Comparative Evaluation of Piper nigrum, Rosmarinus officinalis, Cymbopogon citratus and Juniperus communis L. Essential Oils of Different Origin as Functional Antimicrobials in Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Strains
2.2. Essential Oils
2.3. Analysis of Bioactive Compound of Investigated Essential Oils by Gas Chromatography (GC) Technique
2.4. Antibacterial Activity of Tested EOs
2.5. Estimation of Intracellular Metabolic Activity of P. Orientalis by Flow Cytometry
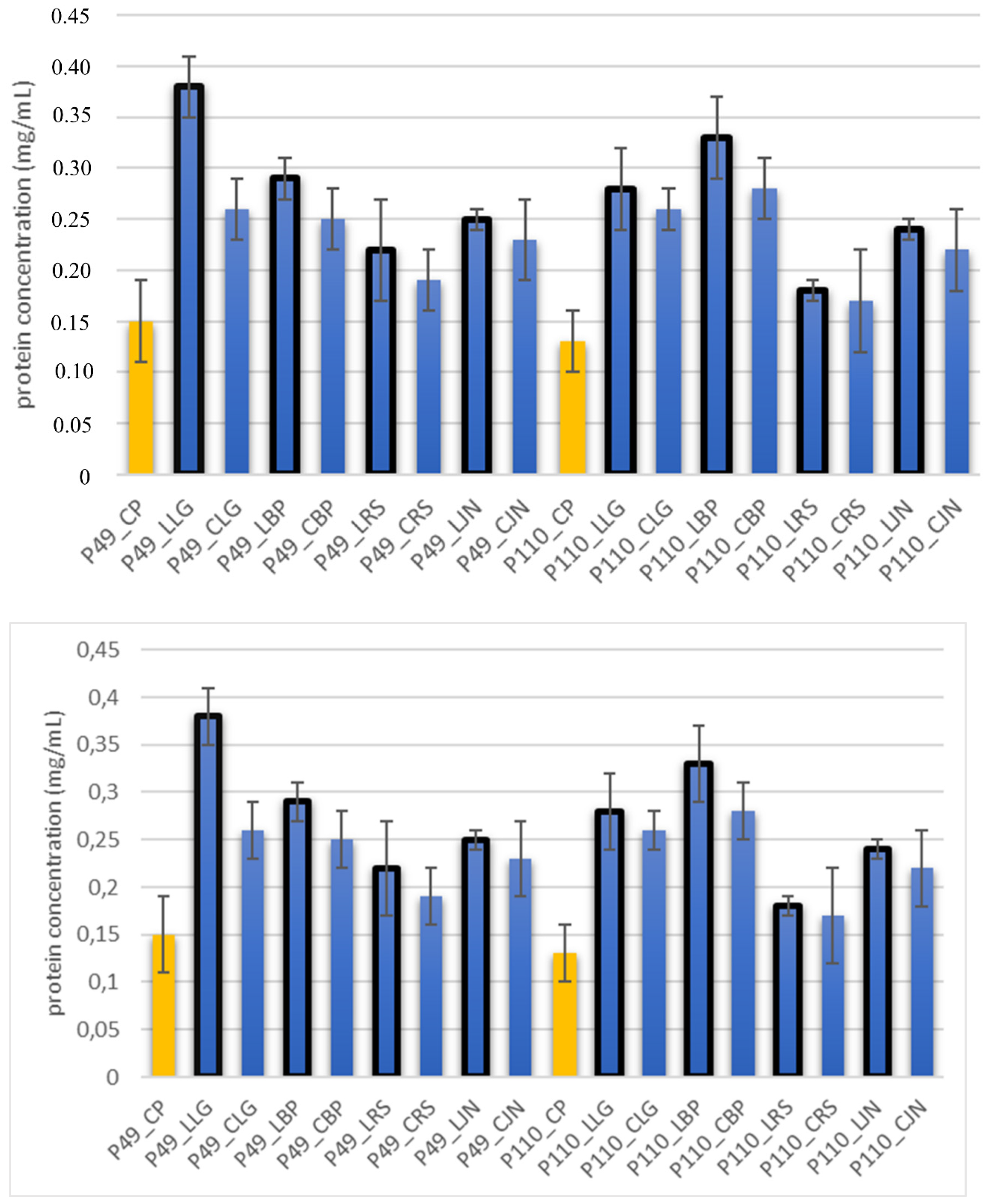
2.6. The Integrity of Bacteria Cell Membrane by Determining Protein Content by the BCA Method
2.7. Observation of the Shape of Bacterial Cells with the Inverted Microscope
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Friedman, M. Nutritional Value of Proteins from Different Food Sources. A Review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Olejnik, A.; Białas, W.; Rybicka, I.; Zielińska-Dawidziak, M.; Siger, A.; Kubiak, P.; Lewandowicz, G. The Nutritional Value and Biological Activity of Concentrated Protein Fraction of Potato Juice. Nutrients 2019, 11, 1523. [Google Scholar] [CrossRef]
- Jarzębski, M.; Fathordoobady, F.; Guo, Y.; Xu, M.; Singh, A.; Kitts, D.D.; Kowalczewski, P.Ł.; Jeżowski, P.; Singh, A.P. Pea Protein for Hempseed Oil Nanoemulsion Stabilization. Molecules 2019, 24, 4288. [Google Scholar] [CrossRef]
- Różańska, M.B.; Kowalczewski, P.Ł.; Tomaszewska-Gras, J.; Dwiecki, K.; Mildner-Szkudlarz, S. Seed-Roasting Process Affects Oxidative Stability of Cold-Pressed Oils. Antioxidants 2019, 8, 313. [Google Scholar] [CrossRef]
- Kujawska, M.; Olejnik, A.; Lewandowicz, G.; Kowalczewski, P.; Forjasz, R.; Jodynis-Liebert, J. Spray-Dried Potato Juice as a Potential Functional Food Component with Gastrointestinal Protective Effects. Nutrients 2018, 10, 259. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Olejnik, A.; Białas, W.; Kubiak, P.; Siger, A.; Nowicki, M.; Lewandowicz, G. Effect of Thermal Processing on Antioxidant Activity and Cytotoxicity of Waste Potato Juice. Open Life Sci. 2019, 14. