Impact of Extending Hard-Cheese Ripening: A Multiparameter Characterization of Parmigiano Reggiano Cheese Ripened up to 50 Months
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cheese Samples
2.2. Cheese Composition Analysis
2.3. Proteolysis Extent
2.3.1. Casein and Peptides
2.3.2. Free Amino Acids
2.4. Volatile Compounds
2.5. Cheese Color
2.6. Cheese Texture Analysis
2.7. Cheese Microstructure
2.8. Statistical Analysis
3. Results and Discussion
3.1. Composition Analysis
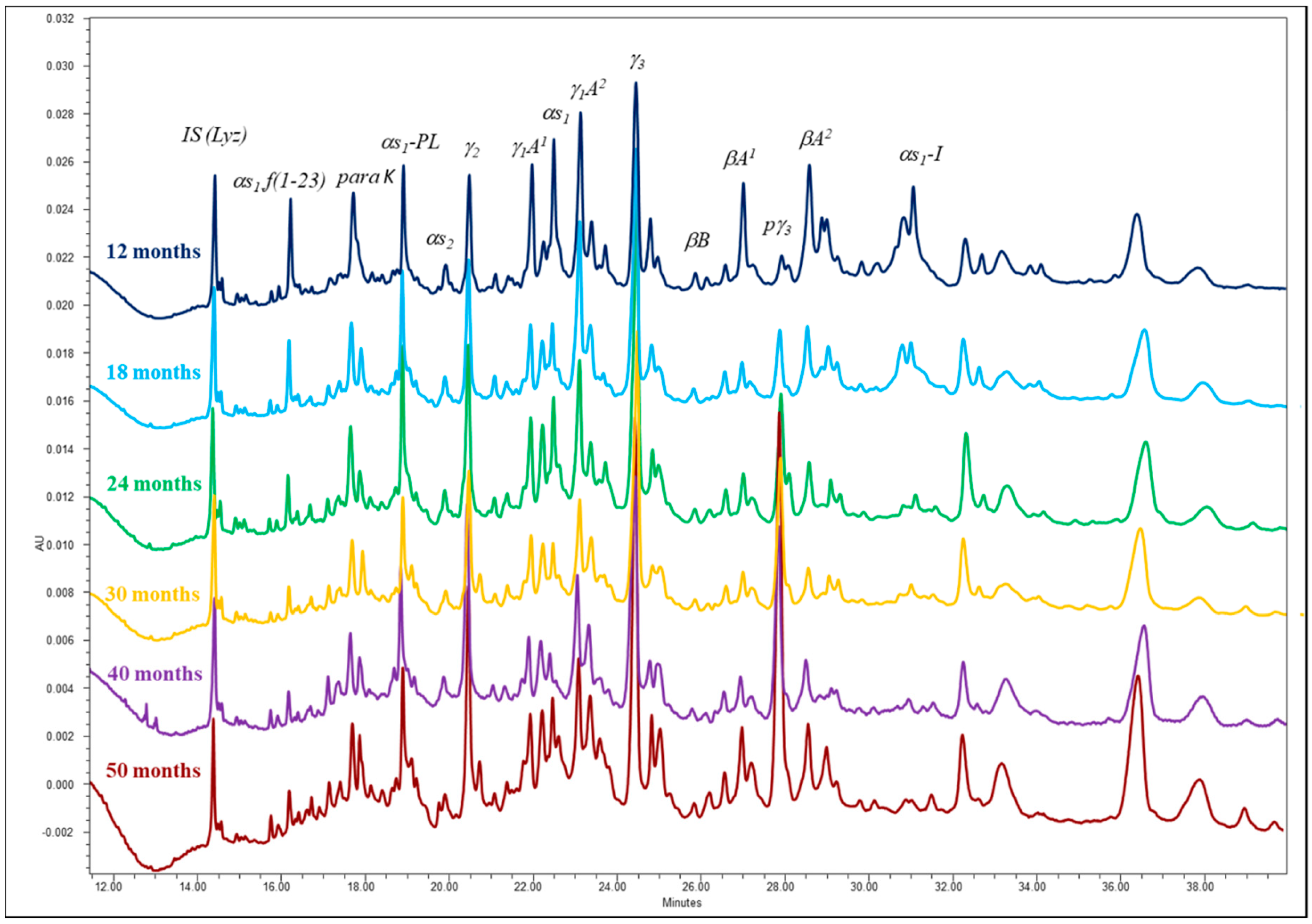
3.2. Proteolysis
3.3. Volatile Compounds
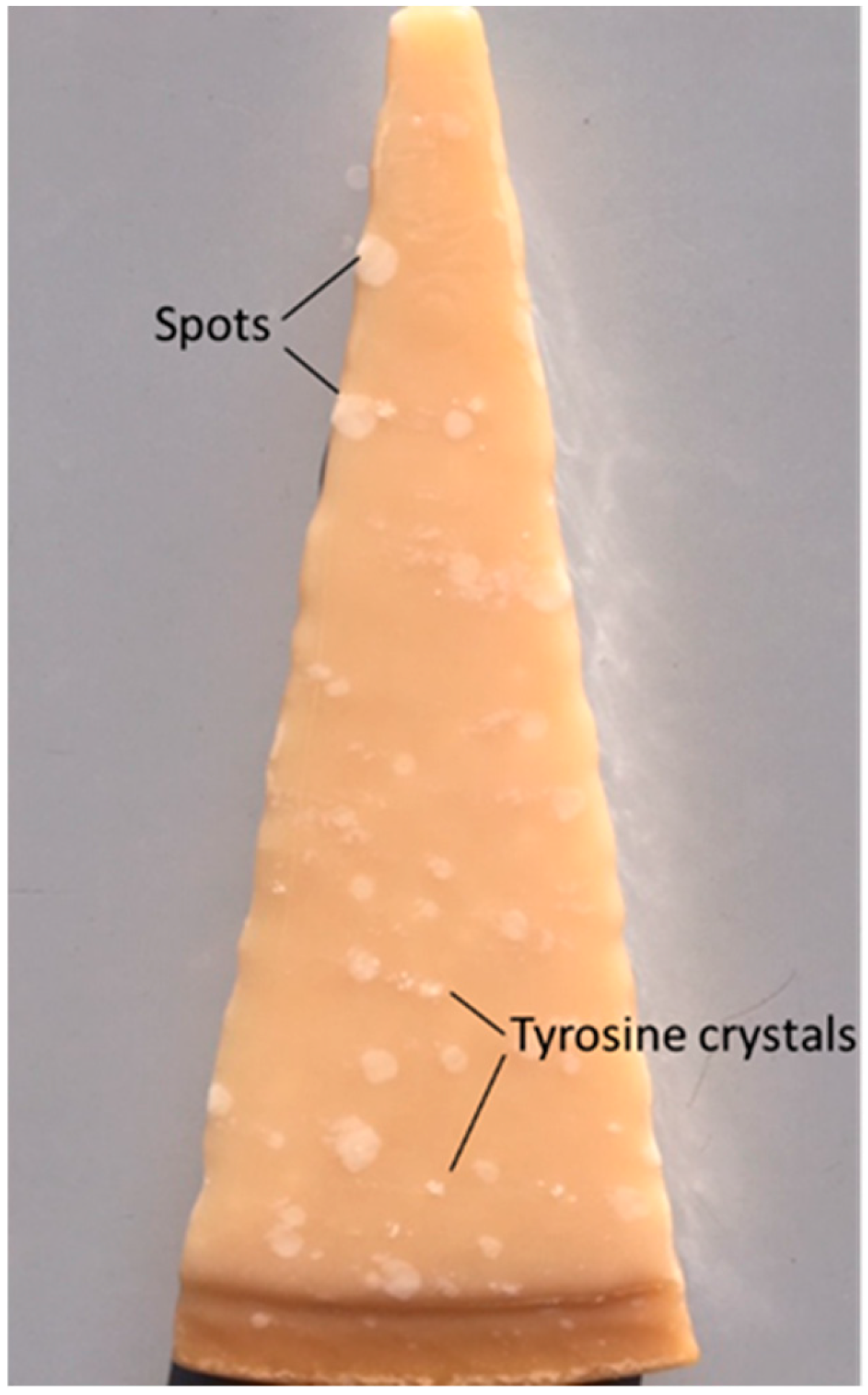
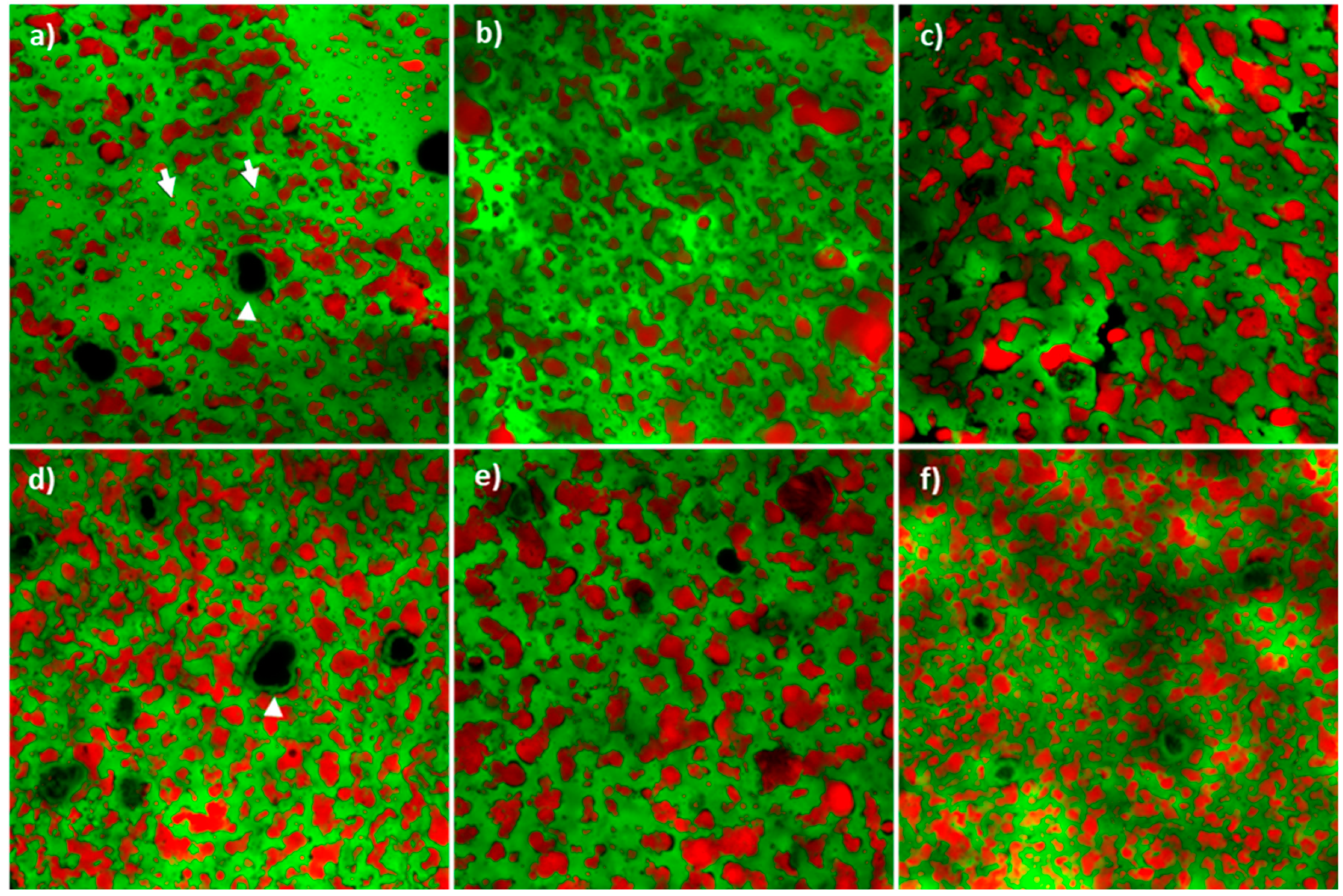
3.4. Cheese Microstructure, Appearance and Texture
3.5. Cheese Color
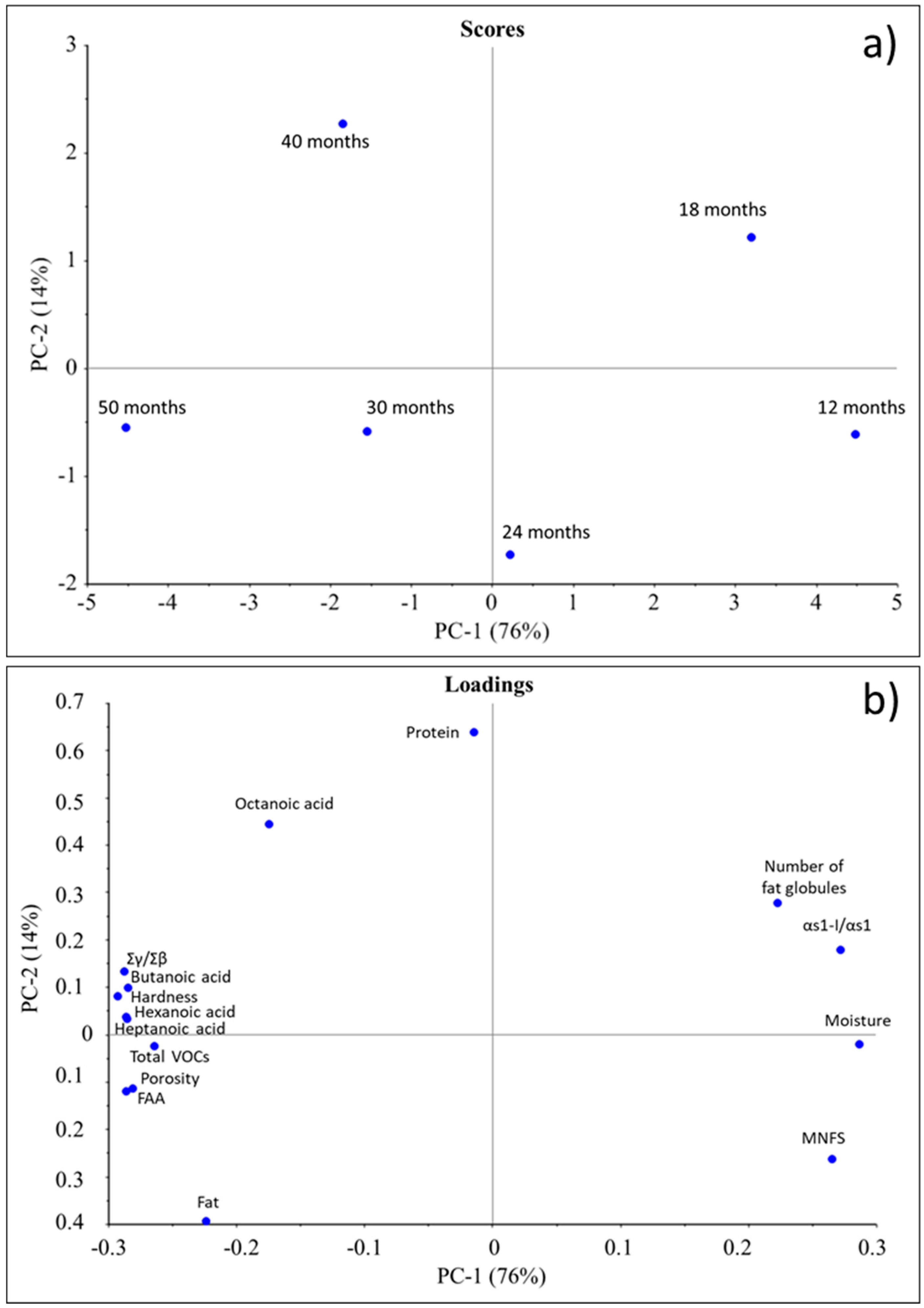
3.6. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Consorzio del Formaggio Parmigiano Reggiano. Specification and Regulations. Available online: https://www.parmigianoreggiano.com/consortium/rules_regulation_2/default.aspx (accessed on 5 December 2019).
- Gatti, M.; Bottari, B.; Lazzi, C.; Neviani, E.; Mucchetti, G. Invited review: Microbial evolution in raw-milk; long-ripened cheeses produced using undefined natural whey starters. J. Dairy Sci. 2014, 97, 573–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montel, M.