ELISA and Chemiluminescent Enzyme Immunoassay for Sensitive and Specific Determination of Lead (II) in Water, Food and Feed Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Chemicals and Reagents
2.3. Synthesis of Artificial Antigens of Lead
2.4. Production of Monoclonal Antibody
2.5. Indirect Competitive ELISA
2.6. Cross-Reactivity
2.7. Indirect Competitive CLEIA
2.8. Graphite Furnace Atomic Absorption Spectrometry (GFAAS)
2.9. Sample Preparation and Spiked Experiment
2.10. Pretreatment of Samples for GFAAS
3. Results
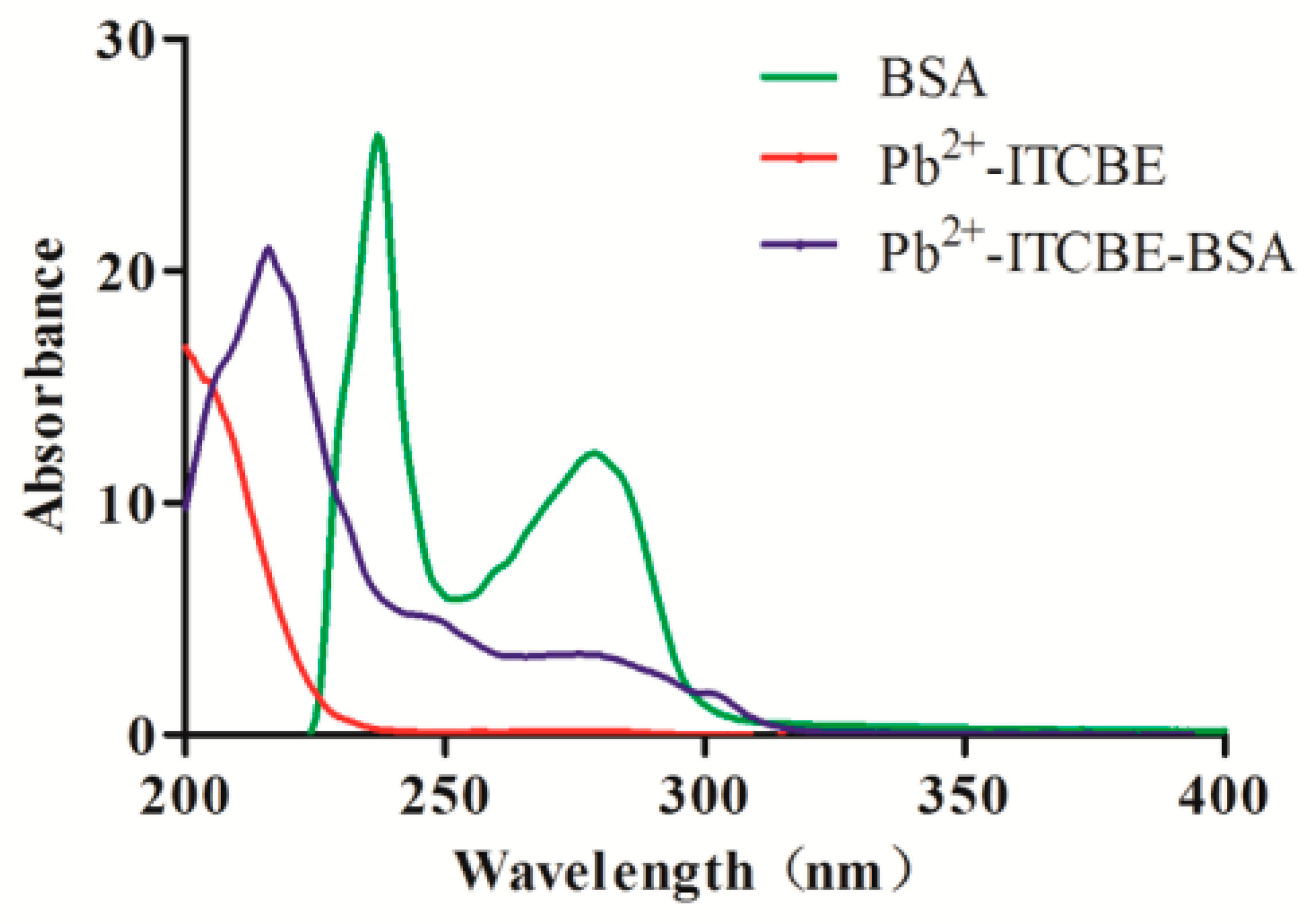
3.1. Characterization of the Artificial Antigen and the Monoclonal Antibody
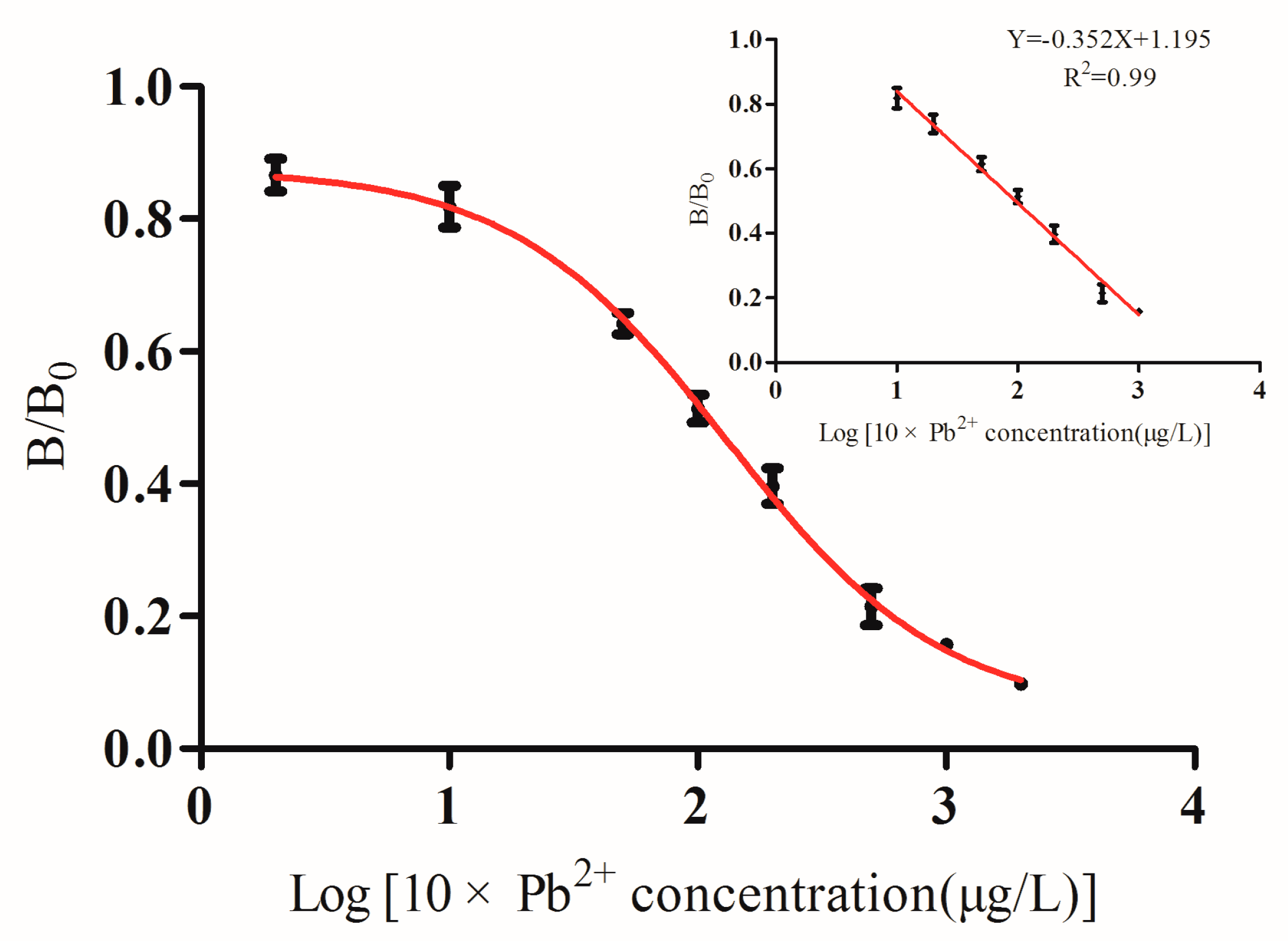
3.2. Development of ic-ELISA
3.3. Cross-Reactivity
3.4. Chemiluminescence Immunoassay
3.5. GFAAS Analysis of Pb2+
3.6. Precision and Recovery in Sample Test
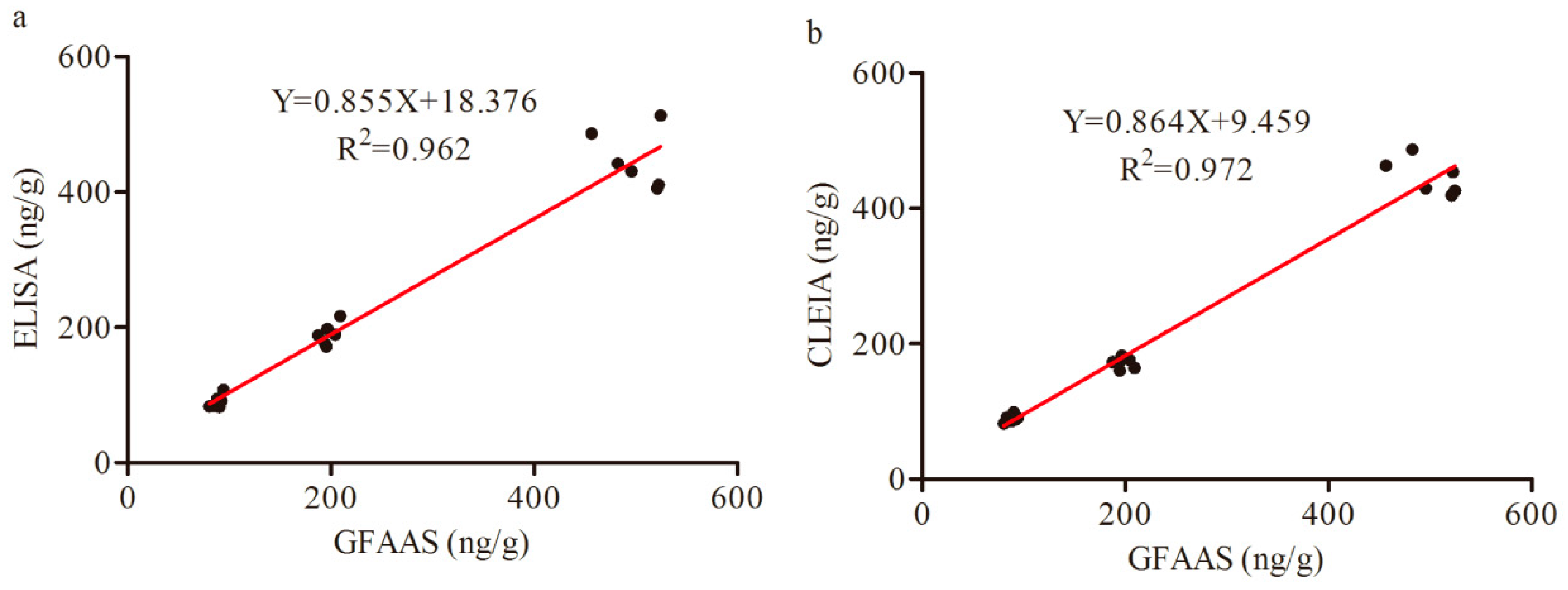
3.7. Comparison of ELISA, CLEIA and GFAAS Results for Lead (II) in Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Molera, J.; Pradell, T.; Salvadó, N.; Vendrell-Saz, M. Interactions between Clay Bodies and Lead Glazes. J. Am. Ceram. Soc. 2001, 84, 1120–1128. [Google Scholar] [CrossRef]
- Szczyglowska, M.; Bodnar, M.; Namiesnik, J.; Konieczka, P. The Use of Vegetables in the Biomonitoring of Cadmium and Lead Pollution in the Environment. Crit. Rev. Anal. Chem. 2014, 44, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Sang