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceutic 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Sutili, F.J.; Gatlin, D.M.; Heinzmann, B.M.; Baldisserotto, B. Plant essential oils as fish diet additives: benefits on fish health and stability in feed. Rev. Aquac. 2018, 10, 716–726. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. Antimicrobial activity of plant essential oils using food model media: Efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009, 26, 142–150. [Google Scholar] [CrossRef]
- Leja, K.; Szudera-Kończal, K.; Myszka, K.; Czaczyk, K. Antibacterial effect of natural oils—an opportunity to solve the problem of antibiotic resistance on the example of Pseudomonas spp. Advanc. Microbiol. 2019, 58, 177–190. [Google Scholar] [CrossRef]
- Hashimoto, G.S.O.; Marinho, N.F.; Ruiz, M.L.; Acchile, M.; Chagas, E.C.; Chaves, F.C.M. Essential oils of Lippia sidoides and Mentha piperita against monogenean parasites and their influence on the hematology of Nile tilapia. Aquaculture 2016, 450, 182–186. [Google Scholar] [CrossRef]
- Burt, S.A.; Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Contr. 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Zidan, Y.E.; Mansour, M.M.A.; El Hadidi, N.M.N.; Elgat, W.A.A. Antifungal activities of two essential oils used in the treatment of three commercial woods deteriorated by five common mold fungi. Int. Biodeterior. Biodegradation. 2016, 106, 88–96. [Google Scholar] [CrossRef]
- Orchard, A.; Van Vuuren, S.F.; Viljoen, A.M.; Kamatou, G. The in vitro antimicrobial evaluation of commercial essential oil sand their combinations against acne. Int. J. Cosmet. Sci. 2018, 40, 226–243. [Google Scholar] [CrossRef]
