition was less evident at higher temperatures (12 and 19 °C). McKellar [56] also reported that a raw milk isolate of P. fluorescens suppressed the growth of E. coli O157:H7 in nutrient broth at 22 °C only when P. fluorescens had reached its maximum population. Similar results were found in a previous study in co-cultures of Listeria spp. with P. fluorescens [38] but without differences between low and high temperatures, as Buchanan and Bagi [18] did. Samelis and Sofos [31] reported that E. coli O157:H7 co-cultured with Pseudomonas reached a population of ca. 6 log CFU/mL after ~2.6 d at 10 °C, not achieving a population of 8 log CFU/mL along the study (14 day); the same co-culture reached a maximum of 7 log CFU/mL (~6.1 day) when the TSB broth was supplemented with 1% of glucose. In our study E. coli spp. did not reach 6 or 8 log CFU/mL at 7 °C. Samelis and Sofos [31] found that E. coli O157:H7 co-cultured at 15 °C with Pseudomonas reached populations of 6 or 8 log CFU/mL after ~0.5 or 2 day, respectively and when the co-cultures were supplemented with 1% of glucose E. coli O157:H7 achieved populations of 6 or 8 log CFU/mL after 1.2 or 7 day, respectively. We found slightly slower growth in our study at 13 °C with a ttr 6 log of 1.6–2.2 day, and a ttr 8 log of 3.5–3.7 day. These authors [31] did not detect changes in pH along the incubation period (14 day) irrespective of the temperature and the type of culture: pH values of 7.3–7.4; in contrast, pH reductions were pronounced when 1% of glucose was added to the medium decreasing to values of 5.0–6.0. The pH values in single cultures or in co-cultures in our study decreased along the study from 6.7–6.8 to about 6.5 after 28 day at 7 or 13 °C (data not shown). At 19 or 25 °C the pH decreased until values of about 4.0–4.5 (data not shown) at the end of the study probably due to E. coli use of the lactose from the milk.
Lebert et al. [57] found that the growth of L. monocytogenes and L. innocua were not affected by Pseudomonas spp. at 6 °C on decontaminated meat but Pseudomonas spp. did affect L. innocua on native-microbiota contaminated meat – when Pseudomonas achieved their stationary phase Listeria was able to grow. These results are in contrast with previous [38,58,59] and current results which found that P. fluorescens exerts similar inhibitory effects on the E. coli strains studied. Besse et al. [58] noted interactions at the end of the exponential phase—when a strain reached its carrying capacity the growth of both strains stopped. McKellar [56] reported that nutrient limitation was the cause of the competition between Pseudomonas and E. coli O157:H7. But quorum sensing stimuli has also been suggested as a mechanism [16,60,61,62,63]. Once a faster growing microorganism reaches its maximum population, the production of signaling molecules also reaches its maximum, indicating to other species of the mixed culture that the carrying capacity of the culture has been achieved. Chu et al. [60] also showed how E. coli indole production inhibited P. aeruginosa factors important for competition.
As P. fluorescens constitutes a major component of native bacteria associated with fresh and minimally processed produce, Liao [32] studied the control of foodborne pathogens by P. fluorescens AG3A and Bacillus YD1 both isolated from fresh peeled baby carrots. Both strains reduced the growth of L. monocytogenes, Yersinia enterocolitica, Salmonella enterica, and E. coli O157:H7 at 20 °C but not at 10 °C. Olanya et al. [34] reported a moderate inhibition of E. coli O157:H7 by P. fluorescens on spinach leaf surfaces. These strains showed similar behaviors when they were co-cultured with nutrient restrictions at 10–35 °C for 48 h [35]; these authors found an E. coli O157:H7 ttr 6 log of 1.5 d at 20 °C, without reaching a population of 8 log CFU/mL; at 35 °C the ttr 6 log was about 1.1 d, and the ttr 8 log 1.6 day.