Yong, E.; Young-Sub, L.; Seul-Gi, L.; Mi-Na, S.; Byung-Sun, C.; Yong-Dae, K.; Ji-Ae, L.; Myung-Sil, H.; Ho-Jang, K.; Yu-Mi, K. Lead, Mercury, and Cadmium Exposure in the Korean General Population. J Korean Med. Sci. 2018, 33, e9. [Google Scholar]
- Wasserman, G.A.; Factor-Litvak, P.; Liu, X.; Todd, A.C.; Graziano, J.H. The Relationship Between Blood Lead, Bone Lead and Child Intelligence. Child Neuropsychol. 2003, 9, 22–34. [Google Scholar] [CrossRef]
- Oliveira de, T.M.; Peres, J.A.; Felsner, M.L.; Justi, K.C. Direct determination of Pb in raw milk by graphite furnace atomic absorption spectrometry (GF AAS) with electrothermal atomization sampling from slurries. Food Chem. 2017, 229, 721–725. [Google Scholar] [CrossRef]
- da Silva, D.L.F.; da Costa, M.A.P.; Silva, L.O.B.; dos Santos, W.N.L. Simultaneous determination of mercury and selenium in fish by CVG AFS. Food Chem. 2019, 273, 24–30. [Google Scholar] [CrossRef]
- Chen, K.y.; Fan, C.; Yuan, H.l.; Bao, Z.a.; Zong, C.l.; Dai, M.n.; Ling, X.; Yang, Y. High-Precision In Situ Analysis of the Lead Isotopic Composition in Copper Using Femtosecond Laser Ablation MC-ICP-MS and the Application in Ancient Coins. Spectrosc. Spect. Anal. 2013, 33, 1342–1349. [Google Scholar]
- Da Col, J.A.; Domene, S.M.A.; Pereira-Filho, E.R. Fast Determination of Cd, Fe, Pb, and Zn in Food using AAS. Food Anal. Methods 2009, 2, 110–115. [Google Scholar] [CrossRef]