- Sacchetti, G.; Medici, A.; Maietti, S.; Radice, M.; Muzzoli, M.; Manfredini, S.; Braccioli, E.; Bruni, R. Composition and Functional Properties of the Essential Oil of Amazonian Basil, Ocimum micranthum Wild., Labiatae in Comparison with Commercial Essential Oils. J. Agric. Food Chem. 2004, 52, 3486–3491. [Google Scholar] [CrossRef]
- Racoti, A.; Buttress, A.J.; Binner, E.; Dodds, C.; Trifan, A.; Calinescu, I. Microwave assisted hydro-distillation of essential oils from fresh ginger root (Zingiber officinale Roscoe). J. Essent. Oil Res. 2017, 29, 471–480. [Google Scholar] [CrossRef]
- Leja, K.; Szudera-Kończal, K.; Świtała, E.; Juzwa, W.; Kowalczewski, P.Ł.; Czaczyk, K. The Influence of Selected Plant Essential Oils on Morphological and Physiological Characteristics in Pseudomonas Orientalis. Foods 2019, 8, 277. [Google Scholar] [CrossRef]
- Leja, K.; Drożdżyńska, A.; Majcher, M.; Kowalczewski, P.Ł.; Czaczyk, K. Influence of sub-inhibitory concentration of selected plant essential oils on physical and biochemical properties of Pseudomonas orientalis strains. Open Chem. 2019, 17, 492–505. [Google Scholar] [CrossRef]
- Ahmad, N.; Fazal, H.; Abbasi, B.H.; Farooq, S.; Ali, M. Biological role of Piper nigrum L. (Black pepper): A review. Asian Pac. J. Trop. Biomed. 2018, 2, 51–59. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012. [CrossRef]
- Myszka, K.; Leja, K.; Majcher, M. A current opinion on the antimicrobial importance of popular pepper essential oil and its application in food industry. J. Essent. Oil Res. 2019, 31. [Google Scholar] [CrossRef]
- Ganjewala, D.; Gupta, A.K.; Muhury, R. An Update on Bioactive Potential of a Monoterpene Aldehyde Citral. J. Biol. Act. Prod. Nat. 2012, 2, 186–199. [Google Scholar] [CrossRef]
- Turgis, M.; Dang, V.K.; Dupont, C.; Lacroix, M. Combined antimicrobial effect of essential oils and bacteriocins against foodborne pathogens and food spoilage bacteria. Food Res. Int. 2012, 48, 696–702. [Google Scholar] [CrossRef]
- Cassella, S.; Cassella, J.P.; Smith, I. Synergistic antifungal activity of tea tree (Melaleuca alternifolia) and lavender (Lavandula angustifolia) essential oils against dermatophyte infection. Int. J. Aromath. 2002, 12, 2–15. [Google Scholar] [CrossRef]