-C.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Traditional cheeses: Rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014, 177, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Sforza, S.; Cavatorta, V.; Lambertini, F.; Galaverna, G.; Dossena, A.; Marchelli, R. Cheese peptidomics: A detailed study on the evolution of the oligopeptide fraction in Parmigiano-Reggiano cheese from curd to 24 months of aging. J. Dairy Sci. 2012, 95, 3514–3526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masotti, F.; Hogenboom, J.A.; Rosi, V.; De Noni, I.; Pellegrino, L. Proteolysis indices related to cheese ripening and typicalness in PDO Grana Padano cheese. Int. Dairy J. 2010, 20, 352–359. [Google Scholar] [CrossRef]
- Malacarne, M.; Summer, A.; Franceschi, P.; Formaggioni, P.; Pecorari, M.; Panari, G.; Mariani, P. Free fatty acid profile of Parmigiano-Reggiano cheese throughout ripening: Comparison between the inner and outer regions of the wheel. Int. Dairy J. 2009, 19, 637–641. [Google Scholar] [CrossRef]
- Cattaneo, S.; Hogenboom, J.A.; Masotti, F.; Rosi, V.; Pellegrino, L.; Resmini, P. Grated Grana Padano cheese: New hints on how to control quality and recognize imitations. Dairy Sci. Technol. 2008, 88, 595–605. [Google Scholar] [CrossRef]
- De Dea Lindner, J.; Bernini, V.; De Lorentiis, A.; Pecorari, A.; Neviani, E.; Gatti, M. Parmigiano–Reggiano cheese: Evolution of cultivable and total lactic microflora and peptidase activities during manufacture and ripening. Dairy Sci. Technol. 2008, 88, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Noël, Y.; Zannoni, M.; Hunter, E.A. Texture of Parmigiano Reggiano cheese: Statistical relationships between rheological and sensory variates. Le Lait 1996, 76, 243–254. [Google Scholar] [CrossRef]
- Zannoni, M. Evolution of the sensory characteristics of Parmigiano-Reggiano cheese to the present day. Food Qual. Prefer. 2010, 21, 901–905. [Google Scholar] [CrossRef]
- D’Incecco, P.; Limbo, S.; Faoro, F.; Hogenboom, J.; Rosi, V.; Morandi, S.; Pellegrino, L. New insight on crystal and spot development in hard and extra-hard cheeses: Association of spots with incomplete aggregation of curd granules. J. Dairy Sci. 2016, 99, 6144–6156. [Google Scholar] [CrossRef]
