Microfiltration of Ovine and Bovine Milk: Effect on Microbial Counts and Biochemical Characteristics
Abstract
1. Introduction
2. Materials and Methods
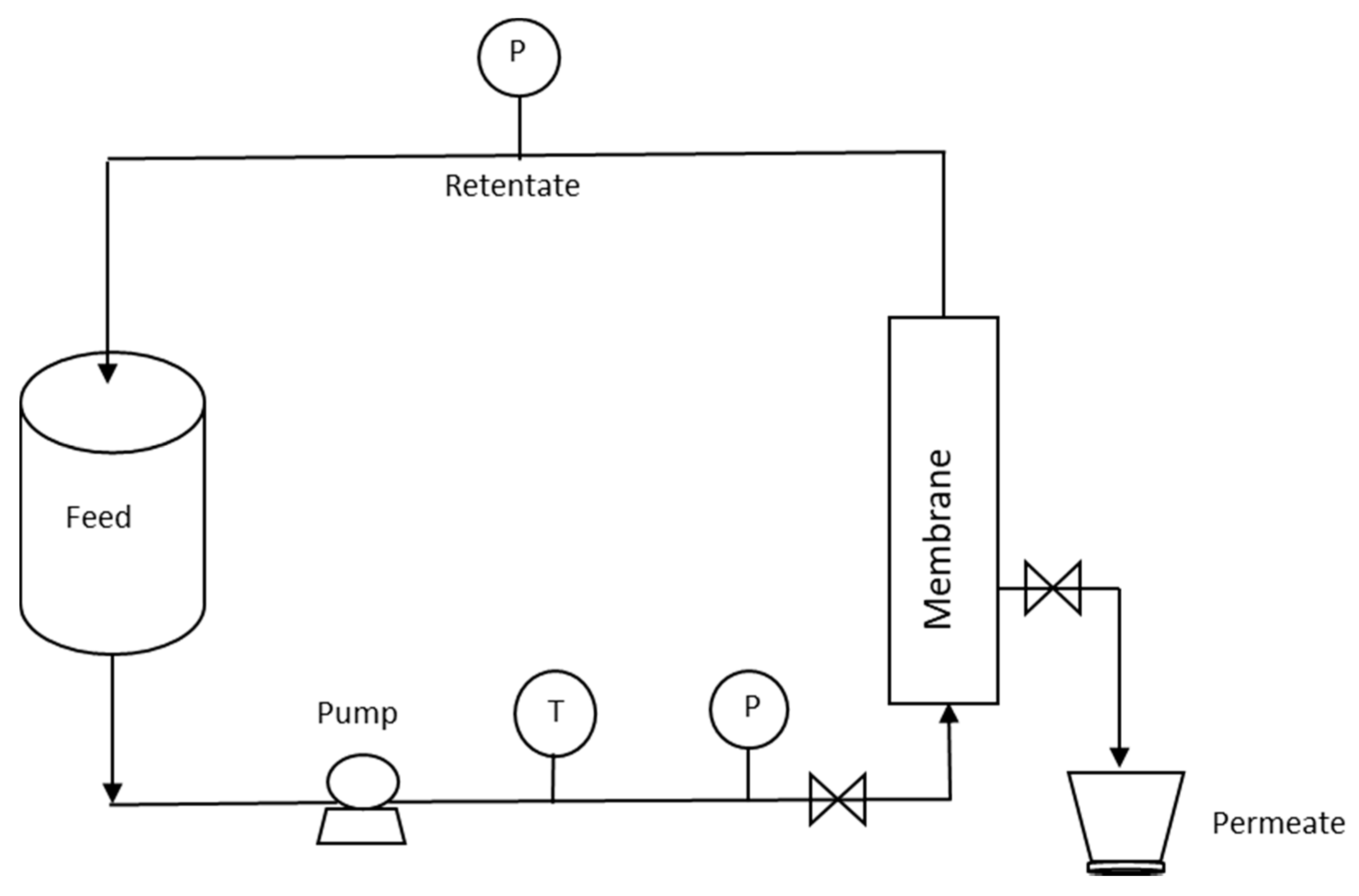
2.1. Microfiltration
2.2. Physicochemical Analyses
2.3. Protein Composition
2.4. Microbial Analyses
2.5. Indigenous Enzymes Activity
2.5.1. Cathepsin D Activity
2.5.2. Alkaline Phosphatase Activity
2.6. Rennet Clotting Behavior
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Composition
3.2. Protein Composition
3.3. Somatic Cell Counts
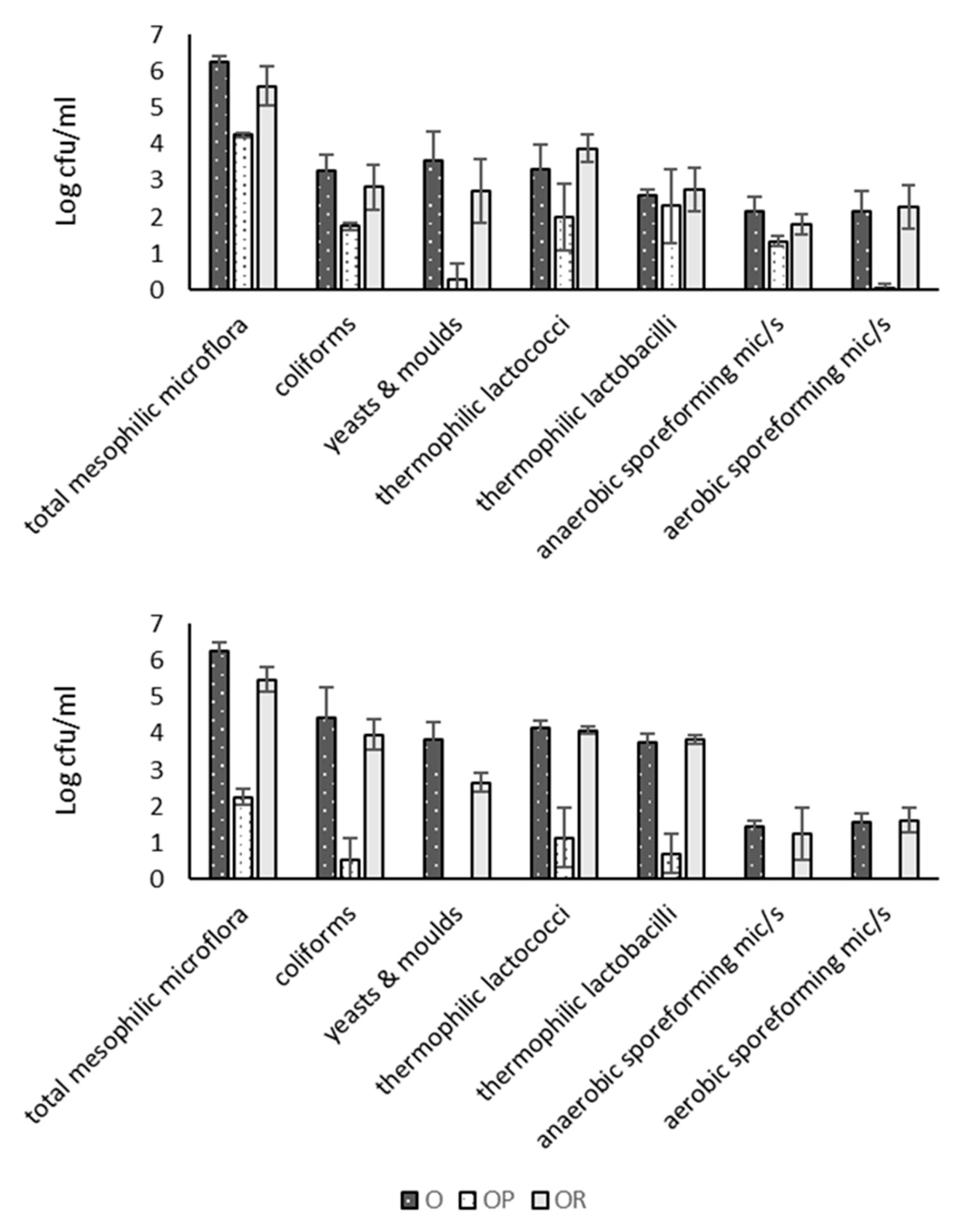
3.4. Microbial Counts
3.5. Alkaline Phosphatase Activity
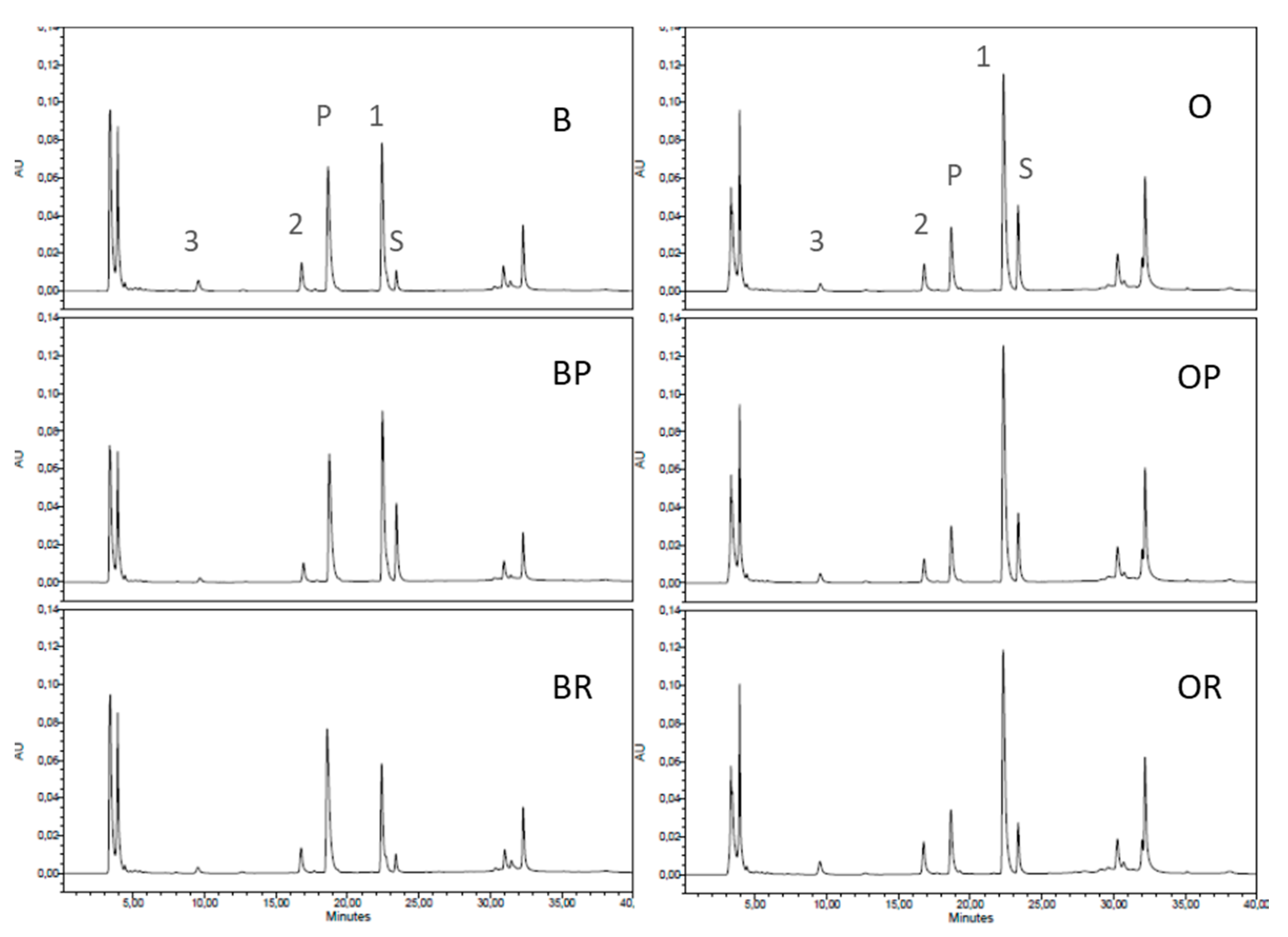
3.6. Cathepsin D Activity
3.7. Renneting Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosenberg, M. Current and future application for membrane processes in the dairy industry. Trends Food Sci. Technol. 1995, 6, 12–19. [Google Scholar] [CrossRef]
- Pouliot, Y. Membrane processes in dairy technology—From a simple idea to worldwide panacea. Int. Dairy J. 2008, 18, 735–740. [Google Scholar] [CrossRef]
- Merin, U.; Dafin, G. Cross flow microfiltration in the dairy industry: State-of-the-art. Le Lait 1990, 70, 281–291. [Google Scholar] [CrossRef]
- Saboya, L.V.; Maubois, J.L. Current developments of microfiltration technology in the dairy industry. Le Lait 2000, 80, 541–553. [Google Scholar]
- Maubois, J.L.; Schuck, P. Membrane technologies for the fractionation of dairy components. Bul. Int. Dairy Federation. 2005, 400, 2–7. [Google Scholar]
- Guerra, A.; Jonsson, G.; Rasmussen, A.; Nielsen, E.W.; Edelsten, D. Low cross flow velocity microfiltration of skim milk for removal of bacterial spores. Int. Dairy J. 1998, 7, 849–861. [Google Scholar] [CrossRef]