- Ncube, N.S.; Afolayan, A.J.; Okoh, A.I. Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. Afr. J. Biotechnol. 2008, 7, 1797–1806. [Google Scholar] [CrossRef]
- Sartorelli, P.; Marquioreto, A.D.; Amaral-Baroli, A.; Lima, M.E.L.; Moreno, P.R.H. Chemical composition and antimicrobial activity of the essential oils from two species of Eucalyptus. Phytother. Res. 2007, 21, 231–233. [Google Scholar] [CrossRef]
- Silva, F.; Ferreira, S.; Queiroz, J.A.; Domingues, F.C. Coriander (Coriandrum sativum L.) essential oil: its antibacterial activity and mode of action evaluated by flow cytometry. J. Med. Microbiol. 2011, 60, 1479–1486. [Google Scholar] [CrossRef]
- Moraes-Lovison, M.; Marostegan, L.F.P.; Peres, M.S.; Menezes, I.F.; Ghiraldi, M.; Rodrigues, R.A.F.; Fernandes, A.M.; Pinho, S.C. Nanoemulsions encapsulating oregano essential oil: Production, stability, antibacterial activity and incorporation in chicken pâté. LWT 2017, 77, 233–240. [Google Scholar] [CrossRef]
- Fakruddin, M.D.; Shahnewaj, K.; Mannan, B.; Andrews, S. Viable but Nonculturable Bacteria: Food Safety and Public Health Perspective. ISRN Microbiol. 2013, 2013, 703813. [Google Scholar] [CrossRef]
- Xu, J.G.; Liu, T.; Hu, Q.P.; Cao, X.M. Chemical Composition, Antibacterial Properties and Mechanism of Action of Essential Oil from Clove Buds against Staphylococcus aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S.Y. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Contr. 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Bouhdid, S.; Abrini, J.; Zhiri, A.; Espuny, M.J.; Manresa, A. Investigation of functional and morphological changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Origanum compactum essential oil. J. Appl. Microbiol. 2009, 106, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Contr. 2014, 35, 109–116. [Google Scholar] [CrossRef]

