The Influence of Millet Flour on Antioxidant, Anti-ACE, and Anti-Microbial Activities of Wheat Wafers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Wafers
2.3. Nutrient Compounds
2.3.1. Preparation of Extracts
2.3.2. Protein Content in the Extracts
2.3.3. Peptide Content in the Extracts
2.3.4. Total Phenolic Content in the Extracts
2.3.5. Reducing Sugar Content in the Extracts
2.4. Texture
2.5. Colour
2.6. Functional Properties
2.6.1. Water Absorption Capacity (WAC)
2.6.2. Oil Absorption Capacity (OAC)
2.7. Nutrients Compounds
2.7.1. Extract Preparation
2.7.2. Protein Content of Extract
2.7.3. Peptides Content of Extract
2.7.4. Total Phenolic Content of Extract
2.7.5. The Reducing Sugar Content of Extract
2.8. In Vitro Hydrolysis
2.9. Antioxidant Activity
2.9.1. ABTS•+
2.9.2. DPPH•
2.9.3. Fe2+ Chelating Activity
2.10. ACE Inhibitory Activity Assay
2.11. Antimicrobial Properties
2.11.1. Determination of the Minimum Inhibitory Concentration (MIC)
2.11.2. Estimation of Biotoxicity Against L. Monocytogenes ATCC BBA-2660 Using Resazurin Reduction Assays
2.12. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gain, W.; Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular. J. Nutr. 2012, 142, 1304–1313. [Google Scholar]
- Alshahrani, S.M.; Fraser, G.E.; Sabat, J.; Knutsen, R.; Shavlik, D.; Mashchak, A.; Lloren, J.I.; Orlich, M.J. Intake population. Nutrients 2019, 2, 1–13. [Google Scholar]
- Kaur, R.; Sharma, M. Cereal polysaccharides as sources of functional ingredient for reformulation of meat products: A review. J. Funct. Foods 2019, 62, 103527. [Google Scholar] [CrossRef]
- Boada, L.D.; Henríquez-Hernández, L.A.; Luzardo, O.P. The impact of red and processed meat consumption on cancer and other health outcomes: Epidemiological evidences. Food Chem. Toxicol. 2016, 92, 236–244. [Google Scholar] [CrossRef]
- Setayesh-Mehr, Z.; Asoodeh, A. The inhibitory activity of HL-7 and HL-10 peptide from scorpion venom (Hemiscorpius lepturus) on angiotensin converting enzyme: Kinetic and docking study. Bioorg. Chem. 2017, 75, 30–37. [Google Scholar] [CrossRef]
- Balti, R.; Bougatef, A.; Sila, A.; Guillochon, D.; Dhulster, P.; Nedjar-Arroume, N. Nine novel angiotensin I-converting enzyme (ACE) inhibitory peptides from cuttlefish (Sepia officinalis) muscle protein hydrolysates and antihypertensive effect of the potent active peptide in spontaneously hypertensive rats. Food Chem. 2015, 170, 519–525. [Google Scholar] [CrossRef]