- Griep, E.R.; Cheng, Y.; Moraru, C. Efficient removal of spores from skim milk using cold microfiltration: Spore size and surface property considerations. J. Dairy Sci. 2018, 101, 9703–9713. [Google Scholar] [CrossRef]
- Hoffmann, W.; Kiesner, C.; Clawin-Rädecker, I.; Martin, D.; Einhoff, K.; Lorenzen, C.P.; Meisel, H.; Hammer, P.; Suhren, G.; Teufel, P. Processing of extended shelf life milk using microfiltration. Int. J. Dairy Technol. 2006, 59, 229–235. [Google Scholar] [CrossRef]
- Wang, D.; Fritsch, J.; Moraru, C. Shelf life and quality of skim milk processed by cold microfiltration with a 1.4-μm pore size membrane, with or without heat treatment. J. Dairy Sci. 2019, 102, 8798–8806. [Google Scholar] [CrossRef]
- Goudédranche, H.; Fauquant, J.; Maubois, J.-L. Fractionation of globular milk fat by membrane microfiltration. Le Lait 2000, 80, 93–98. [Google Scholar] [CrossRef]
- Lawrence, N.D.; Kentish, S.E.; O’Connor, A.J.; Barber, A.R.; Stevens, G.W. Microfiltration of skim milk using polymeric membranes for casein concentrate manufacture. Sep. Purif. Technol. 2008, 60, 237–244. [Google Scholar] [CrossRef]
- Soodam, K.; Guinee, G. The case for milk protein standardisation using membrane filtration for improving cheese consistency and quality. Int. J. Dairy Technol. 2018, 71, 277–291. [Google Scholar] [CrossRef]
- Pafylias, I.; Cheryan, M.; Mehaia, M.A.; Saglam, N. Microfiltration of milk with ceramic membranes. Food Res. Int. 1996, 29, 141–146. [Google Scholar] [CrossRef]
- Elwell, M.W.; Barbano, D.M. Use of microfiltration to improve fluid milk quality. J. Dairy Sci. 2006, 89 (Suppl. E), E20–E30. [Google Scholar] [CrossRef]
- European Union 2006. Commission Regulation (EC) No 1662/2006 of 6 November 2006 amending Regulation (EC) No 853/2004 of the European Parliament and of the Council laying down specific hygiene rules for food of animal origin. Off. J. Eur. Union L 2006, 320, 1–10. [Google Scholar]