In our experiments P. fluorescens grew faster than E. coli spp. at 7 and 13 °C cultured alone as well as in co-culture, with higher µPf values and lower ttr 6 or 8 log; however, P. fluorescens did not affect the µEcO1, µEcO2, and µEc values. Similar behavior was observed when L. monocytogenes were co-cultured with Lactobacillus sakei [64] or P. fluorescens [38] together with higher Nmax of both competitors; in contrast the current study did not find higher Nmax in P. fluorescens single cultured or co-cultured with E. coli spp. At 19 and 25 °C the ttr 6 or 8 log of P. fluorescens were lower than those of E. coli spp. and the µPf values were similar between single cultures and co-cultures showing also similar Nmax: slight maximum population increases (<1 log CFU/mL) of P. fluorescens at 25 °C were observed. These results are not consistent with the Jameson Effect [30,55,65] with regard to the inhibition of one species by another that has reached the stationary phase. There is no correlation between the µPf and its effect on the maximal population densities of E. coli spp. (Pearson’s coefficient correlation of –0.407). The values of E. coli spp. Nmax were high at all temperatures except at 7 °C (Nmax of about 5.2–5.4 log CFU/mL), so the increasing µPf values did not increased E. coli spp. Nmax together with the increase of the temperatures. It would be possible to consider the fermentation of milk lactose by the E. coli spp. as a “high risk, high reward” strategy in the two-species communities studied [66,67]. E. coli spp. must engage additional competitive mechanisms to remain viable such as lactose fermentation [68] although it was far from the aim of this work to explore it. These interactions could also be related to physical location or resource usage overlapping between both populations [67]. Another possible explanation for the absence of the Jameson effect at the higher temperatures studied could be a “counterattack strategy”. Some authors have reported that P. aeruginosa suffering the attack from Vibrio cholerae or Acinetobacter baylyi’s type VI secretion system (T6SS) respond striking back with its own T6SS [69]. The T6SS is a multiprotein contractile-weapon complex that participates in interbacterial competition delivering toxins into both prokaryotic and eukaryotic cells. The T6SS complex does occur in Escherichia coli and Salmonella [70] including enterohemorrhagic E. coli O157:H7 [71]. Decoin et al. [72] described a T6SS involved in P. fluorescens bacterial competition against the potato tuber pathogen Pectobacterium atrosepticum. Although the objective of our study is far from the description of a T6SS P. fluorescens activity against E. coli spp., the results provide evidence for a bacterial ‘‘tit-for-tat’’ [73] or “T6SS dueling” [74] evolutionary strategies that control interactions among different bacterial species.
5. Conclusions
The aim of this work was to study and model the dynamics of the competition between Escherichia coli O157:H7 and Pseudomonas fluorescens co-cultured at 7, 13, 19, and 25 °C in milk. A parametric Bayesian approach was used assuming that the parameters µ (growth rate), k (decline rate), σ (standard deviation of the growth rates), and –σ (standard deviation of the decline rates) are random variables with their own prior distributions. Model results and confidence intervals are based on a probabilistic background. The highest E. coli O157:H7 populations were similar at all temperatures, except at 7 °C: E. coli spp. strains reached their maximal population of 4 log CFU/mL cultured alone, and 5 log CFU/mL co-cultured with P. fluorescens. At 13, 19, and 25 °C E. coli spp. reached their maximal population of 8 log CFU/mL single cultured and co-cultured, with times to reach a population of 6 log CFU/mL after ~48 h at 13 °C or ~24 h at 19 and 25 °C. P. fluorescens achieved its maximal densities of 8–9 log CFU/mL in all cultures at all temperatures, with similar times to reach a population of 6 or 8 log CFU/mL. The results obtained show that the growth rate of P. fluorescens has no direct correlation with its effect on the maximal population of E. coli strains. Modeling the behavior of bacterial communities helps in understanding their dynamics. The inhibition of foodborne pathogens with the use of some species from the natural food microbiota as probiotics may be a tool to improve the safety of refrigerated foods such as milk and dairy products.