| EO | Commercial | Eco-Farm | ||
|---|---|---|---|---|
| Price (10 mL of Essential Oil) | Price (100 g of Plant Material) | Price (Plant Material For Obtaining 10 mL of Oil) | Efficiency of Distillation (%) | |
| BP | 13.36 EUR (57 PLN) | 0.82 EUR 3.50 PLN | 13.13 EUR (56 PLN) | 1.25 |
| RS | 3.28 EUR (14 PLN) | 0.70 EUR (3.00 PLN) | 9.38 EUR (40 PLN) | 0.95 |
| LG | 4.45 EUR (19 PLN) | (0.70 EUR) 3.00 PLN | 12.66 EUR (54 PLN) | 0.18 |
| JN | 4.22 EUR (18 PLN) | 0.82 EUR 3.50 PLN | 5.63 EUR (24 PLN) | 1.46 |
| Bioactive Compound/ Oil Type | RI-SLB-5 | CRS (%) | LRS (%) | CBP (%) | LBP (%) | CLG (%) | LLG (%) | CJN (%) | LJN (%) |
|---|---|---|---|---|---|---|---|---|---|
| α-thujene | 938 | nd | 0.5 | nd | 1.4 | nd | nd | nd | 1.9 |
| α-pinene | 939 | 18.0 | 18.4 | 8.0 | 9.1 | nd | nd | 1.0 | 33.1 |
| camphene | 953 | 2.0 | 2.8 | nd | 0.8 | nd | 0.5 | nd | 0.8 |
| sabinene | 974 | nd | 4.3 | 10.8 | nd | nd | 0.5 | nd | 5.21 |
| ß-pinene | 980 | 8.0 | 1.6 | 7.0 | 9.0 | nd | nd | 18.0 | 12.2 |
| ß-myrcene | 990 | 1.5 | 2.9 | nd | 2.3 | nd | 7.4 | 1.0 | 11.8 |
| α-phellandrene | 1006 | nd | nd | nd | 4.6 | nd | nd | nd | 1.13 |
| α-terpinene | 1015 | 1.0 | 0.5 | nd | 15.8 | nd | nd | nd | 5.5 |
| p-cymene | 1026 | 1.0 | 0.8 | nd | 0.8 | nd | nd | nd | 1.1 |
| limonene | 1030 | 2.5 | 3.0 | 10.0 | 14.8 | 0.2 | 0.4 | nd | 9.1 |
| 1.8-cineole (eucalyptol) | 1031 | 18 | 44.2 | nd | nd | nd | 4.3 | nd | nd |
| β-phellandrene | 1042 | nd | nd | nd | 2.9 | nd | nd | nd | 1.7 |
| γ- terpinene | 1072 | nd | 0.8 | nd | 0.5 | nd | nd | 18.0 | 1.0 |
| α-terpinolene | 1083 | nd | nd | nd | 0.8 | nd | nd | nd | 0.9 |
| linalool | 1100 | nd | 0.8 | nd | 0.8 | nd | 1.2 | nd | nd |
| camphor | 1121 | 20.0 | 9.5 | nd | nd | nd | 4.5 | nd | nd |
| verbenone | 1145 | 0.7 | nd | nd | nd | nd | nd | nd | nd |
| borneol | 1156 | 3.0 | 1.9 | nd | nd | nd | 4.5 | nd | nd |
| terpinen-4-ol | 1180 | nd | 1.1 | nd | 0.5 | nd | 3.0 | nd | 1.1 |