- D’Incecco, P.; Brasca, M.; Rosi, V.; Morandi, S.; Ferranti, P.; Picariello, G.; Pellegrino, L. Bacterial proteolysis of casein leading to UHT milk gelation: An applicative study. Food Chem. 2019, 292, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Hogenboom, J.A.; D’Incecco, P.; Fuselli, F.; Pellegrino, L. Ion-Exchange Chromatographic Method for the Determination of the Free Amino Acid Composition of Cheese and Other Dairy Products: An Inter-Laboratory Validation Study. Food Anal. Methods 2017, 10, 3137–3148. [Google Scholar] [CrossRef] [Green Version]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A. Color measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Romani, S.; Sacchetti, G.; Pittia, P.; Pinnavaia, G.G.; Dalla Rosa, M. Physical, chemical, textural and sensorial changes of portioned Parmigiano Reggiano cheese packed under different conditions. Food Sci. Technol. Int. 2002, 8, 203–211. [Google Scholar] [CrossRef]
- Ong, L.; D’Incecco, P.; Pellegrino, L.; Nguyen, H.T.; Kentish, S.E.; Gras, S.L. The Effect of Salt on the Structure of Individual Fat Globules and the Microstructure of Dry Salted Cheddar Cheese. Food Biophys. 2019. [Google Scholar] [CrossRef]