Supplementary Materials
The following are available online at https://www.mdpi.com/2304-8158/9/3/331/s1, Table S1: Bayesian estimates of the posterior means and Highest Posterior Density intervals (HPD: 2.5 and 97.5%) of the growth (µ; d−1) and decline (k; d−1) rates of E. coli O157:H7 LCDC 86-51, E. coli O157:H7 ATCC 35150, non-pathogenic E. coli, and P. fluorescens cultured alone (EcO1, EcO2, Ec, Pf), or co-cultured (EcO1 + Pf, EcO2 + Pf, Ec + Pf) at 7, 13, 19 or 25°C. Table S2: Bayesian estimates of the posterior means and Highest Posterior Density intervals (HPD: 2.5 and 97.5%) of the standard deviations for growth (σ) and decline (−σ) periods of E. coli O157:H7 LCDC 86-51, E. coli O157:H7 ATCC 35150, non-pathogenic E. coli, and P. fluorescens cultured alone (EcO1, EcO2, Ec, Pf), or co-cultured (EcO1 + Pf, EcO2 + Pf, Ec + Pf) at 7, 13, 19 or 25 °C. Table S3: Maximal population density (Nmax; log CFU/mL) and time to reach (ttr; d) a population density of 6 or 8 log CFU/mL of E. coli spp. strains and P. fluorescens cultured alone or in co-culture at 7, 13, 19, or 25 °C. Figure S1: Hamiltonian Monte Carlo Method (HMCM) diagnosis plots of the growth rates of E. coli O157:H7 LCDC 86-51 (EcO1), E. coli O157:H7 ATCC 35150 (EcO2) or E. coli ATCC 8739 (Ec) co-cultured with P. fluorescens (EcO1(Pf), EcO2(Pf), or Ec(Pf)) at 7 °C. Panels show three plots for the growth rate parameter: Traces or value estimated in each step of the HMM (left); the parameter posterior distributions (middle); and the autocorrelation functions for the parameter estimates (right). mu[1] and mu[2] are the growth rates of E. coli spp or P. fluorescens, respectively. Figure S2: Hamiltonian Monte Carlo Method (HMCM) diagnosis plots of the decline rates of E. coli O157:H7 LCDC 86-51 (EcO1), E. coli O157:H7 ATCC 35150 (EcO2) or E. coli ATCC 8739 (Ec) co-cultured with P. fluorescens at 7 °C. See legends and explanations in Figure S1. k[1] and k[2] are the decline rates of E. coli spp or P. fluorescens, respectively. Figure S3: Hamiltonian Monte Carlo Method (HMCM) diagnosis plots of the growth rates of E. coli O157:H7 LCDC 86-51 (EcO1), E. coli O157:H7 ATCC 35150 (EcO2) or E. coli ATCC 8739 (Ec) co-cultured with P. fluorescens at 25 °C. See legends and explanations in Figure S1. Figure S4: Hamiltonian Monte Carlo Method (HMCM) diagnosis plots of the decline rates of E. coli O157:H7 LCDC 86-51 (EcO1), E. coli O157:H7 ATCC 35150 (EcO2) or E. coli ATCC 8739 (Ec) co-cultured with P. fluorescens at 25 °C. See legends and explanations in Figures S1 and S2.
Author Contributions
Conceptualization, E.J.Q. and D.W.S.; methodology, E.J.Q. and D.W.S.; software, J.M.M.; formal analysis, J.M.M.; investigation, E.J.Q. and I.C.; writing—original draft preparation, E.J.Q., I.C., J.M.M. and J.M.; writing—review and editing, E.J.Q., J.M.M., J.M. and D.W.S.; funding acquisition, E.J.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karmali, M.A. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 1989, 2, 15–38. [Google Scholar] [CrossRef]
- Doyle, M.P.; Zhao, T.; Meng, J.; Zhao, S. Escherichia coli O157:H7. In Food Microbiology: Fundamentals and Frontiers; Doyle, M.D., Beuchat, L.R., Montville, T.J., Eds.; ASM Press: Washington, DC, USA, 1997; pp. 171–191. [Google Scholar]
- Duncan, L.; Mai, V.; Carter, A.; Carlson, J.A.K.; Borczyk, A.; Karmali, M.A. Outbreak of gastrointestinal disease in Sarnia, Ontario. Ontario Dis. Surveill. Rep. 1986, 7, 604–611. [Google Scholar]
- Griffin, P.M.; Tauxe, R.V. The epidemiology of infections caused by Escherichia coli O157: H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 1991, 13, 60–98. [Google Scholar] [CrossRef]