- Wei, C.; Shunbi, X.; Jin, Z.; Dianyong, T.; Ying, T. Immobilized-free miniaturized electrochemical sensing system for Pb2+ detection based on dual Pb2+ -DNAzyme assistant feedback amplification strategy. Biosens. Bioelectron. 2018, 117, 312–318. [Google Scholar]
- Wang, X.Y.; Niu, C.G.; Guo, L.J.; Hu, L.Y.; Wu, S.Q.; Zeng, G.M.; Li, F. A Fluorescence Sensor for Lead (II) Ions Determination Based on Label-Free Gold Nanoparticles (GNPs)-DNAzyme Using Time-Gated Mode in Aqueous Solution. J. Fluoresc. 2017, 27, 643–649. [Google Scholar] [CrossRef]
- Chu, W.; Zhang, Y.; Li, D.; Barrow, C.J.; Wang, H.; Yang, W. A biomimetic sensor for the detection of lead in water. Biosens. Bioelectron. 2015, 67, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Huang, C.C.; Chang, H.T. Highly Selective DNA-Based Sensor for Lead(II) and Mercury(II) Ions. Anal. Chem. 2009, 81, 2383–2387. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.r.; Lu, W.; Gu, F.; Tong, P.; Yan, Z.; Zhang, L. A novel electrochemical sensor for lead ion based on cascade DNA and quantum dots amplification. Electrochim. Acta 2014, 134, 1–7. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, B.; Xiang, Y.; Su, J.; Chai, Y.; Yuan, R. DNAzyme-based highly sensitive electronic detection of lead via quantum dot-assembled amplification labels. Biosens. Bioelectron. 