Anti-Hyperglycemic Effects of Green Crab Hydrolysates Derived by Commercially Available Enzymes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
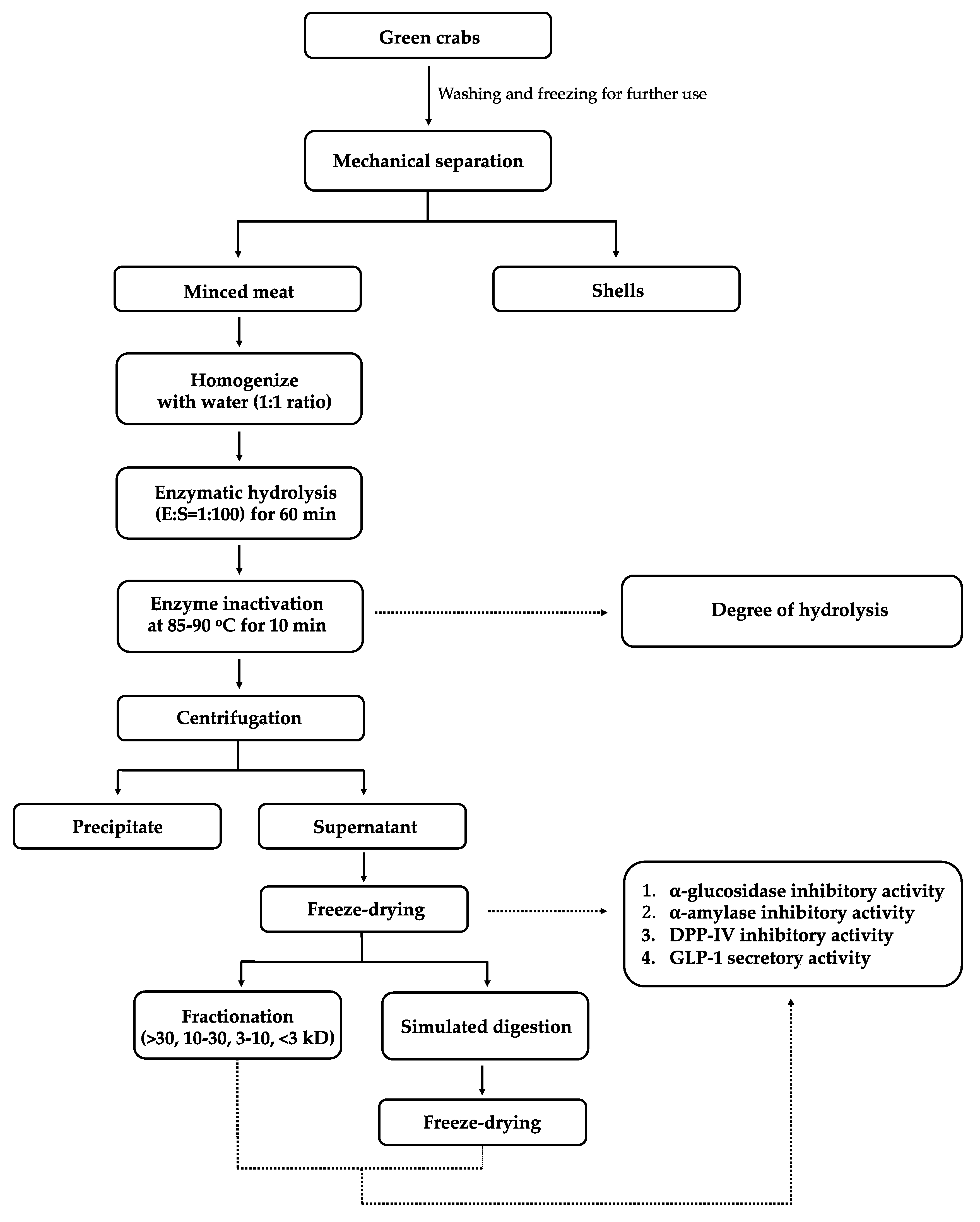
2.2. Preparation of Green Crab
2.3. Enzymatic Hydrolysis of Crab Mince
2.4. Degree of Hydrolysis
2.5. Simulated Gastrointestinal Digestion
2.6. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.7. Fractionation Using Ultrafiltration
2.8. Rat Intestine α-Glucosidase Inhibition Assay
2.9. Porcine Pancreatic α-Amylase Inhibition Assay
2.10. DPP-IV Inhibition Assay
2.11. GLUTag Cell Culture
2.12. Cell Viability Assay
2.13. GLP-1 Secretion Assay
2.14. Statistical Analysis
3. Results
3.1. Degree of Hydrolysis
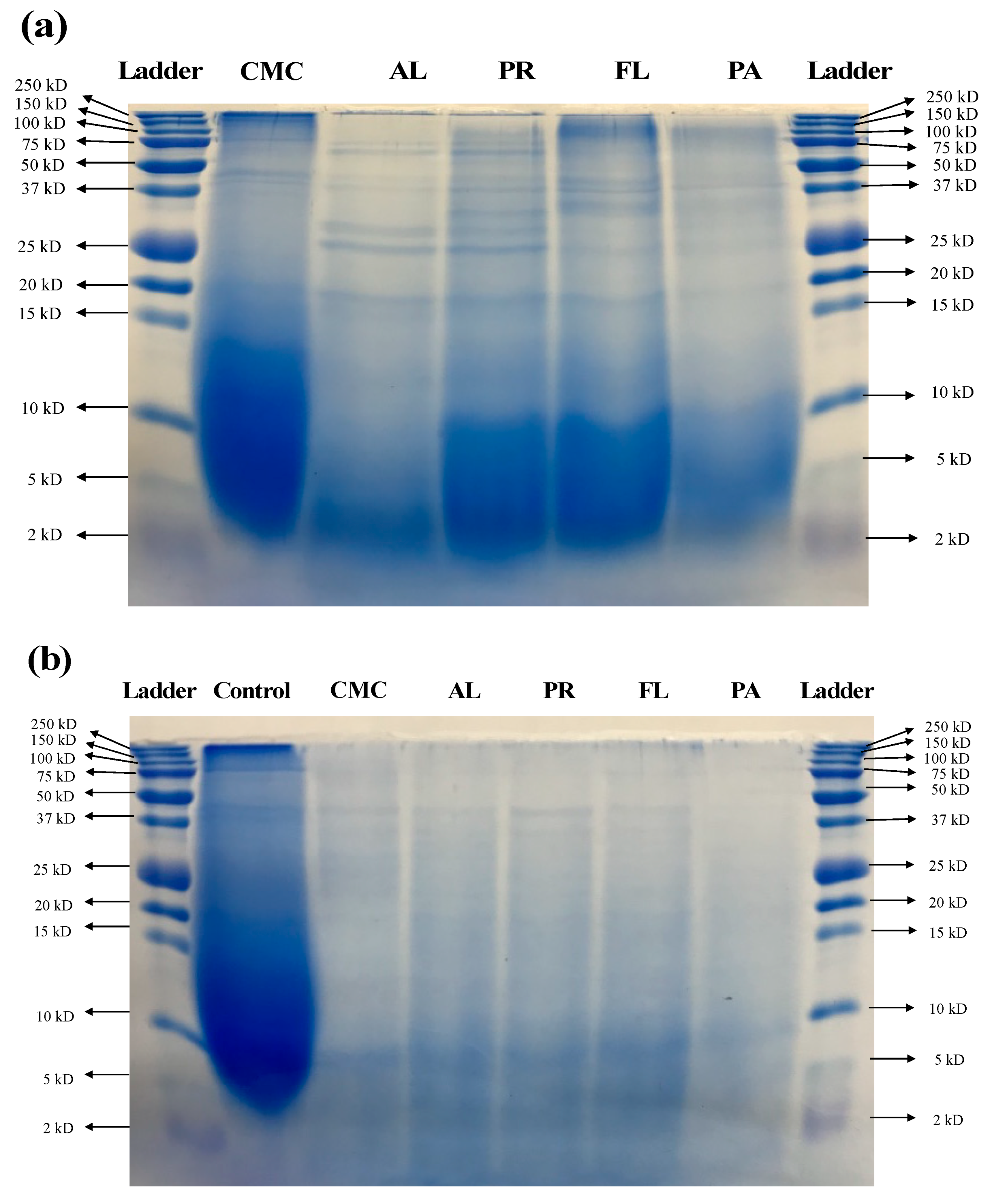
3.2. Molecular Weight Distribution Using SDS-PAGE
3.3. α-Glucosidase and Porcine α-Amylase Inhibitory Activities
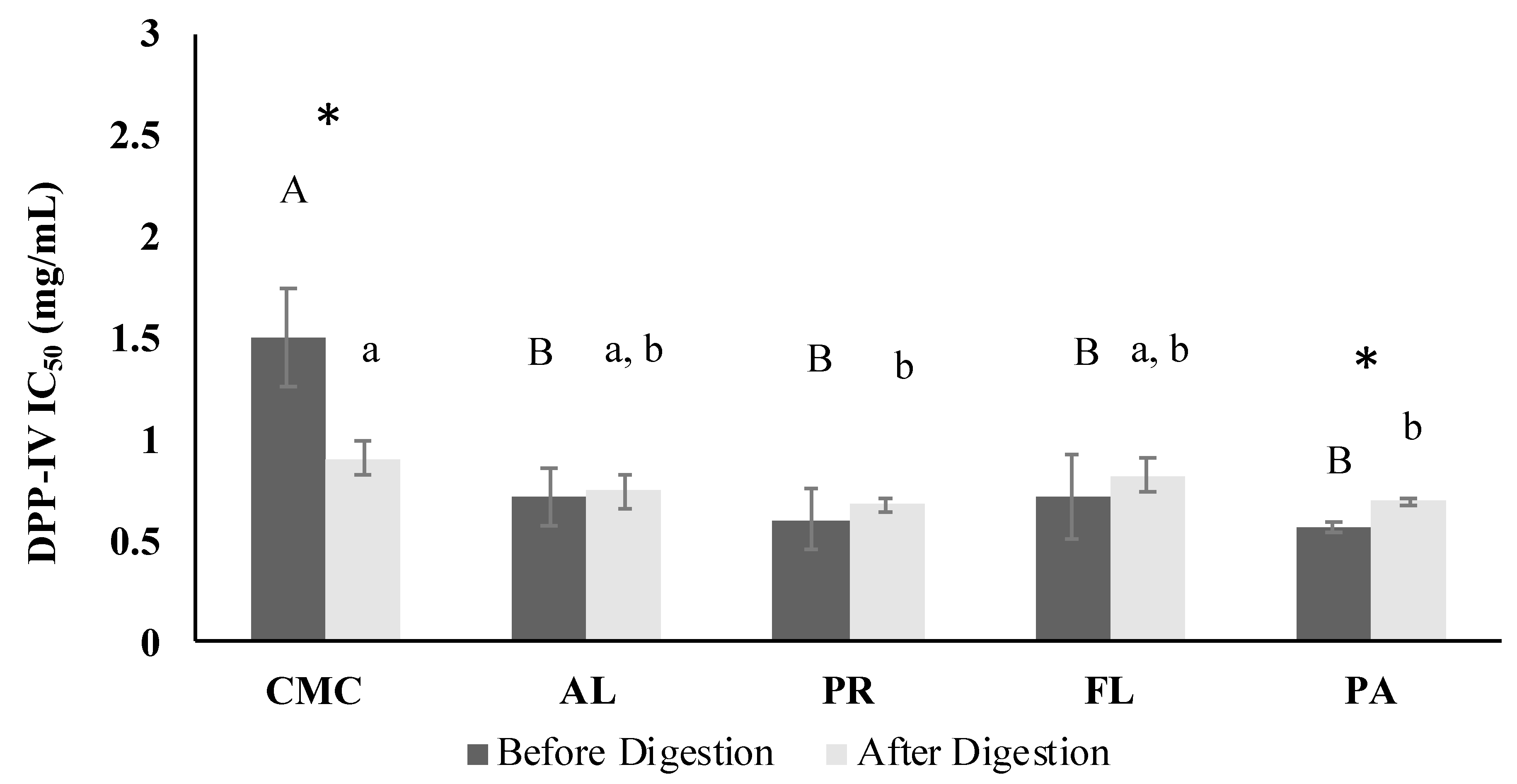
3.4. DPP-IV Inhibitory Activity
3.5. GLP-1 Secretory Activity