- Martin, M.L.; Shipman, L.D.; Potter, M.E.; Wachsmuth, I.K.; Wells, J.G.; Hedberg, K.; Tauxe, R.V.; Davis, J.P.; Arnoldi, J.; Tilleli, J. Isolation of Escherichia coli 0157:H7 from dairy cattle associated with two cases of haemolytic uraemic syndrome. Lancet 1986, 2, 1043. [Google Scholar] [CrossRef]
- Arocha, M.M.; Mcvey, M.; Loder, S.D.; Rupnow, J.H.; Bullerman, L. Behavior of hemorrhagic Escherichia coli O157:H7 during the manufacture of Cottage cheese. J. Food Prot. 1992, 55, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Dineen, S.S.; Takeuchi, K.; Soudah, J.E.; Boor, K.J. Persistence of Escherichia coli O157:H7 in dairy fermentation systems. J. Food Prot. 1998, 61, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.M.; Chen, J.; Hill, A.R.; Griffiths, M.W. Bioluminescence: A rapid indicator of Escherichia coli O157:H7 in selected yogurt and cheese varieties. J. Food Prot. 1997, 60, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Cousin, M.A. Presence and activity of psychrotrophic microorganisms in milk and dairy products: A review. J. Food Prot. 1982, 45, 172–207. [Google Scholar] [CrossRef] [PubMed]
- De Jonghe, V.; Coorevits, A.; Van Hoorde, K.; Messens, W.; Van Landschoot, A.; De Vos, P.; Heyndrickx, M. Influence of storage conditions on the growth of Pseudomonas species in refrigerated raw milk. Appl. Environ. Microbiol. 2011, 77, 460–470. [Google Scholar] [CrossRef]
- de Oliveira, G.B.; Favarin, L.; Luchese, R.H.; McIntosh, D. Psychrotrophic bacteria in milk: How much do we really know? Braz. J. Microbiol. 2015, 46, 313–321. [Google Scholar] [CrossRef]
- Farrag, S.A.; Marth, E.H. Growth of Listeria monocytogenes in the presence of Pseudomonas fluorescens at 7 or 13°C in skim milk. J. Food Prot. 1989, 52, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, F.; Lomonaco, S.; Nucera, D.; Garoglio, D.; Dalmasso, A.; Civera, T. Distribution of Pseudomonas species in a dairy plant affected by occasional blue discoloration. Ital. J. Food Saf. 2014, 3, 245–248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Avendaño-Pérez, G.; Pin, C. Loss of culturability of Salmonella enterica subsp. enterica serovar Typhimurium upon cell-cell contact with human fecal bacteria. Appl. Environ. Microbiol. 2013, 79, 3257–3263. [Google Scholar] [CrossRef] [PubMed]
- Cornforth, D.M.; Foster, K.R. Competition sensing: The social side of bacterial stress responses. Nat. Rev. Microbiol. 2013, 11, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Dubey, G.P.; Ben-Yehuda, S. Intercellular nanotubes mediate bacterial communication. Cell 2011, 144, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Nadell, C.D.; Bassler, B.L. A fitness trade-off between local competition and dispersal in Vibrio cholerae biofilms. Proc. Natl. Acad. Sci. USA 2011, 108, 14181–14185. [Google Scholar] [CrossRef]
- Buchanan, R.; Bagi, L. Microbial competition: Effect of Pseudomonas fluorescens on the growth of Listeria monocytogenes. Food Microbiol. 1999, 16, 523–529. [Google Scholar] [CrossRef]
- Schluter, J.; Nadell, C.D.; Bassler, B.L.; Foster, K.R. Adhesion as a weapon in microbial competition. ISME J. 2015, 9, 139–149. [Google Scholar] [CrossRef]
- Kim, W.; Racimo, F.; Schluter, J.; Levy, S.B.; Foster, K.R. Importance of positioning for microbial evolution. Proc. Natl. Acad. Sci. USA 2014, 111, E1639–E1647. [Google Scholar] [CrossRef]
- Graves, R.R.; Frazier, W.C. Food microorganisms influencing the growth of Staphylococus aureus. Appl. Microbiol. 1963, 11, 513–516. [Google Scholar] [CrossRef]