| decanal | 1206 | nd | nd | nd | nd | 1.5 | nd | nd | nd |
| neral | 1215 | nd | nd | nd | nd | 28.0 | 29.7 | nd | nd |
| geranial | 1222 | nd | nd | nd | nd | 35.0 | 38.5 | nd | nd |
| linalyl acetate | 1246 | nd | nd | 22.0 | nd | nd | nd | nd | nd |
| citral | 1268 | nd | nd | nd | nd | 67.0 | 31.2 | 4.3 | nd |
| bornyl acetate | 1285 | 0.5 | nd | nd | nd | nd | nd | nd | nd |
| ß-sinensal | 1295 | nd | nd | 0.5 | nd | nd | nd | nd | nd |
| β-elemene | 1345 | nd | nd | nd | 1.2 | nd | nd | nd | 0.6 |
| β-cubebene | 1348 | nd | nd | nd | 1.2 | nd | nd | nd | 0.6 |
| eugenol | 1357 | nd | nd | nd | nd | nd | nd | nd | nd |
| geranyl acetate | 1384 | nd | nd | nd | nd | 0.5 | 1.2 | nd | nd |
| ß-caryophyllene | 1442 | nd | 0.9 | 20.0 | 18.8 | nd | 0.7 | nd | 8.5 |
| α-muurolene | 1498 | nd | nd | nd | 1.1 | nd | 0.6 | nd | 1.9 |
| β-bisabolene | 1508 | nd | nd | nd | nd | nd | nd | nd | nd |
| delta-3-Carene | 1518 | nd | nd | 9.0 | nd | nd | nd | nd | nd |
| δ-cadinene | 1520 | nd | nd | nd | 1.5 | nd | nd | nd | 0.8 |
| myristicin | 1546 | nd | nd | nd | nd | nd | nd | nd | nd |
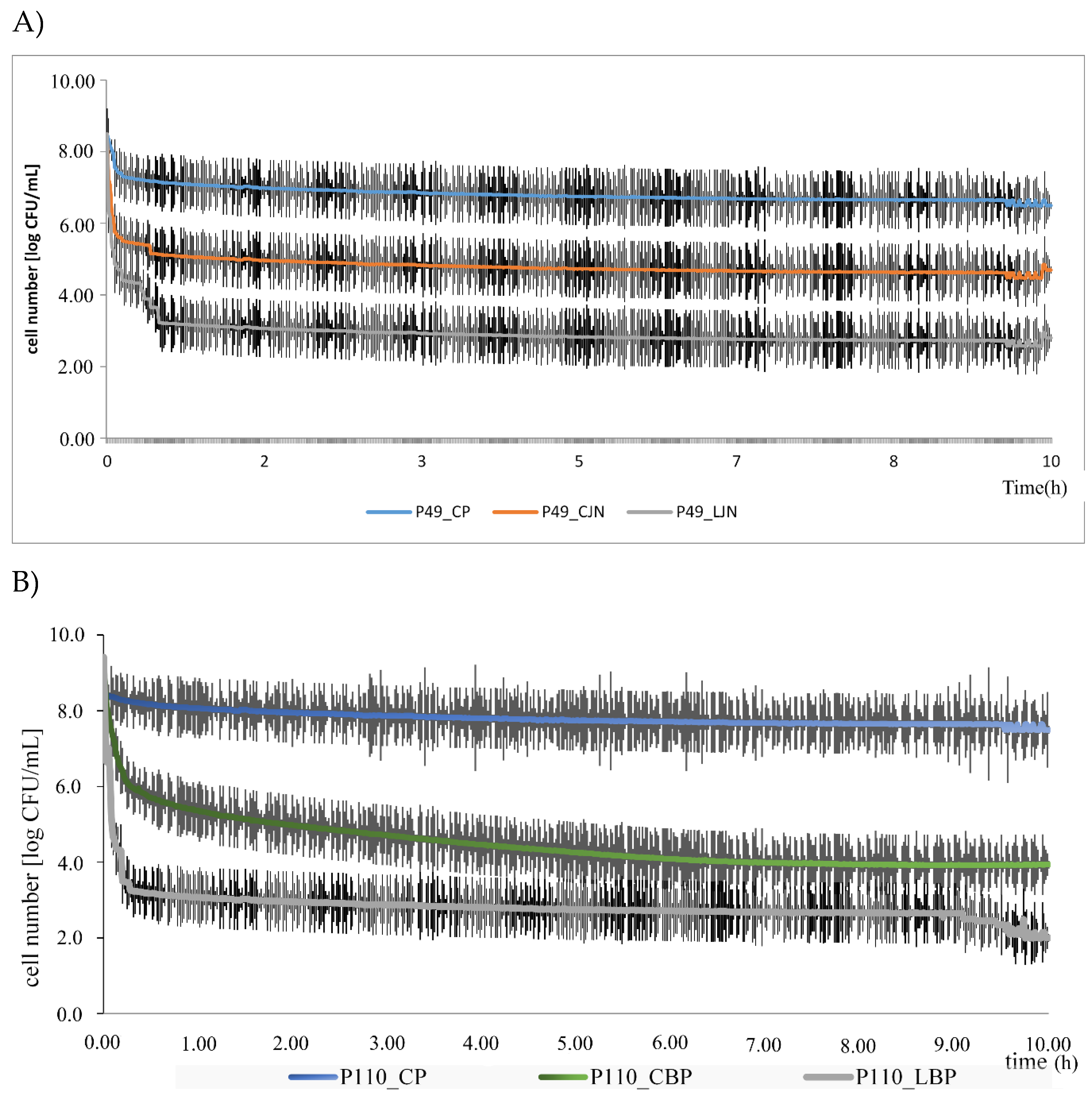
| Bacteria Strain | P49 | P110 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone Inhibition (mm) | MIC (mg/mL) | Growth Inhibition (%) | Zone Inhibition (mm) | MIC (mg/mL) | Growth Inhibition (%) | |||||||
| Oil type | CO | LO | CO | LO | CO | LO | CO | LO | CO | LO | CO | LO |
| RS | 0.4 a ± 0.2 | 0.6 a ± 0.2 | 80.6 a ± 2.1 | 8.85 b ± 1.2 | 24.5 a ± 1.4 | 46.0 b ± 1.2 | 7.0 a ± 0.1 | 27.0 b ± 2.6 | 80.1 a ± 6.3 | 8.12 b ± 1.1 | 64.5 a ± 2.4 | 79.4 b ± 3.6 |
| BP | 2.0 a ± 0.2 | 7.2 b ± 0.3 | 62.9 a ± 2.3 | 6.9 b ± 0.8 | 49.9 a ± 0.7 | 57.0 b ± 2.6 | 0.6 a ± 0.1 | 20.0 b ± 2.1 | 78.7 a ± 2.6 | 7.60 b ± 0.6 | 64.76 a ± 2.0 | 88.5 b ± 3.2 |
| LG | 5.1 a ± 1.1 | 7.6 b ± 0.2 | 65.1 a ± 1.8 | 3.15 b ± 0.2 | 48.5 a ± 1.8 | 58.0 b ± 3.9 | 18.1 a ± 1.9 | 19.0 a ± 1.2 | 70.6 a ± 0.3 | 3.98 b ± 0.5 | 62.7 a ± 5.7 | 82.9 b ± 2.4 |