2011, 28, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.J.; Zhai, Y.f.; Tang, Y.; Wang, H.; Liu, B.; guo, C.W. A competitive indirect enzyme-linked immunoassay for lead ion measurement using mAbs against the lead-DTPA complex. Environ. Pollut. 2010, 158, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhai, Y.F.; Xiang, J.J.; Wang, H.; Guo, C.W. Colloidal gold probe-based immunochromatographic assay for the rapid detection of lead ions in water samples. Biosens. Bioelectron. 2010, 158, 2074–2077. [Google Scholar] [CrossRef]
- Mandappa, I.M.; Ranjini, A.; Haware, D.J.; Manonmani, H.K. Immunoassay for lead ions: analysis of spiked food samples. J. Immunoassay 2014, 35, 1–11. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission (CSC). Codex general standard for contaminants and toxins in food and feed. Codex stan 1995, 193, 229–234. [Google Scholar]
- The commission of the European Community. Commission regulation (EC) NO 1881/2006 setting maximum levels for certain contaminants in foodstuffs. OJEC 2006, 364, 5–24. [Google Scholar]
- National food safety standard for maximum levels of contaminants in foods. Available online: http://www.nhc.gov.cn/ewebeditor/uploadfile/2013/01/20130128114248937.pdf. (accessed on 29 January 2013).
- Australia New Zealand Standard 1.4.1 contaminants and natural toxicants. Available online: https://www.foodstandards.gov.au/code/Documents/Sched%2019%20Contaminant%20MLs%20v157.pdf (accessed on 1 March 2016).