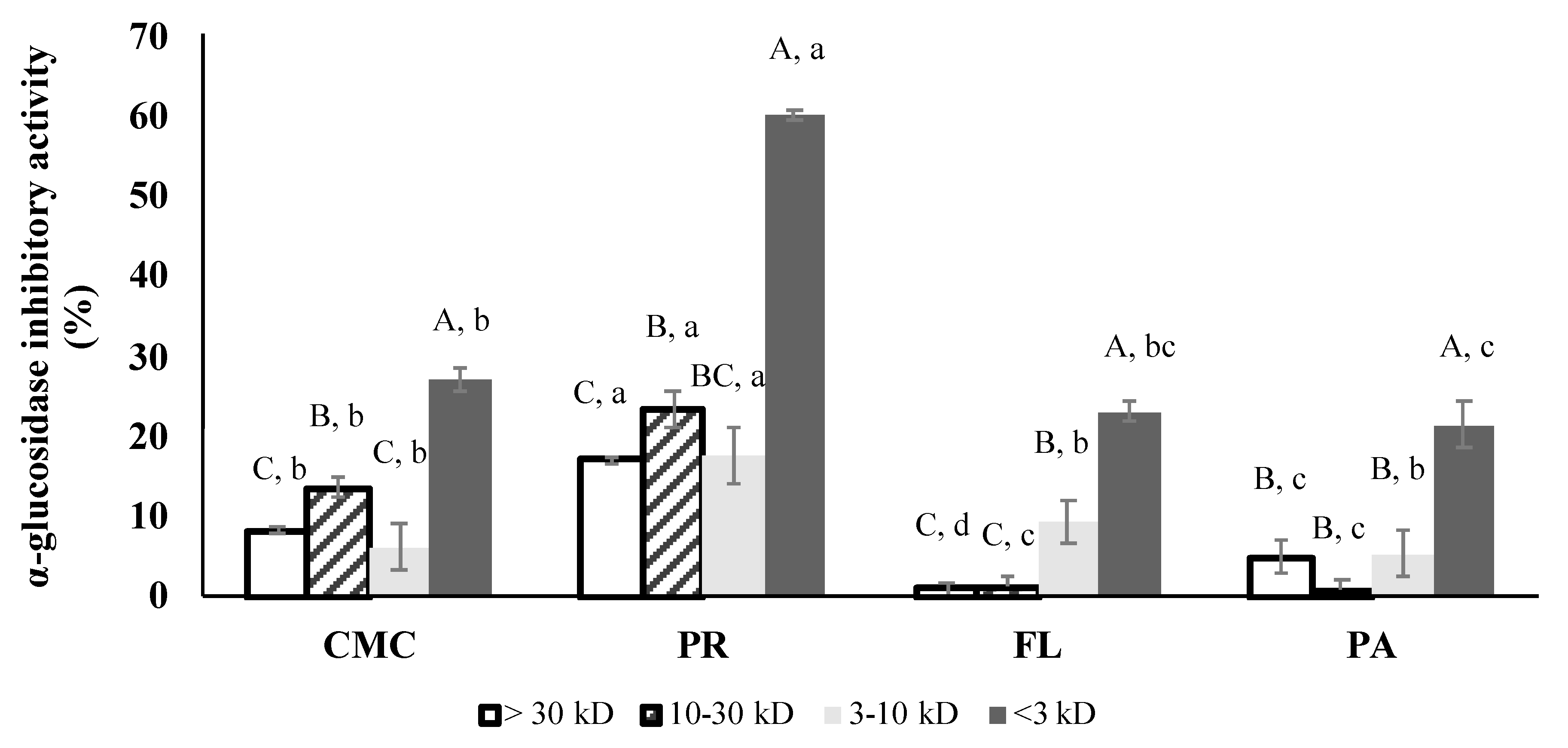
3.6. α-Glucosidase Inhibitory Activity of Fractions
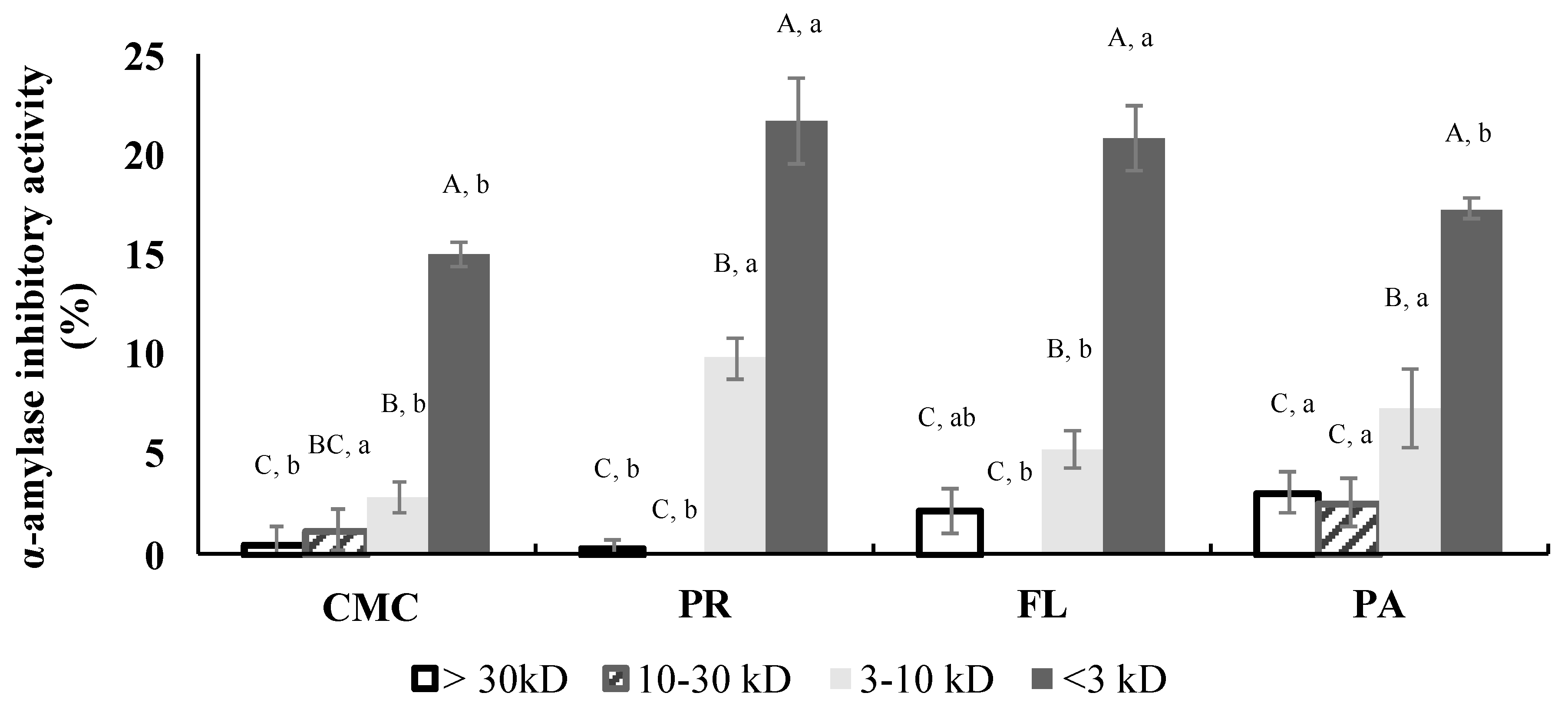
3.7. α-Amylase Inhibitory Activity of Fractions
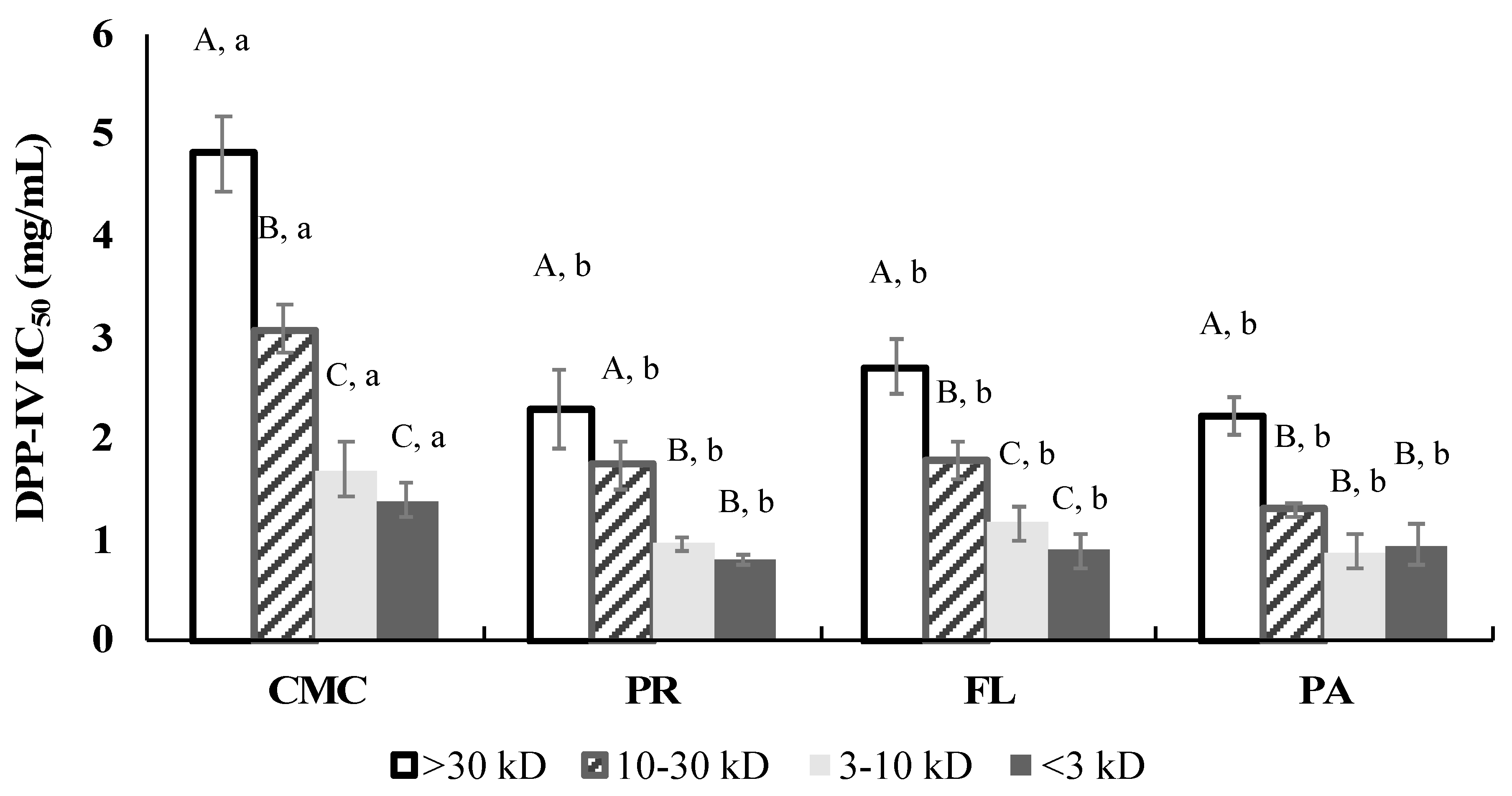
3.8. DPP-IV Inhibitory Activity of Fractions
3.9. GLP-1 Secretory Activity of Fractions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suleria, H.A.R.; Osborne, S.; Masci, P.; Gobe, G. Marine-based nutraceuticals: An innovative trend in the food and supplement industries. Mar. Drugs 2015, 13, 6336–6351. [Google Scholar] [CrossRef]
- Kim, S.K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Leignel, V.; Stillman, J.H.; Baringou, S.; Thabet, R.; Metais, I. Overview on the European green crab Carcinus spp. (Portunidae, Decapoda), one of the most famous marine invaders and ecotoxicological models. Env. Sci. Pollut. Res. 2014, 21, 9129–9144. [Google Scholar] [CrossRef]