| JN | 2.0 a ± 0.3 | 7.6 b ± 0.8 | 81.3 a ± 3.1 | 8.9 b ± 1.4 | 40.7 a ± 2.5 | 88.4 b ± 2.1 | 2.1 a ± 0.4 | 17.0 b ± 2.0 | 76.0 a ± 8.1 | 9.15 b ± 1.2 | 54.9 a ± 1.5 | 64.9 b ± 1.9 |
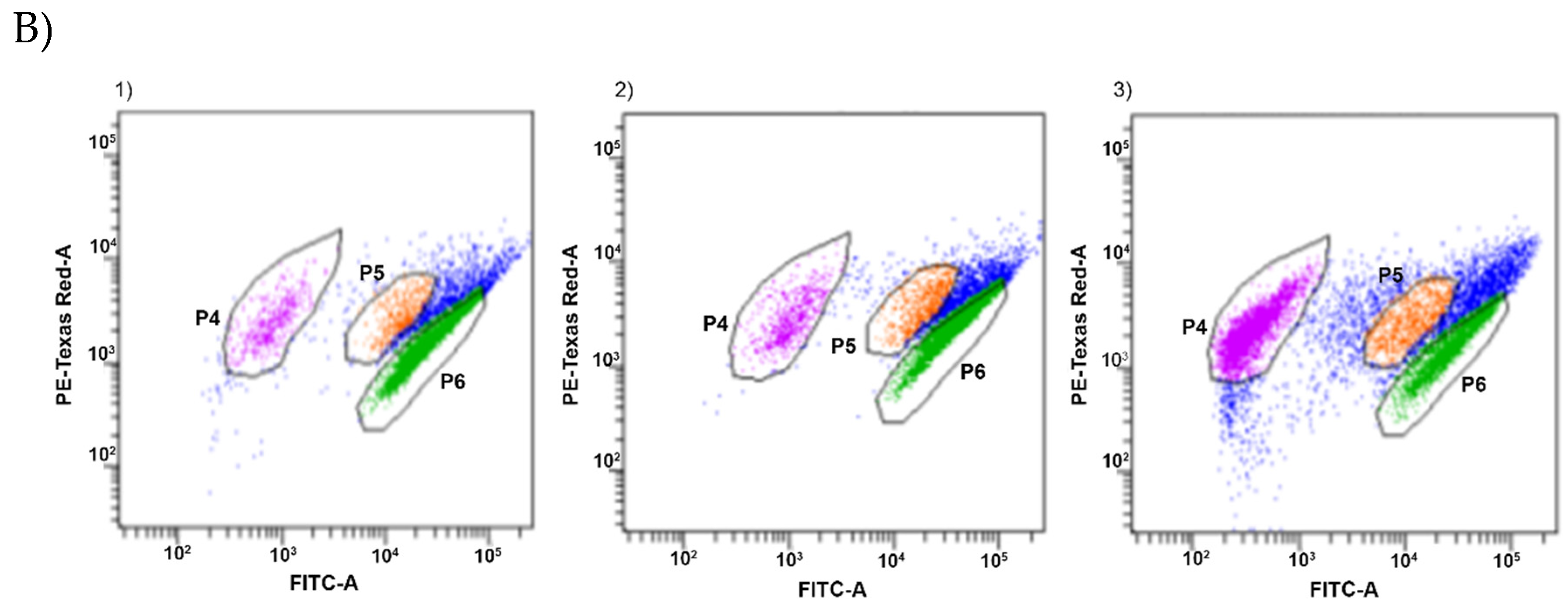
| Essential Oil | Oils Source | Strain | Low Metabolic Activity (%) | Medium Metabolic Activity (%) | High Metabolic Activity (%) |
|---|---|---|---|---|---|
| Control probe | P49 | 2.3 a ± 0.3 | 9.2 a ± 0.6 | 80.1 c ± 0.8 | |
| P110 | 1.4 a ± 0.2 | 4.2 a ± 0.1 | 86.1 c ± 0.6 | ||
| RS oil | LRS | P49 | 49.2 c ± 0.4 | 19.3 b ± 0.3 | 33.2 a ± 0.4 |
| P110 | 33.9 b ± 0.1 | 22.1 b ± 0.2 | 43.2 a ± 0.4 | ||
| CRS | P49 | 2.1 a ± 0.8 | 5.7 a ± 0.9 | 81.8 c ± 1.2 | |
| P110 | 3.2 a ± 0.3 | 9.5 a ± 0.3 | 75.6 b ± 0.7 | ||
| BP oil | LBP | P49 | 53.9 c ± 0.7 | 4.9 a ± 0.6 | 27.1 a ± 0.2 |
| P110 | 50.9 c ± 0.3 | 6.3 a ± 0.6 | 29.3 a ± 0.2 | ||
| CBP | P49 | 2.2 a ± 0.2 | 6.8 a ± 1.2 | 83.1 c ± 2.2 | |
| P110 | 2.0 a ± 0.1 | 2.5 a ± 0.2 | 91.1 c ± 0.2 | ||
| LG oil | LLG | P49 | 87.4 d ± 0.2 | 6.2 b ± 0.5 | 1.6 d ± 0.1 |
| P110 | 92.2 d ± 0.6 | 4.7 b ± 0.5 | 1.2 d ± 0.1 | ||
| CLG | P49 | 36.7 b ± 0.5 | 24.6 c ± 0.6 | 25.9 a ± 0.4 | |
| P110 | 6.8 a ± 0.5 | 5.8 a ± 0.4 | 78.3 b ± 0.5 | ||
| JN oil | LJN | P49 | 36.4 b ± 0.7 | 27.2 c ± 0.9 | 31.9 a ± 1.4 |
| P110 | 39.1 a ± 0.5 | 26.6 a ± 0.3 | 30.7 c ± 1.8 | ||
| CJN | P49 | 4.2 a ± 0.1 | 18.1 b ± 2.6 | 62.8 b ± 3.3 | |
| P110 | 2.7 a ± 0.1 | 6.0 a ± 0.6 | 82.4 c ± 1.1 |
| Control Probe | LRS | CRS | LBP | CBP | LLG | CLG | LJN | CJN | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Length (μm) | Width (μm) | Length (μm) | Width (μm) | Length (μm) | Width (μm) | Length (μm) | Width (μm) | Length (μm) | Width (μm) | Length (μm) | Width (μm) | Length (μm) | Width (μm) | Length (μm) | Width (μm) | Length (μm) | Width (μm) | |
| P49 | 1.02 ± 0.3 | 0.49 ± 0.1 | 0.82 ± 0.2 | 0.73 ± 0.11 | 0.71 ± 0.1 | 0.72 ± 0.2 | 0.78 ± 0.3 | 0.82 ± 0.2 | 0.85 ± 0.2 | 0.83 ± 0.1 | 0.71 ± 0.1 | 0.69 ± 0.1 | 0.76 ± 0.1 | 0.73 ± 0.1 | 0.65 ± 0.2 | 0.67 ± 0.2 | 0.73 ± 0.3 | 0.75 ± 0.2 |