- D’Incecco, P.; Ong, L.; Gras, S.; Pellegrino, L. A fluorescence in situ staining method for investigating spores and vegetative cells of Clostridia by confocal laser scanning microscopy and structured illuminated microscopy. Micron 2018, 110, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Malacarne, M.; Summer, A.; Panari, G.; Pecorari, M.; Mariani, P. Physico-chemical characterization of Parmigiano-Reggiano cheese ripening (in Italian; abstract in English). Sci. Tec. Latt. Casearia 2006, 57, 215–228. [Google Scholar]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L. Microbiology of cheese ripening. In Fundamentals of Cheese Science; Springer: Boston, MA, USA, 2017; pp. 333–390. [Google Scholar]
- Fenelon, M.A.; Guinee, T.P. Primary proteolysis and textural changes during ripening in Cheddar cheeses manufactured to different fat contents. Int. Dairy J. 2000, 10, 151–158. [Google Scholar] [CrossRef]
- Lopez, C.; Briard-Bion, V.; Beaucher, E.; Ollivon, M. Multiscale characterization of the organization of triglycerides and phospholipids in Emmental cheese: From the microscopic to the molecular level. J. Agric. Food Chem. 2008, 56, 2406–2414. [Google Scholar] [CrossRef]
- Codex Alimentarius. Milk and Milk Products. Available online: http://www.fao.org/3/i2085e/i2085e00.pdf (accessed on 5 December 2019).