- Beolchini, F.; Veglio, F.; Barba, D. Microfiltration of Bovine and Ovine Milk for the Reduction of microbial Content in tubular membrane: A preliminary investigation. Desalination 2004, 161, 251–258. [Google Scholar] [CrossRef]
- Beolchini, F.; Cimini, S.; Mosca, L.; Veglio, F.; Barba, D. Microfiltration of Bovine and Ovine Milk for the Reduction of Microbial Content: Effect of Some Operating Conditions on Permeate Flux and Microbial Reduction. Sep. Sci. Technol. 2005, 40, 757–772. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 12th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1975; p. 254. [Google Scholar]
- IDF Standard 42: 2006. Milk—Determination of Total Phosphorous Content—Method Using Molecular Absorption Spectrometry; International Dairy Federation: Brussels, Belgium, 2006. [Google Scholar]
- IDF Standard 119: 2007. Milk and Milk Products—Determination of Calcium, Sodium, Potassium and Magnesium Content-Atomic Absorption Spectrometric Method; International Dairy Federation: Brussels, Belgium, 2007. [Google Scholar]
- Moatsou, G.; Moschopoulou, E.; Molle, D.; Gagnaire, V.; Kandarakis, I.; Leonil, J. Comparative study of the protein fraction of goat milk from the Indigenous Greek breed and from international breeds. Food Chem. 2008, 106, 509–520. [Google Scholar] [CrossRef]
- O’Driscoll, B.M.; Rattray, F.P.; McSweeney, P.L.H.; Kelly, A.L. Protease activities in raw milk determined using a synthetic heptapeptide substrate. J. Food Sci. 1999, 64, 606–611. [Google Scholar] [CrossRef]
- Hurley, M.J.; Larsen, L.B.; Kelly, A.L.; McSweeney, P.L.H. Cathepsin D activity in quarg. Int. Dairy J. 2000, 10, 453–458. [Google Scholar] [CrossRef]