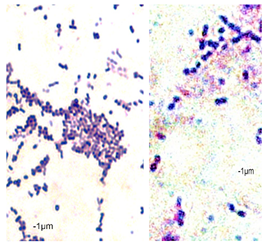 | 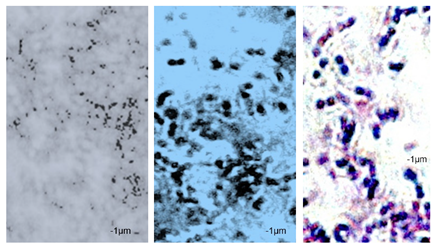 | 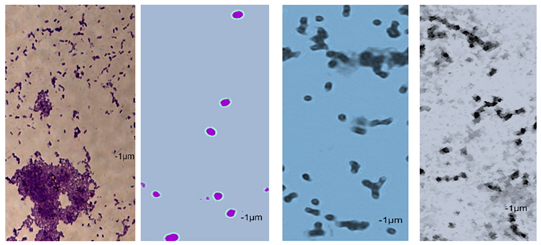 | ||||||||||||||||
| P110 | 1.21 ± 0.1 | 0.62 ± 0.1 | 0.69 ± 0.1 | 0.67 ± 0.17 | 0.65 ± 0.1 | 0.68 ± 0.1 | 0.71 ± 0.2 | 0.74 ± 0.1 | 0.84 ± 0.2 | 0.81 ± 0.1 | 0.80 ± 0.2 | 0.76 ± 0.2 | 0.82 ± 0.3 | 0.84 ± 0.1 | 0.75 ± 0.1 | 0.73 ± 0.2 | 0.80 ± 0.2 | 0.78 ± 0.2 |
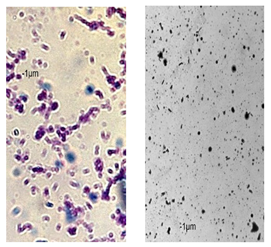 | 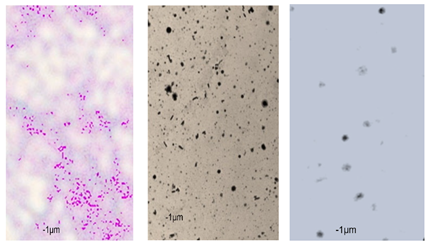 | 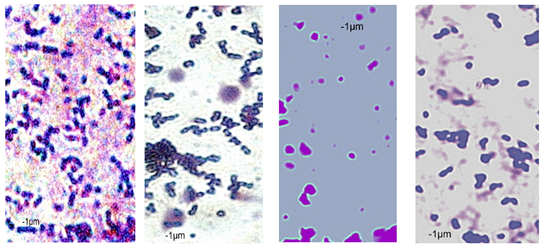 | ||||||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leja, K.; Majcher, M.; Juzwa, W.; Czaczyk, K.; Komosa, M. Comparative Evaluation of Piper nigrum, Rosmarinus officinalis, Cymbopogon citratus and Juniperus communis L. Essential Oils of Different Origin as Functional Antimicrobials in Foods. Foods 2020, 9, 141. https://doi.org/10.3390/foods9020141
Leja K, Majcher M, Juzwa W, Czaczyk K, Komosa M. Comparative Evaluation of Piper nigrum, Rosmarinus officinalis, Cymbopogon citratus and Juniperus communis L. Essential Oils of Different Origin as Functional Antimicrobials in Foods. Foods. 2020; 9(2):141. https://doi.org/10.3390/foods9020141
Chicago/Turabian StyleLeja, Katarzyna, Małgorzata Majcher, Wojciech Juzwa, Katarzyna Czaczyk, and Marcin Komosa. 2020. "Comparative Evaluation of Piper nigrum, Rosmarinus officinalis, Cymbopogon citratus and Juniperus communis L. Essential Oils of Different Origin as Functional Antimicrobials in Foods" Foods 9, no. 2: 141. https://doi.org/10.3390/foods9020141
APA StyleLeja, K., Majcher, M., Juzwa, W., Czaczyk, K., & Komosa, M. (2020). Comparative Evaluation of Piper nigrum, Rosmarinus officinalis, Cymbopogon citratus and Juniperus communis L. Essential Oils of Different Origin as Functional Antimicrobials in Foods. Foods, 9(2), 141. https://doi.org/10.3390/foods9020141