- Guang, C.; Phillips, R.D.; Jiang, B.; Milani, F. Three key proteases—Angiotensin-I-converting enzyme (ACE), ACE2 and renin—Within and beyond the renin-angiotensin system. Arch. Cardiovasc. Dis. 2012, 105, 373–385. [Google Scholar] [CrossRef]
- Forghani, B.; Zarei, M.; Ebrahimpour, A.; Philip, R.; Bakar, J.; Abdul Hamid, A.; Saari, N. Purification and characterization of angiotensin converting enzyme-inhibitory peptides derived from Stichopus horrens: Stability study against the ACE and inhibition kinetics. J. Funct. Foods 2016, 20, 276–290. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; De Caterina, R. Oxidative stress and vascular stiffness in hypertension: A renewed interest for antioxidant therapies? Vasc. Pharmacol. 2019, 116, 45–50. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Vidal, C.M.M.; Canniatti-Brazaca, S.G.; Shahidi, F. Fortification of cookies with peanut skins: Effects on the composition, polyphenols, antioxidant properties, and sensory quality. J. Agric. Food Chem. 2014, 62, 11228–11235. [Google Scholar] [CrossRef]
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Nawaz, H. Preparation of fiber and mineral enriched defatted rice bran supplemented cookies. Pak. J. Nutr. 2009, 8, 571–577. [Google Scholar] [CrossRef]
- Nasir, M.; Siddiq, M.; Ravi, R.; Harte, J.B.; Dolan, K.D.; Butt, M.S. Physical quality characteristics and sensory evaluation of cookies made with added defatted maize germ flour. J. Food Qual. 2010, 33, 72–84. [Google Scholar] [CrossRef]
- Sharma, B.; Gujral, H.S. Modulation in quality attributes of dough and starch digestibility of unleavened flat bread on replacing wheat flour with different minor millet flours. Int. J. Biol. Macromol. 2019, 141, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 2002, 27, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Oxidants and Antioxidants—Part A: Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Khattab, R.Y.; Arntfield, S.D. Functional properties of raw and processed canola meal. LWT-Food Sci. Technol. 2009, 42, 1119–1124. [Google Scholar] [CrossRef]
- Durak, A.; Baraniak, B.; Jakubczyk, A.; Świeca, M. Biologically active peptides obtained by enzymatic hydrolysis of Adzuki bean seeds. Food Chem. 2013, 141, 2177–2183. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved Abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Osaka, I.; Hefty, P.S. Simple resazurin-based microplate assay for measuring chlamydia. Antimicrob. Agents Chemother. 2013, 57, 2838–2840. [Google Scholar] [CrossRef] [PubMed]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Krystyna, J.; Sławomir, L.; Urszula, Z. Different temperature treatments of millet grains affect the biological activity of protein hydrolyzates. Nutrients 2019, 11, 550. [Google Scholar] [CrossRef] [PubMed]