- Sigurdsson, G.M.; Rochette, R. Predation by green crab and sand shrimp on settling and recently settled American lobster postlarvae. J. Crustac. Biol. 2013, 33, 10–14. [Google Scholar] [CrossRef]
- Malyshev, A.; Quijón, P.A. Disruption of essential habitat by a coastal invader: New evidence of the effects of green crabs on eelgrass beds. Ices. J. Mar. Sci. 2011, 68, 1852–1856. [Google Scholar] [CrossRef]
- Grosholz, E.D.; Ruiz, G.M. Predicting the impact of introduced marine species: Lessons from the multiple invasions of the European green crab Carcinus maenas. Biol. Conserv. 1996, 78, 59–66. [Google Scholar] [CrossRef]
- Kang, B.; Myracle, A.D.; Skonberg, D.I. Potential of recovered proteins from invasive green crabs (Carcinus maenas) as a functional food ingredient. J. Sci. Food Agric 2019, 99, 1748–1754. [Google Scholar] [CrossRef]
- Galetti, J.A.; Calder, B.L.; Skonberg, D.I. Mechanical separation of green crab (carcinus maenas) meat and consumer acceptability of a value-added food product. J. Aquat. Food Prod. T. 2017, 26, 172–180. [Google Scholar] [CrossRef]