- Perrin, C.L.; Kim, Y.J. Symmetry of Metal Chelates. Inorg. Chem. 2000, 39, 3902–3910. [Google Scholar] [CrossRef]
- Love, R.A.; Villafranca, J.E.; Aust, R.M.; Nakamura, K.K.; Jue, R.A.; Major, J.G.; Radhakrishnan, R.; Butler, W.F. How the anti-(metal chelate) antibody CHA255 is specific for the metal ion of its antigen: X-ray structures for two Fab’/hapten complexes with different metals in the chelate. Biochemistry 1993, 32, 10950–10959. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hu, B.; Lou, Y.; Xu, L.; Yang, F.; Yu, H.; Blake, D.A.; Liu, F. Characterization of monoclonal antibodies for lead chelate complexes: applications in antibody-based assays. J. Agric. Food Chem. 2007, 55, 4993–4998. [Google Scholar] [CrossRef] [PubMed]
- Safari, Y.; Karimaei, M.; Sharafi, K.; Arfaeinia, H.; Moradi, M.; Fattahi, N. Persistent sample circulation microextraction combined with graphite furnace atomic absorption spectroscopy for trace determination of heavy metals in fish species marketed in Kermanshah, Iran and human health risk assessment. J. Sci. Food Agric. 2017, 98, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Alqadami, A.A.; Mu, N.; Abdalla, M.A.; Khan, M.R.; Alothman, Z.A.; Wabaidur, S.M.; Ghfar, A.A. Determination of heavy metals in skin-whitening cosmetics using microwave digestion and inductively coupled plasma atomic emission spectrometry. IET Nanobiotechnol. 2017, 11, 597–603. [Google Scholar] [CrossRef]
- Hadiani, M.R.; Farhangi, R.; Soleimani, H.; Rastegar, H.; Cheraghali, A.M. Evaluation of heavy metals contamination in Iranian foodstuffs: Canned tomato paste and tomato sauce (ketchup). Food Addit. Contam. Part B 2014, 7, 74–78. [Google Scholar] [CrossRef]
- Baxter, M.J.; Burrell, J.A.; Crews, H.M.; Massey, R.C.; McWeeny, D.J. A procedure for the determination of lead in green vegetables at concentrations down to 1 μg/kg. Food Addit. Contam. 1989, 6, 341–349. [Google Scholar] [CrossRef]


| Fresh Vegetable (mg/kg) | Cereals (mg/kg) | Fresh Fruits (mg/kg) | Mushroom (mg/kg) | Beans (mg/kg) | Livestock and Poultry Meat (mg/kg) | Livestock and Poultry Gut (mg/kg) | Fish (mg/kg) | Salt (mg/kg) | Drinking Water (mg/L) | |
|---|---|---|---|---|---|---|---|---|---|---|
| CAC [18] | 0.1 | 0.2 | 0.1 | - | 0.2 | 0.1 | 0.5 | 0.3 | 2 | 0.01 |
| EFSA [19] | 0.1 | 0.2 | 0.1 | 0.3 | 0.2 | 0.1 | 0.5 | 0.3 | - | 0.01 |
| CFDA [20] | 0.1 | 0.2 | 0.1 | 1 | 0.2 | 0.2 | 0.5 | 0.5 | 2 | 0.01 |
| FSANZ [21] | 0.1 | 0.2 | 0.1 | - | 0.2 | 0.1 | 0.5 | 0.5 | - | 0.05 |
| Compounds | IC50 (µg/L) | Cross-Reactivity (%) |
|---|---|---|
| Pb2+ -ITCBE | 9.4 | 100 |
| Hg2+ -ITCBE | >1×103 | <0.943 |
| Cd2+ -ITCBE | >1×103 | <0.943 |
| Cr3+ -ITCBE | >1×103 | <0.943 |
| Cu2+ -ITCBE | >1×103 | <0.943 |
| Co2+ -ITCBE | >1×103 | <0.943 |
| Ni2+ -ITCBE | >1×103 | <0.943 |
| Zn2+ -ITCBE | >1×103 | <0.943 |
| Fe2+ -ITCBE | >1×103 | <0.943 |
| Samples | ELISA | CLEIA | AAS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spiked | Mea-sured | Recovery | RSD | Mea-sured | Recovery | RSD | Mea-sured | Recovery | RSD | |