- Moatsou, G.; Katsaros, G.; Bakopanos, C.; Kandarakis, I.; Taoukis, P.; Politis, I. Effect of high-pressure treatment at various temperatures on activity of indigenous proteolytic enzymes and denaturation of whey proteins in ovine milk. Int. Dairy J. 2008, 18, 1119–1125. [Google Scholar] [CrossRef]
- IDF Standard 63: 2009. Milk—Determination of Alkaline Phosphatase; International Dairy Federation: Brussels, Belgium, 2009. [Google Scholar]
- Park, Y.W.; Juarez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep. Small Rum. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Piry, A.; Kuhnl, W.; Grein, T.; Tolkach, A.; Ripperger, S.; Kulozik, U. Length dependency of flux and protein permeation in crossflow microfiltration of skimmed milk. J. Membr. Sci. 2008, 325, 887–894. [Google Scholar] [CrossRef]
- Tziboula, A.; Steele, W.; West, I.; Muir, D.D. Microfiltration of milk with ceramic membranes: Influence on casein composition and heat stability. Milchwissensichaft 1998, 53, 8–11. [Google Scholar]
- Heidebrecht, H.J.; Kulozik, U. Fractionation of casein micelles and minor proteins by microfiltration in diafiltration mode. Study of the transmission and yield of the immunoglobulins IgG, IgA and IgM. Int. Dairy J. 2019, 93, 1–10. [Google Scholar] [CrossRef]
- Te Giffel, M.C.; van der Horst, H.C. Comparison between bactofugation and microfiltration regarding efficiency of somatic cell and bacteria removal. Bul. Int. Dairy Federation. 2004, 389, 49–53. [Google Scholar]
- Trouve, E.; Maubois, J.L.; Piot, M.; Madec, M.N.; Fauquant, J.; Rouault, A.; Tabard, J.; Brinkman, G. Retention de differentes especes microbiennes lors de l’epuration du lait par microfiltration en flux tangential. Le Lait 1991, 72, 327–332. [Google Scholar]