- Giuberti, G.; Rocchetti, G.; Sigolo, S.; Fortunati, P.; Lucini, L.; Gallo, A. Exploitation of alfalfa seed (Medicago sativa L.) flour into gluten-free rice cookies: Nutritional, antioxidant and quality characteristics. Food Chem. 2018, 239, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Shafi, M.; Baba, W.N.; Ahmad, F.; Rafiya, M. Wheat-water chestnut flour blends: Effect of baking on antioxidant properties of cookies. J. Food Sci. Technol. 2016, 53, 4278–4288. [Google Scholar] [CrossRef] [PubMed]
- Złotek, U.; Szymanowska, U.; Jakubczyk, A.; Świeca, M. Effect of arachidonic and jasmonic acid elicitation on the content of phenolic compounds and antioxidant and anti-inflammatory properties of wheatgrass (Triticum aestivum L.). Food Chem. 2019, 288, 256–261. [Google Scholar]
- Jiménez, C.; Caleja, C.; Prieto, M.A.; Sokovic, M.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Stability of a cyanidin-3-O-glucoside extract obtained from Arbutus unedo L. and incorporation into wafers for colouring purposes. Food Chem. 2019, 275, 426–438. [Google Scholar]
- Szymanowska, U.; Baraniak, B. Antioxidant and potentially anti-inflammatory activity of anthocyanin fractions from pomace obtained from enzymatically treated raspberries. Antioxidants 2019, 8, 299. [Google Scholar] [CrossRef]
- Auwal, M.; Bester, M.J.; Neitz, A.W.; Gaspar, A.R.M. Biomedicine & Pharmacotherapy Rational in silico design of novel α-glucosidase inhibitory peptides and in vitro evaluation of promising candidates. Biomed. Pharm. 2018, 107, 234–242. [Google Scholar]
- Sreerama, Y.N.; Sasikala, V.B.; Pratape, V.M. Nutritional implications and flour functionality of popped/expanded horse gram. Food Chem. 2008, 108, 891–899. [Google Scholar] [CrossRef]
- Patricia, S.; Acuña, C.; Humberto, J.; González, G.; Darío, I.; Torres, A. Physicochemical characteristics and functional properties of vitabosa (mucuna deeringiana) and soybean (glycine max). Food Sci. Technol. 2012, 32, 98–105. [Google Scholar]
- Baumgartner, B.; Ozkaya, B.; Saka, I.; Ozkaya, H. Functional and physical properties of cookies enriched with dephytinized oat bran. J. Cereal Sci. 2018, 80, 24–30. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, P.; Kumar, V.; Panghal, A.; Kaur, J.; Gat, Y. Effect of addition of flaxseed flour on phytochemical, physicochemical, nutritional, and textural properties of cookies. J. Saudi Soc. Agric. Sci. 2019, 18, 372–377. [Google Scholar] [CrossRef]