- McSweeney, P.L.H. Biochemistry of cheese ripening. Int. J. Dairy Technol. 2004, 57, 127–144. [Google Scholar] [CrossRef]
- Vélez, M.A.; Bergamini, C.V.; Ramonda, M.B.; Candioti, M.C.; Hynes, E.R.; Perotti, M.C. Influence of cheese making technologies on plasmin and coagulant associated proteolysis. LWT Food Sci. Technol. 2015, 64, 282–288. [Google Scholar] [CrossRef]
- Hynes, E.R.; Aparo, L.; Candioti, M.C. Influence of residual milk-clotting enzyme on αs1-casein hydrolysis during ripening of Reggianito Argentino cheese. J. Dairy Sci. 2004, 87, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Mayer, H.K.; Rockenbauer, C.; Mlcak, H. Evaluation of proteolysis in Parmesan cheese using electrophoresis and HPLC. Le Lait 1998, 78, 425–438. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, L.; Rosi, V.; D’Incecco, P.; Stroppa, A.; Hogenboom, J.A. Changes in the soluble nitrogen fraction of milk throughout PDO Grana Padano cheese-making. Int. Dairy J. 2015, 47, 128–135. [Google Scholar] [CrossRef]
- Brasca, M.; Hogenboom, J.A.; Morandi, S.; Rosi, V.; D’Incecco, P.; Silvetti, T.; Pellegrino, L. Proteolytic activity and production of γ-aminobutyric acid by Streptococcus thermophilus cultivated in microfiltered pasteurized milk. J. Agric. Food Chem. 2016, 64, 8604–8614. [Google Scholar] [CrossRef]
- D’Incecco, P.; Gatti, M.; Hogenboom, J.A.; Bottari, B.; Rosi, V.; Neviani, E.; Pellegrino, L. Lysozyme affects the microbial catabolism of free arginine in raw-milk hard cheeses. Food Microbiol. 2016, 57, 16–22. [Google Scholar] [CrossRef]
- Pedersen, T.B.; Vogensen, F.K.; Ardö, Y. Effect of heterofermentative lactic acid bacteria of DL-starters in initial ripening of semi-hard cheese. Int. Dairy J. 2016, 57, 72–79. [Google Scholar] [CrossRef]
- Khattab, A.R.; Guirguis, H.A.; Tawfik, S.M.; Farag, M.A. Cheese ripening: A review on modern technologies towards flavor enhancement; process acceleration and improved quality assessment. Trends Food Sci. Technol. 2019, 88, 343–360. [Google Scholar] [CrossRef]
- Frank, D.C.; Owen, C.M.; Patterson, J. Solid phase microextraction (SPME) combined with gas-chromatography and olfactometry-mass spectrometry for characterization of cheese aroma compounds. LWT Food Sci. Technol. 2004, 37, 139–154. [Google Scholar] [CrossRef]
- Qian, M.C.; Burbank, H.M. Hard Italian Cheeses: Parmigiano-Reggiano and Grana Padano. In Improving the Flavor of Cheese; Weimer, B.C., Ed.; Woodhead Publishing: Cambridge, UK, 2007; pp. 421–443. [Google Scholar]
- Kilcawley, K.N. Cheese flavor. In Fundamentals of Cheese Science; Fox, P.F., Guinee, T.P., Cogan, T.M., McSweeney, P.L., Eds.; Springer: Boston, MA, USA, 2017; pp. 443–474. [Google Scholar]
- Moio, L.; Addeo, F. Grana Padano cheese aroma. J. Dairy Res. 1998, 65, 317–333. [Google Scholar] [CrossRef]
- Abbatangelo, M.; Núñez-Carmona, E.; Sberveglieri, V. Application of a novel S3 nanowire gas sensor device in parallel with GC-MS for the identification of Parmigiano Reggiano from US and European competitors. J. Food Eng. 2018, 236, 36–43. [Google Scholar] [CrossRef]
- Lazzi, C.; Povolo, M.; Locci, F.; Bernini, V.; Neviani, E.; Gatti, M. Can the development and autolysis of lactic acid bacteria influence the cheese volatile fraction? The case of Grana Padano. Int. J. Food Microbiol. 2016, 233, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ceruti, R.J.; Zorrilla, S.E.; Sihufe, G.A. Volatile profile evolution of Reggianito cheese during ripening under different temperature–time combinations. Eur. Food Res. Technol. 2016, 242, 1369–1378. [Google Scholar] [CrossRef]