- Fernandez Garcia, L.; Alvarez Blanco, S.; Riera Rondriguez, A. Microfiltration applied to dairy streams: Removal of bacteria. J. Sci. Food Agric. 2013, 93, 187–196. [Google Scholar] [CrossRef]
- Malmberg, M.; Holm, S. Low bacteria skim milk by microfiltration. North Eur. Food Dairy J. 1988, 54, 75–78. [Google Scholar]
- Shakeel-ur-Rehman, S.; Fleming, C.M.; Farkye, N.Y.; Fox, P.F. Indigenous phosphatases in milk. In Advanced Dairy Chemistry Volume I —Proteins; Fox, P.F., McSweeney, P.L.H., Eds.; Kluwer Academic-Plenum Publishers: New York, NY, USA, 2003; pp. 523–543. [Google Scholar]
- Dumitraşcu, L.; Stănciuc, N.; Stanciu, S.; Râpeanu, G. Inactivation Kinetics of Alkaline Phosphatase from Different Species of Milk Using Quinolyl Phosphate as a Substrate. Food Sci. Biotechnol. 2014, 23, 1773–1778. [Google Scholar] [CrossRef]
- Moatsou, G. Indigenous enzymatic activities in ovine and caprine milks. Int. J. Dairy Technol. 2010, 63, 16–31. [Google Scholar] [CrossRef]
| Bovine | Ovine | |||||
|---|---|---|---|---|---|---|
| B | BP | BR | O | OP | OR | |
| pH | 6.68 ± 0.02 | 6.67 ± 0.03 | 6.68 ± 0.02 | 6.60 ± 0.07 | 6.53 ± 0.07 | 6.49 ± 0.08 |
| Acidity | 0.15 ± 0.02 a,* | 0.13 ± 0.00 b | 0.14 ± 0.01 a,b | 0.22 ± 0.02 a | 0.19 ± 0.02 b | 0.22 ± 0.02 a |
| Fat | 0.29 ± 0.10 a,b | 0.05 ± 0.02 a | 0.46 ± 0.19 b | 0.41 ± 0.09 a | 0.16 ± 0.01 b | 0.43 ± 0.11 a |
| Protein | 3.43 ± 0.15 a | 3.03 ± 0.21 b | 3.63 ± 0.15 a | 5.71 ± 0.28 a | 4.68 ± 0.17 b | 5.78 ± 0.28 a |
| Lactose | 4.91 ± 0.12 | 4.96 ± 0.23 | 4.96 ± 0.22 | 4.83 ± 0.05 | 4.76 ± 0.04 | 4.80 ± 0.05 |
| Total Solids | 8.88 ± 0.29 a,b | 8.3 ± 0.21 a | 9.24 ± 0.44 b | 10.87 ± 0.46 a | 9.32 ± 0.21 b | 10.95 ± 0.46 a |
| Ash | 0.78 ± 0.01 a | 0.75 ± 0.02 b | 0.81 ± 0.02 a | 0.94 ± 0.04 a | 0.82 ± 0.03 b | 0.96 ± 0.04 a |