- Marshall, D.L.; Schmidt, R.H. Growth of Listeria monocytogenes at 10°C in milk preincubated with selected Pseudomonads. J. Food Prot. 1988, 51, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.L.; Schmidt, R.H. Physiological evaluation of stimulated growth of Listeria monocytogenes by Pseudomonas species in milk. Can. J. Microbiol. 1991, 37, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Al-Zeyara, S.A.; Jarvis, B.; Mackey, B.M. The inhibitory effect of natural microflora of food on growth of Listeria monocytogenes in enrichment broths. Int. J. Food Microbiol. 2011, 145, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.L.; Bagi, L.K. Microbial competition: Effect of culture conditions on the suppression of Listeria monocytogenes Scott A by Carnobacterium piscicola. J. Food Prot. 1997, 60, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-M.; Doyle, M.P.; Luchansky, J.B. Identification of Pseudomonas fluorescens strains isolated from raw pork and chicken that produce siderophores antagonistic towards foodborne pathogens. J. Food Prot. 1995, 58, 1340–1344. [Google Scholar] [CrossRef]
- Farrag, S.A.; Marth, E.H. Variation in initial populations of Pseudomonas fluorescens affects behavior of Listeria monocytogenes in skim milk at 7 or 13 °C. Milchwissenschaft 1989, 46, 718–721. [Google Scholar]
- Fgaier, H.; Eberl, H.J. A competition model between Pseudomonas fluorescens and pathogens via iron chelation. J. Theor. Biol. 2010, 263, 566–578. [Google Scholar] [CrossRef]
- Freedman, D.J.; Kondo, J.K.; Willrett, D.L. Antagonism of foodborne bacteria by Pseudomonas spp.: A possible role for iron. J. Food Prot. 1989, 52, 484–489. [Google Scholar] [CrossRef]
- Mellefont, L.A.; McMeekin, T.A.; Ross, T. Effect of relative inoculum concentration on Listeria monocytogenes growth in co-culture. Int. J. Food Microbiol. 2008, 121, 157–168. [Google Scholar] [CrossRef]
- Samelis, J.; Sofos, J.N. Role of glucose in enhancing the temperature-dependent growth inhibition of Escherichia coli O157:H7 ATCC 43895 by a Pseudomonas sp. Appl. Environ. Microbiol. 2002, 68, 2600–2604. [Google Scholar] [CrossRef]
- Liao, C.-H. Control of foodborne pathogens and soft-rot bacteria on bell pepper by three strains of bacterial antagonists. J. Food Prot. 2009, 72, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-H.; Cooke, P.H.; Niemira, B.A. Localization, growth, and inactivation of Salmonella Saintpaul on jalapeño peppers. J. Food Sci. 2010, 75, M377–M382. [Google Scholar] [CrossRef] [PubMed]
- Olanya, O.M.; Annous, B.A.; Niemira, B.A.; Ukuku, D.O.; Sommers, C. Effects of media on recovery of Escherichia coli O157:H7 and Pseudomonas fluorescens from spinach. J. Food Saf. 2012, 32, 492–501. [Google Scholar] [CrossRef]
- Olanya, O.M.; Ukuku, D.O.; Niemira, B.A. Effects of temperatures and storage time on resting populations of Escherichia coli O157:H7 and Pseudomonas fluorescens in vitro. Food Control 2014, 39, 128–134. [Google Scholar] [CrossRef]
- Rickett, L.M.; Pullen, N.; Hartley, M.; Zipfel, C.; Kamoun, S.; Baranyi, J.; Morris, R.J. Incorporating prior knowledge improves detection of differences in bacterial growth rate. BMC Syst. Biol. 2015, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Chatzilena, A.; van Leeuwen, E.; Ratmann, O.; Baguelin, M.; Demiris, N. Contemporary statistical inference for infectious disease models using Stan. Epidemics 2019, 29, 100367. [Google Scholar] [CrossRef] [PubMed]