| (ng/g) | (ng/g) | (%) | (%) | (ng/g) | (%) | (%) | (ng/g) | (%) | (%) | |
| Chicken | 0 | <LOD | -- | --- | <LOD | -- | --- | <LOD | -- | --- |
| 100 | 91.0 | 91.0 | 10.1 | 88.2 | 88.2 | 16.0 | 92.1 | 92.1 | 8.9 | |
| 200 | 197.5 | 98.7 | 11.4 | 182.2 | 91.1 | 2.5 | 196.4 | 98.2 | 0.2 | |
| 500 | 442.3 | 88.5 | 15.0 | 487.4 | 97.5 | 4.3 | 482.5 | 96.5 | 0.3 | |
| Rice | 0 | <LOD | -- | --- | <LOD | -- | --- | <LOD | -- | --- |
| 100 | 82.1 | 82.1 | 5.5 | 98.8 | 98.8 | 12.1 | 90.2 | 90.2 | 9.1 | |
| 200 | 189.1 | 94.6 | 8.4 | 176.4 | 88.2 | 9.3 | 204.0 | 102.0 | 4.7 | |
| 500 | 405.2 | 81.0 | 1.7 | 419.3 | 83.9 | 11.7 | 521.2 | 104.2 | 0.2 | |
| Chicken feed | 0 | <LOD | -- | --- | <LOD | -- | --- | <LOD | -- | --- |
| 100 | 83.8 | 83.8 | 6.8 | 91.3 | 91.3 | 6.8 | 83.3 | 83.3 | 8.7 | |
| 200 | 216.5 | 108.3 | 12.1 | 164.5 | 82.2 | 12.0 | 209.2 | 104.6 | 0.3 | |
| 500 | 430.8 | 86.1 | 6.8 | 429.9 | 85.9 | 10.6 | 495.7 | 99.1 | 0.2 | |
| Rat feed | 0 | <LOD | -- | --- | <LOD | -- | --- | <LOD | -- | --- |
| 100 | 83.0 | 83.0 | 9.6 | 82.0 | 82.0 | 11.0 | 80.3 | 80.3 | 1.4 | |
| 200 | 171.9 | 85.9 | 8.5 | 175.2 | 87.6 | 8.2 | 195.5 | 97.7 | 0.3 | |
| 500 | 410.5 | 82.1 | 9.6 | 453.5 | 90.7 | 10.9 | 522.5 | 104.5 | 1.6 | |
| Milk | 0 | <LOD | -- | --- | <LOD | -- | --- | <LOD | -- | --- |
| 100 | 94.5 | 94.5 | 12.0 | 85.6 | 85.6 | 12.3 | 88.01 | 88.01 | 8.0 | |
| 200 | 175.0 | 87.5 | 10.5 | 160.3 | 80.1 | 2.3 | 194.6 | 97.3 | 0.6 | |
| 500 | 486.7 | 97.3 | 14.1 | 463.2 | 92.6 | 8.4 | 456.5 | 91.3 | 0.3 | |
| Tap water | 0 | <LOD | -- | --- | <LOD | -- | --- | <LOD | -- | --- |
| 100 | 107.7 | 107.7 | 10.2 | 90.9 | 90.9 | 8.7 | 94.2 | 94.2 | 9.3 | |
| 200 | 188.3 | 94.1 | 9.8 | 172.8 | 86.4 | 5.0 | 187.4 | 93.7 | 0.5 | |
| 500 | 513.0 | 102.6 | 3.6 | 426.3 | 85.3 | 4.2 | 524.5 | 104.9 | 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Suo, X.-y.; Zhang, Q.; Li, X.-p.; Chen, C.; Zhang, X.-y. ELISA and Chemiluminescent Enzyme Immunoassay for Sensitive and Specific Determination of Lead (II) in Water, Food and Feed Samples. Foods 2020, 9, 305. https://doi.org/10.3390/foods9030305
Xu L, Suo X-y, Zhang Q, Li X-p, Chen C, Zhang X-y. ELISA and Chemiluminescent Enzyme Immunoassay for Sensitive and Specific Determination of Lead (II) in Water, Food and Feed Samples. Foods. 2020; 9(3):305. https://doi.org/10.3390/foods9030305
Chicago/Turabian StyleXu, Long, Xiao-yi Suo, Qi Zhang, Xin-ping Li, Chen Chen, and Xiao-ying Zhang. 2020. "ELISA and Chemiluminescent Enzyme Immunoassay for Sensitive and Specific Determination of Lead (II) in Water, Food and Feed Samples" Foods 9, no. 3: 305. https://doi.org/10.3390/foods9030305
APA StyleXu, L., Suo, X.-y., Zhang, Q., Li, X.-p., Chen, C., & Zhang, X.-y. (2020). ELISA and Chemiluminescent Enzyme Immunoassay for Sensitive and Specific Determination of Lead (II) in Water, Food and Feed Samples. Foods, 9(3), 305. https://doi.org/10.3390/foods9030305