| Phosphorus (mg/100 g) | 105.35 ± 5.73 a | 97.54 ± 1.43 b | 107.19 ± 1.65 a | 149.62 ± 5.46 a | 127.85 ± 5.50 b | 154.70 ± 8.36 |
| SCC | 417500.00 ± 43100.00 a | 0 | 653000.00 ± 73500.00 b | 660400.00 ± 199600.00 a | 0 | 570600.00 ± 159000.00 b |
| Bovine | Ovine | |||||
|---|---|---|---|---|---|---|
| B | BP | BR | O | OP | OR | |
| Total nitrogen (TN) | 0.55 ± 0.03 a | 0.48 ± 0.02 b | 0.55 ± 0.02 a | 0.88 ± 0.04 a | 0.71 ± 0.02 b | 0.90 ± 0.03 a |
| Casein nitrogen (CN) | 0.43 ± 0.04 a | 0.35 ± 0.02 b | 0.43 ± 0.03 a | 0.67 ± 0.03 a | 0.51 ± 0.04 b | 0.68 ± 0.03 a |
| Water soluble nitrogen | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.02 | 0.21 ± 0.04 | 0.20 ± 0.03 | 0.22 ± 0.04 |
| CN/TN | 0.75 ± 0.01 | 0.74 ± 0.04 | 0.75 ± 0.07 | 0.78 ± 0.01 a | 0.74 ± 0.02 a | 0.78 ± 0.0 b |
| Bovine | Ovine | |||||
|---|---|---|---|---|---|---|
| B | BP | BR | O | OP | OR | |
| κ-CN | 11.02 ± 0.30 a | 11.47 ± 0.48 b | 11.56 ± 0.24 b | 9.71 ± 0.43 a | 10.14 ± 0.51 b | 9.77 ± 0.17 a |
| αs1-CN | 28.62 ± 0.82 | 28.19 ± 0.60 | 28.90 ± 1.26 | 29.02 ± 0.81 a | 27.42 ± 0.53 b | 29.41 ± 0.82 a |
| αs2-CN | 8.96 ± 0.77 | 9.03 ± 0.63 | 8.93 ± 0.83 | 12.51 ± 0.95 a | 11.66 ± 0.88 b | 12.58 ± 0.70 a |
| β-CN | 34.09 ± 0.77 a | 32.59 ± 0.58 b | 33.11 ± 1.00 b | 32.01 ± 1.66 a | 30.67 ± 1.03 b | 31.83 ± 0.85 a |
| α-la | 2.69 ± 0.28 a,b | 2.91 ± 0.33 a | 2.52 ± 0.23 b | 3.72 ± 0.24 a | 4.41 ± 0.28 b | 3.80 ± 0.46 a |
| β-lg | 8.07 ± 0.71 | 8.49 ± 0.91 | 7.75 ± 0.65 | 8.11 ± 0.57 a | 9.48 ± 0.62 b | 7.70 ± 0.29 a |
| Bovine | Ovine | |||||
|---|---|---|---|---|---|---|
| B | BP | BR | O | OP | OR | |
| ALP | 810 ± 127 a | 407 ± 111 b | 964 ± 217 a | 4728 ± 958 a | 2832 ± 757 b | 4132 ± 1009 a |
| Proteases in whey | ||||||