- Quinto, E.J.; Marín, J.M.; Caro, I.; Mateo, J.; Schaffner, D.W. Bayesian modeling of two- and three-species bacterial competition in milk. Food Res. Int. 2018, 105, 952–961. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Cornu, M.; Billoir, E.; Bergis, H.; Beaufort, A.; Zuliani, V. Modeling microbial competition in food: Application to the behavior of Listeria monocytogenes and lactic acid flora in pork meat products. Food Microbiol. 2011, 28, 639–647. [Google Scholar] [CrossRef]
- Cornu, M. Modelling the competitive growth of Listeria monocytogenes and food flora in situ. Acta Hortic. 2001, 566, 151–157. [Google Scholar] [CrossRef]
- Ghasemi, O.; Lindsey, M.L.; Yang, T.; Nguyen, N.; Huang, Y.; Jin, Y.-F. Bayesian parameter estimation for nonlinear modelling of biological pathways. BMC Syst. Biol. 2011, 5, S9. [Google Scholar] [CrossRef] [PubMed]
- Dormand, J.R.; Prince, P.J. A family of embedded Runge-Kutta formulae. J. Comput. Appl. Math. 1980, 6, 19–26. [Google Scholar] [CrossRef]
- Vinet, L.; Zhedanov, A. A “missing” family of classical orthogonal polynomials. J. Phys. A Math. Theor. 2011, 44, 085201. [Google Scholar] [CrossRef]
- Carpenter, B.; Gelman, A.; Hoffman, M.D.; Lee, D.; Goodrich, B.; Betancourt, M.; Brubaker, M.; Guo, J.; Li, P.; Riddell, A. Stan: A probabilistic programming language. J. Stat. Softw. 2017, 76, 1–32. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.R-project.org/ (accessed on 11 March 2020).
- Stan Development Team. Stan Modeling Language. Stan User’s Guide and Reference Mannual. 2017. Available online: http://www.mc-stan.org/ (accessed on 11 March 2020).
- Metris, A. Distribution of turbidity detection times produced by single cell-generated bacterial populations. J. Microbiol. Methods 2003, 55, 821–827. [Google Scholar] [CrossRef]
- Pin, C.; Baranyi, J. Kinetics of single cells: Observation and modeling of a stochastic process. Appl. Environ. Microbiol. 2006, 72, 2163–2169. [Google Scholar] [CrossRef]
- Akkermans, S.; Logist, F.; Van Impe, J.F. Parameter estimations in predictive microbiology: Statistically sound modelling of the microbial growth rate. Food Res. Int. 2018, 106, 1105–1113. [Google Scholar] [CrossRef]
- Park, S.; Worobo, R.W.; Durst, R.A. Escherichia coli O157:H7 as an emerging foodborne pathogen: A literature review. Crit. Rev. Biotechnol. 2001, 21, 27–48. [Google Scholar] [CrossRef]
- Ternström, A.; Lindberg, A.-M.; Molin, G. Classification of the spoilage flora of raw and pasteurized bovine milk, with special reference to Pseudomonas and Bacillus. J. Appl. Bacteriol. 1993, 75, 25–34. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Conway, W.S.; Leverentz, B. Biological control of postharvest decays of apple can prevent growth of Escherichia coli O157:H7 in apple wounds. J. Food Prot. 1999, 62, 1372–1375. [Google Scholar] [CrossRef]
- Vold, L.; Holck, A.; Wasteson, Y.; Nissen, H. High levels of background flora inhibits growth of Escherichia coli O157:H7 in ground beef. Int. J. Food Microbiol. 2000, 56, 219–225. [Google Scholar] [CrossRef]
- Jameson, J.E. A discussion of the dynamics of Salmonella enrichment. J. Hyg. 1962, 60, 193–207. [Google Scholar] [CrossRef]
- McKellar, R.C. Role of nutrient limitation in the competition between Pseudomonas fluorescens and Escherichia coli O157:H7. J. Food Prot. 2007, 70, 1739–1743. [Google Scholar] [CrossRef] [PubMed]
- Lebert, I.; Robles-Olvera, V.; Lebert, A. Application of polynomial models to predict growth of mixed cultures of Pseudomonas spp. and Listeria in meat. Int. J. Food Microbiol. 2000, 61, 27–39. [Google Scholar] [CrossRef]