- Naczk, M.; Williams, J.; Brennan, K.; Liyanapathirana, C.; Shahidi, F. Compositional characteristics of green crab (Carcinus maenas). Food Chem. 2004, 88, 429–434. [Google Scholar] [CrossRef]
- Skonberg, D.I.; Perkins, B.L. Nutrient composition of green crab (Carcinus maenus) leg meat and claw meat. Food Chem. 2002, 77, 401–404. [Google Scholar] [CrossRef]
- Baharuddin, N.A.; Halim, N.R.A.; Sarbon, N.M. Effect of degree of hydrolysis (DH) on the functional properties and angiotensin I-converting enzyme (ACE) inhibitory activity of eel (Monopterus sp.) protein hydrolysate. Int. Food Res. J. 2016, 23, 1424–1431. [Google Scholar]
- Intarasirisawat, R.; Benjakul, S.; Visessanguan, W.; Wu, J. Antioxidative and functional properties of protein hydrolysate from defatted skipjack (Katsuwonous pelamis) roe. Food Chem. 2012, 135, 3039–3048. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Ndiaye, F.; Vuong, T.; Duarte, J.; Aluko, R.E.; Matar, C. Anti-oxidant, anti-inflammatory and immunomodulating properties of an enzymatic protein hydrolysate from yellow field pea seeds. Eur. J. Nutr. 2012, 51, 29–37. [Google Scholar] [CrossRef]
- Pan, D.; Cao, J.; Guo, H.; Zhao, B. Studies on purification and the molecular mechanism of a novel ACE inhibitory peptide from whey protein hydrolysate. Food Chem. 2012, 130, 121–126. [Google Scholar] [CrossRef]
- Slizyte, R.; Rommi, K.; Mozuraityte, R.; Eck, P.; Five, K.; Rustad, T. Bioactivities of fish protein hydrolysates from defatted salmon backbones. Biotechnol. Rep. 2016, 11, 99–109. [Google Scholar] [CrossRef]
- Ahn, C.B.; Je, J.Y.; Cho, Y.S. Antioxidant and anti-inflammatory peptide fraction from salmon byproduct protein hydrolysates by peptic hydrolysis. Food Res. Int. 2012, 49, 92–98. [Google Scholar] [CrossRef]
- Ahn, C.B.; Jeon, Y.J.; Kim, Y.T.; Je, J.Y. Angiotensin I converting enzyme (ACE) inhibitory peptides from salmon byproduct protein hydrolysate by Alcalase hydrolysis. Process Biochem. 2012, 47, 2240–2245. [Google Scholar] [CrossRef]
- Ovissipour, M.; Rasco, B.; Shiroodi, S.G.; Modanlow, M.; Gholami, S.; Nemati, M. Antioxidant activity of protein hydrolysates from whole anchovy sprat (Clupeonella engrauliformis) prepared using endogenous enzymes and commercial proteases. J. Sci. Food Agric. 2013, 93, 1718–1726. [Google Scholar] [CrossRef]
- Liu, F.R.; Wang, L.; Wang, R.; Chen, Z.X. Calcium-binding capacity of wheat germ protein hydrolysate and characterization of peptide–calcium complex. J. Agr. Food Chem. 2013, 61, 7537–7544. [Google Scholar] [CrossRef]
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 10 January 2020).
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/chronicdisease/about/costs/index.htm (accessed on 10 January 2020).