| Cathepsin D-like product | 0.99 ± 0.08 a | 0.97 ± 0.08 a | 1.24 ± 0.03 b | 0.39 ± 0.06 | 0.34 ± 0.05 | 0.36 ± 0.01 |
| Peak 1 | 1.04 ± 0.08 a | 1.06 ± 0.14 a | 0.64 ± 0.11 b | 2.02 ± 0.25 | 1.93 ± 0.15 | 1.81 ± 0.18 |
| Peak 2 | 0.21 ± 0.04 | 0.19 ± 0.07 | 0.21 ± 0.04 | 0.07 ± 0.01 a | 0.16 ± 0.02 b | 0.19 ± 0.02 b |
| Peak 3 | 0.11 ± 0.01 a | 0.04 ± 0.01 b | 0.04 ± 0.01 b | 0.14 ± 0.05 | 0.13 ± 0.06 | 0.25 ± 0.03 |
| Bovine | Ovine | |||||
|---|---|---|---|---|---|---|
| B | BP | BR | O | OP | OR | |
| r (min) | 20.71 ± 1.13 | 22.04 ± 2.41 | 21.25 ± 2.08 | 14.15 ± 2.44 | 14.41 ± 2.25 | 14.34 ± 2.38 |
| A30 (mm) | 19.12 ± 2.93 a | 12.58 ± 3.22 b | 17.81 ± 2.26 a,b | 42.71 ± 3.73 a | 39.70 ± 4.35 a,b | 46.29 ± 4.55 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panopoulos, G.; Moatsou, G.; Psychogyiopoulou, C.; Moschopoulou, E. Microfiltration of Ovine and Bovine Milk: Effect on Microbial Counts and Biochemical Characteristics. Foods 2020, 9, 284. https://doi.org/10.3390/foods9030284
Panopoulos G, Moatsou G, Psychogyiopoulou C, Moschopoulou E. Microfiltration of Ovine and Bovine Milk: Effect on Microbial Counts and Biochemical Characteristics. Foods. 2020; 9(3):284. https://doi.org/10.3390/foods9030284
Chicago/Turabian StylePanopoulos, George, Golfo Moatsou, Chrysanthi Psychogyiopoulou, and Ekaterini Moschopoulou. 2020. "Microfiltration of Ovine and Bovine Milk: Effect on Microbial Counts and Biochemical Characteristics" Foods 9, no. 3: 284. https://doi.org/10.3390/foods9030284
APA StylePanopoulos, G., Moatsou, G., Psychogyiopoulou, C., & Moschopoulou, E. (2020). Microfiltration of Ovine and Bovine Milk: Effect on Microbial Counts and Biochemical Characteristics. Foods, 9(3), 284. https://doi.org/10.3390/foods9030284