- Ghaboos, H.; Ardabili, S. Physico-chemical, textural and sensory evaluation of sponge cake supplemented with pumpkin flour. Int. Food Res. J. 2018, 25, 854–860. [Google Scholar]
- Ghanbari, S.; Nader, H. Study of texture and color of the sponge cake. Indian J. Fundam. Appl. Life Sci. 2015, 5, 1084–1089. [Google Scholar]
- Chalamaiah, M.; Keskin, S.; Hong, H.; Wu, J. Regulatory requirements of bioactive peptides (protein hydrolysates) from food proteins. J. Funct. Foods 2019, 58, 123–129. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Karaś, M.; Złotek, U.; Szymanowska, U. Identification of potential inhibitory peptides of enzymes involved in the metabolic syndrome obtained by simulated gastrointestinal digestion of fermented bean (Phaseolus vulgaris L.) seeds. Food Res. Int. 2017, 100, 489–496. [Google Scholar] [CrossRef]
- Lao, F.; Sigurdson, G.T. Health Benefits of Purple Corn (Zea mays L.) Phenolic Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 234–246. [Google Scholar] [CrossRef]
- Giacco, R.; Costabile, G.; Fatati, G.; Frittitta, L. Effects of polyphenols on cardio-metabolic risk factors and risk of type 2 diabetes. A joint position statement of the diabetes and nutrition study group of the Italian Society of Diabetology (SID), the Italian association of dietetics and clinical nut. Nutr. Metab. Cardiovasc. Dis. 2019, in press. [Google Scholar] [CrossRef]
- Hao, M.; Beta, T. Development of Chinese steamed bread enriched in bioactive compounds from barley hull and flaxseed hull extracts. Food Chem. 2012, 133, 1320–1325. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Sanda, V.; Braicu, C.; Socaciu, C. Antioxidant/prooxidant activity of a polyphenolic grape seed extract. Food Chem. 2010, 121, 132–139. [Google Scholar]
- Dandona, P.; Aljada, A.; Chaudhuri, A. Metabolic syndrome a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhong, F.; Go, H.D.; Li, Y. Study on starch-protein interactions and their effects on physicochemical and digestible properties of the blends. Food Chem. 2019, 280, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Świeca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Czy, J. The influence of protein—Flavonoid interactions on protein digestibility in vitro and the antioxidant quality of breads enriched with onion skin. Food Chem. 2013, 141, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Sęczyk, Ł.; Świeca, M.; Gawlik-Dziki, U. Soymilk enriched with green coffee phenolics—Antioxidant and nutritional properties in the light of phenolics-food matrix interactions. Food Chem. 2017, 223, 1–7. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Jridi, M.; Jemil, I.; Mora, L.; Toldrá, F.; Aristoy, M.; Boualga, A.; Nasri, M.; Nasri, R. Combined biocatalytic conversion of smooth hound viscera: Protein hydrolysates elaboration and assessment of their antioxidant, anti-ACE and antibacterial activities. Food Res. Int. 2016, 86, 9–23. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I.; Hernández-Ledesma, B. Antihypertensive peptides from food proteins: A review food & function antihypertensive peptides from food proteins: A review. Food Funct. 2012, 4, 1–12. [Google Scholar]
- Oliver-salvador, M.D.E.L.C.; Ariza-ortega, T.D.J.; Zenón-briones, Y.; Luis, J. Angiotensin-I-converting enzyme inhibitory, antimicrobial, and antioxidant effect of bioactive peptides obtained from different varieties of common beans (Phaseolus vulgaris L.) with in vivo antihypertensive activity in spontaneously hypertensive rats. Eur. Food Res. Technol. 2014, 239, 785–794. [Google Scholar]
- Salas, C.E.; Badillo-corona, J.A.; Ramírez-sotelo, G.; Oliver-salvador, C. Biologically active and antimicrobial peptides from plants. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kaen, K. Screening antimicrobial activity against pathogens from protein hydrolysate of rice bran and Nile Tilapia by-products. Int. Food Res. J. 2018, 25, 2157–2163. [Google Scholar]

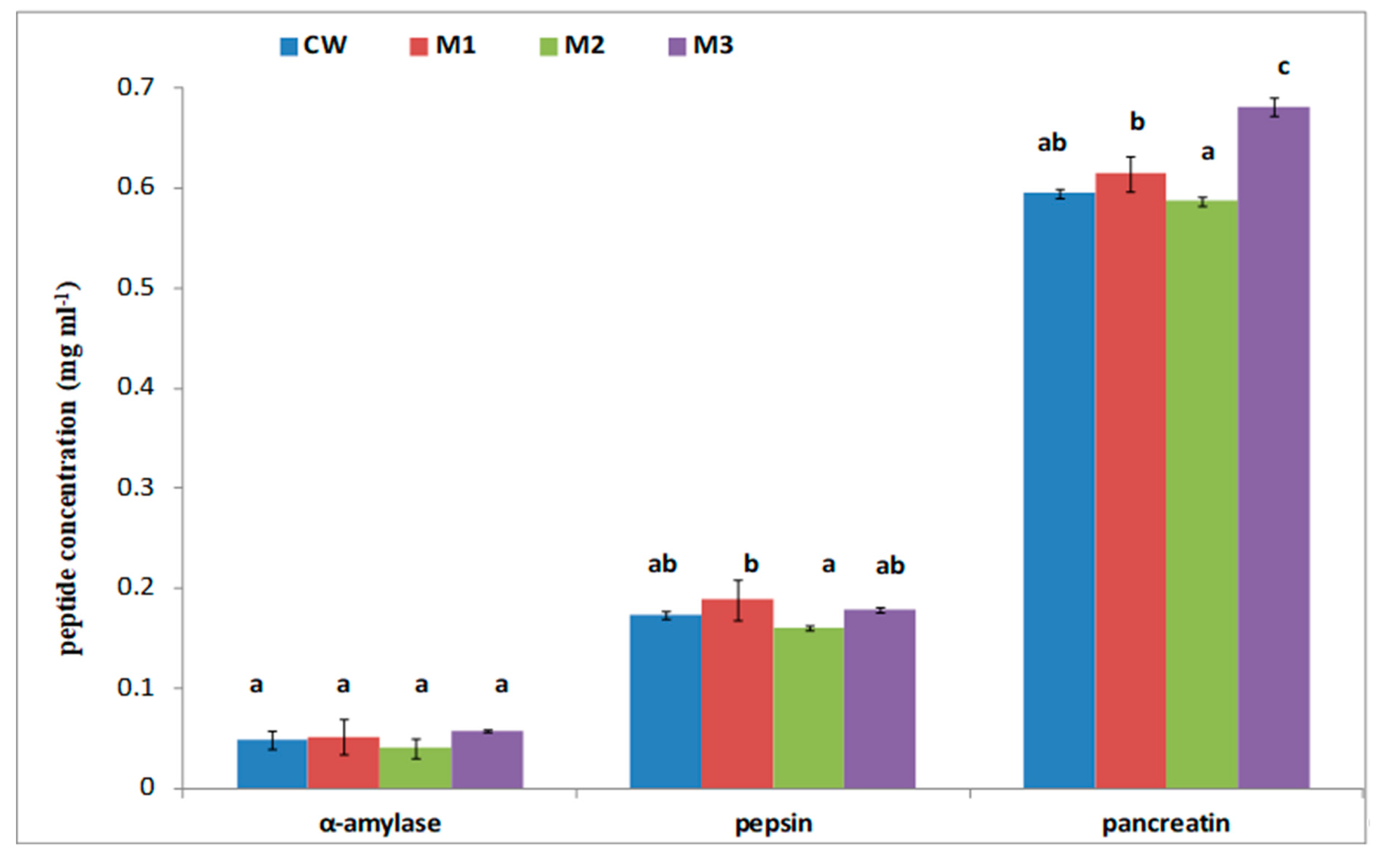
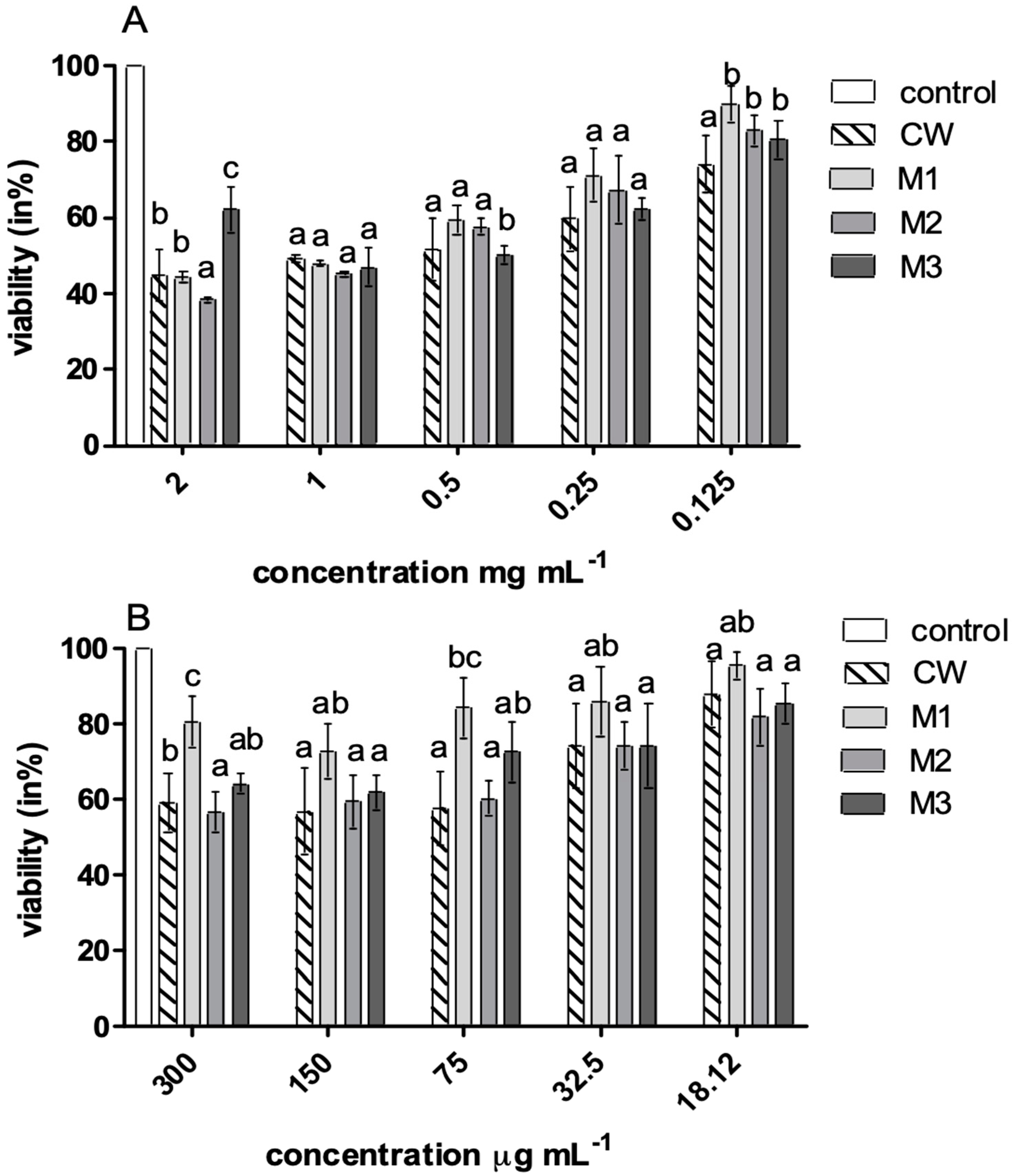
| Color | Rheological Properties | ||||||
|---|---|---|---|---|---|---|---|
| Sample | L* | a* | b* | ΔTC | Hardness (g) | Fracturability (mm) | Puncture Strength (N/mm2) |
| CW | 61.92 ± 5.36 a | 8.77 ± 1.04 a | 23.75 ± 1.95 a | - | 911.02 ± 108.53 a | 68.35 ± 0.39 a | 2.63 ± 0.32 a |
| M1 | 64.80 ± 2.92 a | 8.53 ± 1.02 a | 23.93 ± 1.96 a | 2.89 ± 0.14 a | 870.26 ± 140.58 a | 67.60 ± 0.18 b | 2.16 ± 0.42 a |
| M2 | 64.84 ± 1.87 a | 8.76 ± 1.27 a | 25.04 ± 1.46 a | 3.19 ± 0.08 b | 862.85 ± 119.84 a | 67.49 ± 0.63 b | 2.47 ± 0.60 a |
| M3 | 65.96 ± 1.83 a | 7.30 ± 1.55 a | 23.22 ± 2.51 a | 4.33 ± 0.06 c | 856.68 ± 139.26 a | 67.06 ± 0.42 b | 3.7 ± 0.90 a |
| Sample | Bioactive Compound Content | Functional Properties | |||||
|---|---|---|---|---|---|---|---|
| Protein (mg mL−1) | Polyphenols (mg mL−1) | Flavonoids (mg mL−1) | Phenolic Acids (µg mL−1) | Reducing Sugars (mg mL−1) | WAC (%) | OAC (%) | |