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Steyn, N.P.; Mann, J.; Bennett, P.H.; Temple, N.; Zimmet, P.; Tuomilehto, J.; Louheranta, A. Diet, nutrition and the prevention of type 2 diabetes. Public Health Nut. 2004, 7, 147–165. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. Costs associated with the primary prevention of type 2 diabetes mellitus in the diabetes prevention program. Diabetes Care 2003, 26, 36–47. [Google Scholar] [CrossRef] [PubMed]
- de Campos Zani, S.C.; Wu, J.; Chan, C.B. Egg and soy-derived peptides and hydrolysates: A review of their physiological actions against diabetes and obesity. Nutrients 2018, 10, 549. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.; Thibodeau, J.; Bryl, P.; Carbonneau, M.É. Characterization of enzymatic hydrolyzed snow crab (Chionoecetes opilio) by-product fractions: A source of high-valued biomolecules. Bioresour. Technol. 2009, 100, 3332–3342. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Karayannakidis, P.D.; Apostolidis, E.; Lee, C.M. Comparison of direct steam injection and steam-jacketed heating in squid protein hydrolysis for energy consumption and hydrolysis performance. Lwt Food Sci. Technol. 2014, 57, 134–140. [Google Scholar] [CrossRef]
- González-Montoya, M.; Hernández-Ledesma, B.; Silván, J.M.; Mora-Escobedo, R.; Martínez-Villaluenga, C. Peptides derived from in vitro gastrointestinal digestion of germinated soybean proteins inhibit human colon cancer cells proliferation and inflammation. Food Chem. 2018, 242, 75–82. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680. [Google Scholar] [CrossRef]
- Worthington, V. Maltase-α-glucosidase. In Worthington Enzyme Manual, 1st ed.; Worthington Biochemical Corp: Freehold, NJ, USA, 1993; p. 261. [Google Scholar]
- Kwon, Y.I.; Apostolidis, E.; Kim, Y.C.; Shetty, K. Health benefits of traditional corn, beans, and pumpkin: In vitro studies for hyperglycemia and hypertension management. J. Med. Food. 2007, 10, 266–275. [Google Scholar] [CrossRef]
- Worthington, V. α-amylase. In Worthington Enzyme Manual, 1st ed.; Worthington Biochemical Corp: Freehold, NJ, USA, 1993; pp. 36–41. [Google Scholar]
- Li-Chan, E.C.; Hunag, S.L.; Jao, C.L.; Ho, K.P.; Hsu, K.C. Peptides derived from Atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J. Agric. Food. Chem. 2012, 60, 973–978. [Google Scholar] [CrossRef]
- Ojo, O.O.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Frog skin peptides (tigerinin-1R, magainin-AM1,-AM2, CPF-AM1, and PGla-AM1) stimulate secretion of glucagon-like peptide 1 (GLP-1) by GLUTag cells. Biochem. Biophys. Res. Commun. 2013, 431, 14–18. [Google Scholar] [CrossRef]
- Chakka, A.K.; Elias, M.; Jini, R.; Sakhare, P.Z.; Bhaskar, N. In-vitro antioxidant and antibacterial properties of fermentatively and enzymatically prepared chicken liver protein hydrolysates. J. Food Sci. Technol. 2015, 52, 8059–8067. [Google Scholar] [CrossRef]
- Lacroix, I.M.; Li-Chan, E.C. Inhibition of dipeptidyl peptidase (DPP)-IV and α-glucosidase activities by pepsin-treated whey proteins. J. Agric. Food. Chem. 2013, 61, 7500–7506. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.P.M.; FitzGerald, R.J. Blue whiting (Micromesistius poutassou) muscle protein hydrolysate with in vitro and in vivo antidiabetic properties. J. Funct. Foods. 2018, 40, 137–145. [Google Scholar] [CrossRef]
- Jamdar, S.N.; Rajalakshmi, V.; Pednekar, M.D.; Juan, F.; Yardi, V.; Sharma, A. Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem. 2010, 121, 178–184. [Google Scholar] [CrossRef]
- Smyth, M.; FitzGerald, R.J. Relationship between some characteristics of WPC hydrolysates and the enzyme complement in commercially available proteinase preparations. Int. Dairy J. 1998, 8, 819–827. [Google Scholar] [CrossRef]
- Aspmo, S.I.; Horn, S.J.; Eijsink, V.G. Enzymatic hydrolysis of Atlantic cod (Gadus morhua L.) viscera. Process Biochem. 2005, 40, 1957–1966. [Google Scholar] [CrossRef]
- Hirsh, A.J.; Yao, S.Y.; Young, J.D. Inhibition of glucose absorption in the rat jejunum: A novel action of alpha-D-glucosidase inhibitors. Gastroenterology 1997, 113, 205–211. [Google Scholar] [CrossRef]
- Matsui, T.; Oki, T.; Osajima, Y. Isolation and identification of peptidic α -glucosidase inhibitors derived from sardine muscle hydrolyzate. Z. Nat. C 1999, 54, 259–263. [Google Scholar] [CrossRef]