- Curioni, P.M.G.; Bosset, J.O. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Soodam, K.; Ong, L.; Powell, I.B.; Kentish, S.E.; Gras, S.L. Effect of elevated temperature on the microstructure of full fat Cheddar cheese during ripening. Food Struct. 2017, 14, 8–16. [Google Scholar] [CrossRef]
- Soodam, K.; Ong, L.; Powell, I.B.; Kentish, S.E.; Gras, S.L. Effect of calcium chloride addition and draining pH on the microstructure and texture of full fat Cheddar cheese during ripening. Food Chem. 2015, 181, 111–118. [Google Scholar] [CrossRef]
- Tansman, G.F.; Kindstedt, P.S.; Hughes, J.M. Crystal fingerprinting: Elucidating the crystals of Cheddar, Parmigiano-Reggiano, Gouda, and soft washed-rind cheeses using powder x-ray diffractometry. Dairy Sci. Technol. 2015, 95, 651–664. [Google Scholar] [CrossRef] [Green Version]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L. Cheese: Structure; Rheology and Texture. In Fundamentals of Cheese Science; Springer: Boston, MA, USA, 2017; pp. 475–532. [Google Scholar]
- Mokrzycki, W.S.; Tatol, M. Color difference Delta E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Cerquaglia, O.; Sottocorno, M.; Pellegrino, L.; Ingi, M. Detection of cow’s milk; fat or whey in ewe and buffalo ricotta by HPLC determination of β-carotene. Ital. J. Food Sci. 2011, 23, 367. [Google Scholar]
| Ripening Time (Months) | Moisture | Protein | Fat | FDM1 | MNFS2 |
|---|---|---|---|---|---|
| 12 | 31.72 c ± 0.07 | 31.17 a ± 0.07 | 30.78 ab ± 0.02 | 45.08 b ± 0.03 | 45.83 c ± 0.09 |
| 18 | 30.61 bc ± 2.42 | 33.72 d ± 0.15 | 29.23 a ± 2.33 | 42.08 a ± 1.87 | 43.20 b ± 2.02 |
| 24 | 29.39 bc ± 1.83 | 31.37 ab ± 0.32 | 32.61 bc ± 2.14 | 46.15 b ± 1.84 | 43.59 bc ± 1.32 |
| 30 | 28.86 abc ± 0.48 | 31.75 bc ± 0.16 | 32.48 bc ± 0.38 | 45.65 b ± 0.25 | 42.73 b ± 0.48 |
| 40 | 28.03 ab ± 0.31 | 33.79 d ± 0.11 | 30.43 ab ± 0.30 | 42.29 a ± 0.26 | 40.30 a ± 0.29 |
| 50 | 25.34 a ± 0.52 | 31.93 c ± 0.66 | 35.74 c ± 0.10 | 47.87 b ± 0.43 | 39.43 a ± 0.85 |
| Ripening Time (Months) | αs1-I/αs1 (1) | αs1 f(1-23)/αs1 (2) | Σγ/Σβ (3) | Calculated Cheese Age (4) | FAA |
|---|---|---|---|---|---|
| 12 | 0.7 | 0.4 | 2.7 | 13 | 24.2 ± 0.08 a |
| 18 | 0.6 | 0.3 | 5.0 | 22 | 24.6 ± 0.17 a |
| 24 | 0.3 | 0.2 | 5.4 | 25 | 28.4 ± 0.25 b |
| 30 | 0.3 | 0.2 | 8.7 | 34 | 28.6 ± 1.30 b |
| 40 | 0.4 | 0.3 | 10.0 | 39 | 28.4 ± 0.26 b |
| 50 | 0.2 | 0.2 | 12.1 | 49 | 30.0 ± 0.86 c |
| Compound | LRI a | Identification b | Ripening Time (Months) | |||||
|---|---|---|---|---|---|---|---|---|
| 12 | 18 | 24 | 30 | 40 | 50 | |||
| Acids | ||||||||
| Acetic acid | 1455 | MS, PI | 1000 ± 200 | 850 ± 270 | 950 ± 60 | 1300 ± 120 | 1100 ± 110 | 1200 ± 90 |
| Propanoic acid | 1546 | MS, PI | 3 ± 0 | 24 ± 2 | 4 ± 2 | 7 ± 1 | 8 ± 1 | 6 ± 1 |
| Butanoic acid | 1640 | MS, PI | 4300 ± 300 | 4500 ± 700 | 5500 ± 300 | 8100 ± 500 | 9300 ± 400 | 11000 ± 890 |
| Pentanoic acid | 1755 | MS, PI | 18 ± 1 | 21 ± 4 | 41 ± 14 | 73 ± 8 | 97 ± 9 | 91 ± 7 |
| Hexanoic acid | 1870 | MS, PI | 4800 ± 900 | 5600 ± 760 | 7000 ± 700 | 12000 ± 1200 | 12000 ± 890 | 17000 ± 1300 |
| Heptanoic acid | 1957 | MS, PI | ND | 6 ± 3 | 28 ± 21 | 74 ± 50 | 23 ± 15 | 58 ± 23 |