- Gnanou Besse, N.; Barre, L.; Buhariwalla, C.; Vignaud, M.L.; Khamissi, E.; Decourseulles, E.; Nirsimloo, M.; Chelly, M.; Kalmokoff, M. The overgrowth of Listeria monocytogenes by other Listeria spp. in food samples undergoing enrichment cultivation has a nutritional basis. Int. J. Food Microbiol. 2010, 136, 345–351. [Google Scholar] [CrossRef]
- Cornu, M.; Kalmokoff, M.; Flandrois, J.-P. Modelling the competitive growth of Listeria monocytogenes and Listeria innocua in enrichment broths. Int. J. Food Microbiol. 2002, 73, 261–274. [Google Scholar] [CrossRef]
- Chu, W.; Zere, T.R.; Weber, M.M.; Wood, T.K.; Whiteley, M.; Hidalgo-Romano, B.; Valenzuela, E.; McLean, R.J.C. Indole production promotes Escherichia coli mixed-culture growth with Pseudomonas aeruginosa by inhibiting quorum signaling. Appl. Environ. Microbiol. 2012, 78, 411–419. [Google Scholar] [CrossRef]
- Diggle, S.P.; Griffin, A.S.; Campbell, G.S.; West, S.A. Cooperation and conflict in quorum-sensing bacterial populations. Nature 2007, 450, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.-L.; Bassler, B.L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009, 43, 197–222. [Google Scholar] [CrossRef]
- West, S.A.; Griffin, A.S.; Gardner, A.; Diggle, S.P. Social evolution theory for microorganisms. Nat. Rev. Microbiol. 2006, 4, 597–607. [Google Scholar] [CrossRef]
- Quinto, E.J.; Marín, J.M.; Schaffner, D.W. Effect of the competitive growth of Lactobacillus sakei MN on the growth kinetics of Listeria monocytogenes Scott A in model meat gravy. Food Control 2016, 63, 34–45. [Google Scholar] [CrossRef]
- Ross, T. Predictive modelling of the growth and survival of Listeria in fishery products. Int. J. Food Microbiol. 2000, 62, 231–245. [Google Scholar] [CrossRef]
- Mao, J.; Blanchard, A.E.; Lu, T. Slow and steady wins the race: A bacterial exploitative competition strategy in fluctuating environments. ACS Synth. Biol. 2015, 4, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Stubbendieck, R.M.; Vargas-Bautista, C.; Straight, P.D. Bacterial communities: Interactions to scale. Front. Microbiol. 2016, 7, 1234. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ramsey, M.M.; Chen, X.; Koley, D.; Whiteley, M.; Bard, A.J. Real-time mapping of a hydrogen peroxide concentration profile across a polymicrobial bacterial biofilm using scanning electrochemical microscopy. Proc. Natl. Acad. Sci. USA 2011, 108, 2668–2673. [Google Scholar] [CrossRef]
- Basler, M.; Ho, B.T.; Mekalanos, J.J. Tit-for-Tat: Type VI secretion system counterattack during bacterial cell-cell interactions. Cell 2013, 152, 884–894. [Google Scholar] [CrossRef]
- Journet, L.; Cascales, E. The type VI secretion system in Escherichia coli and related species. EcoSal Plus 2016, 7. [Google Scholar] [CrossRef]
- Wan, B.; Zhang, Q.; Ni, J.; Li, S.; Wen, D.; Li, J.; Xiao, H.; He, P.; Ou, H.; Tao, J.; et al. Type VI secretion system contributes to Enterohemorrhagic Escherichia coli virulence by secreting catalase against host reactive oxygen species (ROS). PLoS Pathog. 2017, 13, e1006246. [Google Scholar] [CrossRef]
- Decoin, V.; Barbey, C.; Bergeau, D.; Latour, X.; Feuilloley, M.G.J.; Orange, N.; Merieau, A. A type VI secretion system is involved in Pseudomonas fluorescens bacterial competition. PLoS ONE 2014, 9, e89411. [Google Scholar] [CrossRef]
- Sachs, J.L.; Mueller, U.G.; Wilcox, T.P.; Bull, J.J. The evolution of cooperation. Q. Rev. Biol. 2004, 79, 135–160. [Google Scholar] [CrossRef]
- Basler, M.; Mekalanos, J.J. Type 6 secretion dynamics within and between bacterial cells. Science 2012, 337, 815. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).