| CW | 0.69 ± 0.017 a | 0.015 ± 0.002 a | 0.85 ± 0.07 a | 0.13 ± 0.004 a | 0.59 ± 0.004 a | 631.08 ± 14.54 a | 38.43 ± 1.44 a |
| M1 | 0.75 ± 0.008 b | 0.019 ± 0.003 b | 1.88 ± 0.012 b | 0.14 ± 0.002 b | 0.61 ± 0.003 b | 644.92 ± 18.36 a | 39.12 ± 1.48 a b |
| M2 | 0.99 ± 0.008 c | 0.020 ± 0.002 c | 1.96 ± 0.013 c | 0.16 ± 0.003 c | 0.62 ± 0.002 b c | 714.77 ± 20.38 b | 41.34 ± 1.24 a b |
| M3 | 1.03 ± 0.012 d | 0.021 ± 0.003 d | 2.26 ± 0.010 d | 0.17 ± 0.004 d | 0.63 ± 0.008 c | 803.91 ± 28.71 c | 42.79 ± 1.86 b |
| Sample | Properties (IC50 ug mL−1) | Antibacterial Activity (L. monocytogenes ATCC BBA-2660) | |||
|---|---|---|---|---|---|
| ABTS·+ | DPPH· | Fe2+ Chelation | ACE | MIC | |
| Hyrolysates | |||||
| CW | 67.22 ± 1.78 a | 404.26 ± 3.18 a | 0.58 ± 0.03 a | 163.93 ± 1.25 a | 0.25 * |
| M 1 | 80.08 ± 1.99 b | 287.23 ± 2.41 b | 0.62 ± 0.01 a | 205.76 ± 1.57 b | 0.50 * |
| M 2 | 101.54 ± 2.15 c | 198.09 ± 1.18 c | 0.49 ± 0.01 b | 155.76 ± 2.08 c | 0.50 * |
| M 3 | 113.34 ± 1.06 d | 191.04 ± 2.83 d | 0.46 ± 0.02 b | 157.73 ± 1.69 c | 0.50 * |
| Fractions <3.0 kDa | |||||
| CW | 26.67 ± 0.73 a | 31.71 ± 0.98 a | 0.32 ± 0.01 ab | 21.11 ± 0.77 a | 75 ** |
| M 1 | 62.58 ± 1.13 b | 129.45 ± 1.01 b | 0.33 ± 0.02 ab | 24.17 ± 0.51 b | - |
| M 2 | 39.42 ± 1.08 c | 227.01 ± 1.58 c | 0.31 ± 0.02 a | 15.67 ± 1.03 c | 75 ** |
| M 3 | 41.50 ± 2.014 d | 3.46 ± 0.14 d | 0.26 ± 0.01 c | 16.27 ± 0.89 c | 150 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubczyk, A.; Ćwiek, P.; Rybczyńska-Tkaczyk, K.; Gawlik-Dziki, U.; Złotek, U. The Influence of Millet Flour on Antioxidant, Anti-ACE, and Anti-Microbial Activities of Wheat Wafers. Foods 2020, 9, 220. https://doi.org/10.3390/foods9020220
Jakubczyk A, Ćwiek P, Rybczyńska-Tkaczyk K, Gawlik-Dziki U, Złotek U. The Influence of Millet Flour on Antioxidant, Anti-ACE, and Anti-Microbial Activities of Wheat Wafers. Foods. 2020; 9(2):220. https://doi.org/10.3390/foods9020220
Chicago/Turabian StyleJakubczyk, Anna, Paula Ćwiek, Kamila Rybczyńska-Tkaczyk, Urszula Gawlik-Dziki, and Urszula Złotek. 2020. "The Influence of Millet Flour on Antioxidant, Anti-ACE, and Anti-Microbial Activities of Wheat Wafers" Foods 9, no. 2: 220. https://doi.org/10.3390/foods9020220
APA StyleJakubczyk, A., Ćwiek, P., Rybczyńska-Tkaczyk, K., Gawlik-Dziki, U., & Złotek, U. (2020). The Influence of Millet Flour on Antioxidant, Anti-ACE, and Anti-Microbial Activities of Wheat Wafers. Foods, 9(2), 220. https://doi.org/10.3390/foods9020220