| Octanoic acid | 2066 | MS, PI | ND | 71 ± 10 | 170 ± 60 | 1300 ± 1200 | 500 ± 67 | 1700 ± 500 |
| 2-Methylbutanoic acid | T | 4 ± 0.3 | 4 ± 0.3 | 3 ± 0.5 | 2 ± 0.4 | 3 ± 0.2 | 3 ± 0.2 | |
| 3-Methylbutanoic acid | T | 40 ± 0.3 | 36 ± 5 | 16 ± 5 | 20 ± 3 | 6 ± 1 | 20 ± 3 | |
| Sum of areas | 10165 | 11112 | 13712 | 22876 | 23037 | 31078 | ||
| Ketones | ||||||||
| 2-Pentanone | 990 | MS, PI, ST | 150 ± 12 | 400 ± 90 | 400 ± 110 | 300 ± 70 | 300 ± 50 | 340 ± 50 |
| 2-Heptanone | 1190 | MS, PI, ST | 200 ± 40 | 570 ± 180 | 700 ± 40 | 600 ± 130 | 600 ± 70 | 630 ± 120 |
| 2-Nonanone | 1400 | MS, PI, ST | 80 ± 2 | 170 ± 40 | 400 ± 100 | 290 ± 100 | 270 ± 60 | 700 ± 200 |
| 8-Nonen-2-one | 1495 | MS, PI | ND | 15 ± 2 | 30 ± 15 | 40 ± 4 | 20 ± 4 | 70 ± 21 |
| Sum of areas | 430 | 1155 | 1530 | 1230 | 1190 | 1740 | ||
| Alcohols | ||||||||
| 2-Pentanol | 1172 | MS, PI, ST | 95 ± 8 | 97 ± 76 | 86 ± 83 | 45 ± 8 | 18 ± 4 | 48 ± 18 |
| 2-Heptanol | 1320 | MS, PI | 45 ± 4 | 33 ± 23 | 38 ± 23 | 17 ± 2 | 17 ± 4 | 37 ± 17 |
| Sum of areas | 140 | 130 | 124 | 62 | 35 | 85 | ||
| Esters | ||||||||
| Ethyl butanoate | 1055 | MS, PI | 250 ± 1 | 440 ± 49 | 150 ± 14 | 580 ± 96 | 330 ± 96 | 500 ± 87 |
| Ethyl hexanoate | 1240 | MS, PI | 430 ± 54 | 1000 ± 170 | 230 ± 70 | 2000 ± 75 | 720 ± 132 | 1300 ± 170 |
| Ethyl octanoate | 1440 | MS, PI | 35 ± 2 | 98 ± 8 | 11 ± 4 | 287 ± 35 | 81 ± 17 | 249 ± 30 |
| Sum of areas | 715 | 1538 | 391 | 2867 | 1131 | 2049 | ||
| Total sum of areas | 11450 | 13935 | 15757 | 27035 | 25393 | 34952 | ||
| Ripening Time (Months) | Number of Fat Globules | Porosity | Hardness | Brittleness |
|---|---|---|---|---|
| 12 | 676 ± 28 c | 0.11 ± 0.01 a | 11.96 ± 1.57 a | 5.02 ± 0.34 a |
| 18 | 574 ± 149 bc | 0.11 ± 0.01 a | 15.98 ± 0.29 abc | 6.31 ± 0.38 ab |
| 24 | 292 ± 96 a | 0.17 ± 0.01 b | 18.93 ± 0.49 abcd | 6.52 ± 0.82 b |
| 30 | 410 ± 114 abc | 0.16 ± 0.01 b | 21.48 ± 0.49 bcd | 6.30 ± 0.94 ab |
| 40 | 476 ± 65 abc | 0.17 ± 0.01 b | 22.65 ± 4.31 cbcd | 6.52 ± 0.69 ab |
| 50 | 363 ± 150 ab | 0.19 ± 0.01 c | 25.20 ± 3.53 d | 5.53 ± 0.69 a |
| Ripening Time (Months) | ΔE* (6) | YI (5) | h* (7) |
|---|---|---|---|
| 12 | 35.07 a ± 0.62 | 88.65 a ± 0.19 | |
| 18 | 3.66 a ± 0.74 | 31.42 a ± 1.32 | 82.75 bc ± 0.31 |
| 24 | 3.26 a ± 1.22 | 31.04 a ± 2.97 | 82.69 bc ± 1.62 |
| 30 | 6.72 b ± 1.31 | 26.28 b ± 2.94 | 80.57 c ± 1.07 |
| 40 | 7.73 b ± 0.91 | 26.24 b ± 3.02 | 84.80 b ± 0.55 |
| 50 | 6.38 b ± 1.50 | 31.40 a ± 2.14 | 87.64 a ± 2.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Incecco, P.; Limbo, S.; Hogenboom, J.; Rosi, V.; Gobbi, S.; Pellegrino, L. Impact of Extending Hard-Cheese Ripening: A Multiparameter Characterization of Parmigiano Reggiano Cheese Ripened up to 50 Months. Foods 2020, 9, 268. https://doi.org/10.3390/foods9030268
D’Incecco P, Limbo S, Hogenboom J, Rosi V, Gobbi S, Pellegrino L. Impact of Extending Hard-Cheese Ripening: A Multiparameter Characterization of Parmigiano Reggiano Cheese Ripened up to 50 Months. Foods. 2020; 9(3):268. https://doi.org/10.3390/foods9030268
Chicago/Turabian StyleD’Incecco, Paolo, Sara Limbo, John Hogenboom, Veronica Rosi, Serena Gobbi, and Luisa Pellegrino. 2020. "Impact of Extending Hard-Cheese Ripening: A Multiparameter Characterization of Parmigiano Reggiano Cheese Ripened up to 50 Months" Foods 9, no. 3: 268. https://doi.org/10.3390/foods9030268