- Yoon, S.; Wong, N.A.; Chae, M.; Auh, J.H. Comparative characterization of protein hydrolysates from three edible insects: Mealworm larvae, adult crickets, and silkworm pupae. Foods 2019, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Ambigaipalan, P.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities of date seed protein hydrolysates prepared using Alcalase, Flavourzyme and Thermolysin. J. Funct. Foods 2015, 18, 1125–1137. [Google Scholar] [CrossRef]
- Chiasson, J.L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M. STOP-NIDDM Trial Research Group. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet 2002, 359, 2072–2077. [Google Scholar] [CrossRef]
- Bharatham, K.; Bharatham, N.; Park, K.H.; Lee, K.W. Binding mode analyses and pharmacophore model development for sulfonamide chalcone derivatives, a new class of α-glucosidase inhibitors. J. Mol. Graph. Model. 2008, 26, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Addison, D.; Aguilar, D. Diabetes and cardiovascular disease: Thepotential benefit of incretin-based therapies. Curr. Atheroscler. Rep. 2011, 13, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Nguyen, C.; Callebaut, C.; Jacotot, E.; Krust, B.; Mazaleyrat, J.P.; Wakselman, M.; Hovanessian, A.G. Dipeptidyl-peptidase IV-b. Further characterization and comparison to dipeptidyl- peptidase IV activity of CD26. Eur. J. Biochem. 1998, 256, 369–378. [Google Scholar] [CrossRef]
- Hildebrandt, M.; Reutter, W.; Arck, P.; Rose, M.; Klapp, B.F. A guardian angel: The involvement of dipeptidyl peptidase IV in psychoneuroendocrine function, nutrition and immune defence. Clin. Sci. 2000, 99, 93–104. [Google Scholar] [CrossRef]
- Lacroix, I.M.; Li-Chan, E.C. Evaluation of the potential of dietary proteins as precursors of dipeptidyl peptidase (DPP)-IV inhibitors by an in silico approach. J. Funct. Foods 2012, 4, 403–422. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. An in silico model to predict the potential of dietary proteins as sources of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides. Food Chem. 2014, 165, 489–498. [Google Scholar] [CrossRef]
- Deacon, C.F.; Nauck, M.A.; Toft-Nielsen, M.; Pridal, L.; Willms, B.; Holst, J.J. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 1995, 44, 1126–1131. [Google Scholar] [CrossRef]
- Reimann, F.; Williams, L.; da Silva Xavier, G.; Rutter, G.A.; Gribble, F.M. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia 2004, 47, 1592–1601. [Google Scholar] [CrossRef] [PubMed]
- Reimann, F.; Ward, P.S.; Gribble, F.M. Signaling mechanisms underlying the release of glucagon-like peptide 1. Diabetes 2006, 55, S78–S85. [Google Scholar] [CrossRef]
- Reimer, R.A. Meat hydrolysate and essential amino acid-induced glucagon-like peptide-1 secretion, in the human NCI-H716 enteroendocrine cell line, is regulated by extracellular signal-regulated kinase1/2 and p38 mitogen-activated protein kinases. J. Endocrinol. 2006, 191, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, A.; Reimann, F.; Habib, A.M.; O’malley, D.; Williams, L.; Simpson, A.K.; Gribble, F.M. The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line. J. Physiol. 2005, 569, 761–772. [Google Scholar] [CrossRef]
- Tolhurst, G.; Zheng, Y.; Parker, H.E.; Habib, A.M.; Reimann, F.; Gribble, F.M. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology 2011, 152, 405–413. [Google Scholar] [CrossRef]
- Diakogiannaki, E.; Pais, R.; Tolhurst, G.; Parker, H.E.; Horscroft, J.; Rauscher, B.; Zietek, T.; Daniel, H.; Gribble, F.M.; Reimann, F. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia 2013, 56, 2688–2696. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, B.; Skonberg, D.I.; Myracle, A.D. Anti-Hyperglycemic Effects of Green Crab Hydrolysates Derived by Commercially Available Enzymes. Foods 2020, 9, 258. https://doi.org/10.3390/foods9030258
Kang B, Skonberg DI, Myracle AD. Anti-Hyperglycemic Effects of Green Crab Hydrolysates Derived by Commercially Available Enzymes. Foods. 2020; 9(3):258. https://doi.org/10.3390/foods9030258
Chicago/Turabian StyleKang, Bouhee, Denise I. Skonberg, and Angela D. Myracle. 2020. "Anti-Hyperglycemic Effects of Green Crab Hydrolysates Derived by Commercially Available Enzymes" Foods 9, no. 3: 258. https://doi.org/10.3390/foods9030258
APA StyleKang, B., Skonberg, D. I., & Myracle, A. D. (2020). Anti-Hyperglycemic Effects of Green Crab Hydrolysates Derived by Commercially Available Enzymes. Foods, 9(3), 258. https://doi.org/10.3390/foods9030258



