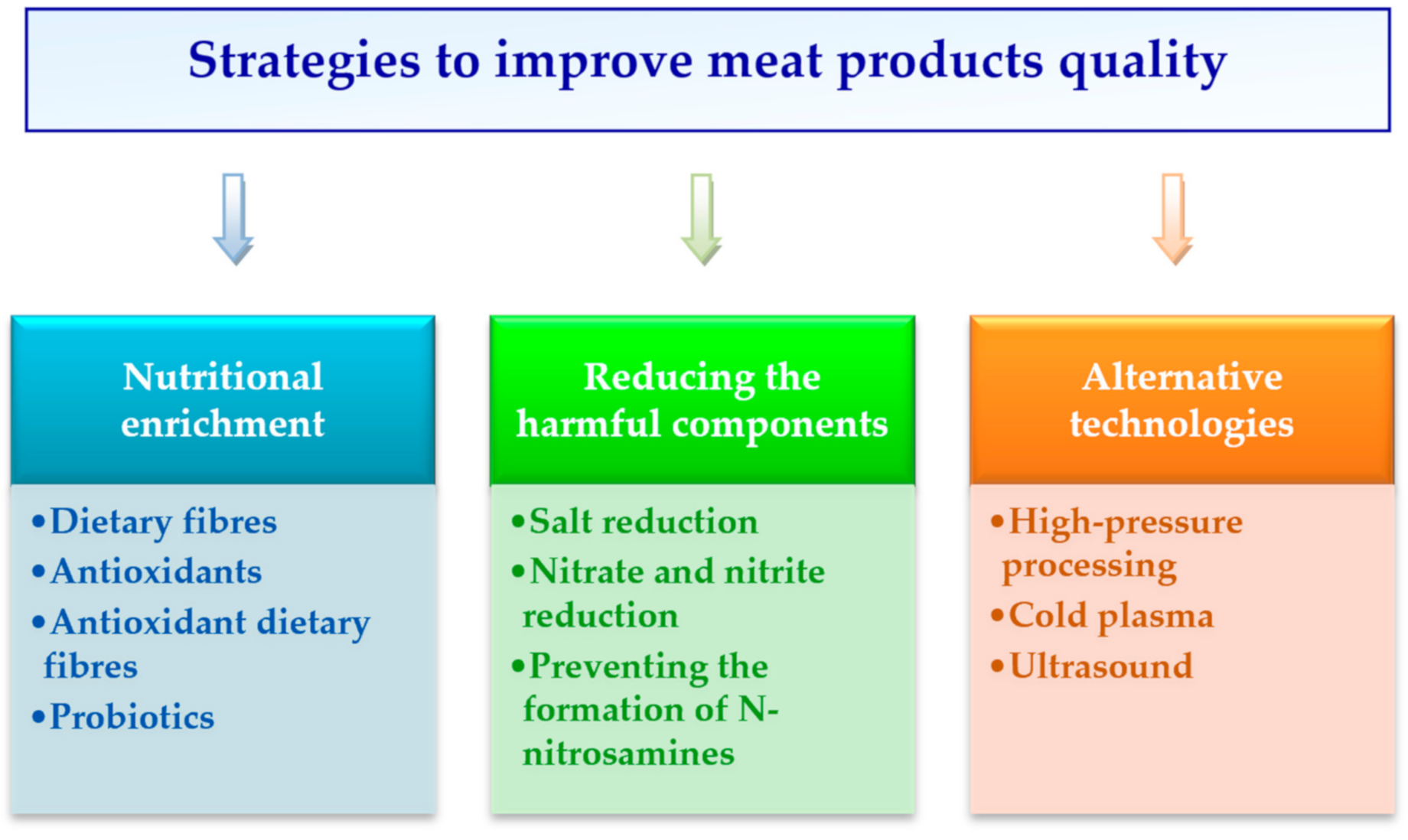
Strategies to Improve Meat Products’ Quality
Abstract
1. Introduction
2. Nutritional Enrichment
2.1. Dietary Fibres
2.2. Antioxidants
2.3. Antioxidant Dietary Fibres
2.4. Probiotics
3. Reducing the Harmful Components
3.1. Salt Reduction
3.2. Nitrate and Nitrite Reduction
3.3. Preventing the Formation of N-nitrosamines
4. Alternative Technologies
4.1. High-Pressure Processing
4.2. Cold Plasma
4.3. Ultrasound
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Commission. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. J. Eur. Union 2004, 139, 55–205. [Google Scholar]
- Simonin, H.; Duranton, F.; de Lamballerie, M. New Insights into the High-Pressure Processing of Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2012, 11, 285–306. [Google Scholar] [CrossRef]
- Higgs, J.D. The changing nature of red meat: 20 years of improving nutritional quality. Trends Food Sci. Technol. 2000, 11, 85–95. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Pereira, P.M.; Vicente, A.F. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef]
- Kulczynski, B.; Sidor, A.; Gramza-Michalowska, A. Characteristics of Selected Antioxidative and Bioactive Compounds in Meat and Animal Origin Products. Antioxidants 2019, 8, 335. [Google Scholar] [CrossRef]
- Skibska, B.; Goraca, A. The protective effect of lipoic acid on selected cardiovascular diseases caused by age-related oxidative stress. Oxid. Med. Cell. Longev. 2015, 2, 1–11. [Google Scholar] [CrossRef]
- Albenzio, M.; Santillo, A.; Caroprese, M.; Della Malva, A.; Marino, R. Bioactive Peptides in Animal Food Products. Foods 2017, 6, 35. [Google Scholar] [CrossRef]
- McBey, D.; Watts, D.; Johnstone, A.M. Nudging, formulating new products, and the lifecourse: A qualitative assessment of the viability of three methods for reducing Scottish meat consumption for health, ethical, and environmental reasons. Appetite 2019, 142, 104349. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Carcinogenicity of consumption of red meat and processed meat: A review of scientific news since the IARC decision. Food Chem. Toxicol. 2017, 105, 256–261. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.; Bonham, M.P.; Fearon, A.M. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef]
- Wolk, A. Potential health hazards of eating red meat. J. Intern. Med. 2017, 281, 106–122. [Google Scholar] [CrossRef]
- Gagaoua, M.; Picard, B. Current Advances in Meat Nutritional, Sensory and Physical Quality Improvement. Foods 2020, 9, 321. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, S.; Samaraweera, H.; Lee, E.J.; Ahn, D.U. Improving functional value of meat products. Meat Sci. 2010, 86, 15–31. [Google Scholar] [CrossRef]
- Hathwar, S.C.; Rai, A.K.; Modi, V.K.; Narayan, B. Characteristics and consumer acceptance of healthier meat and meat product formulations-a review. J. Food Sci. Technol. 2012, 49, 653–664. [Google Scholar] [CrossRef]
- Rothstein, W.G. Dietary fat, coronary heart disease, and cancer: A historical review. Prev. Med. 2006, 43, 356–360. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar] [CrossRef]
- Dai, F.-J.; Chau, C.-F. Classification and regulatory perspectives of dietary fiber. J. Food Drug Anal. 2017, 25, 37–42. [Google Scholar] [CrossRef]
- Mehta, N.; Ahlawat, S.S.; Sharma, D.P.; Dabur, R.S. Novel trends in development of dietary fiber rich meat products-a critical review. J. Food Sci. Technol. 2015, 52, 633–647. [Google Scholar] [CrossRef]
- Kim, H.J.; Paik, H.-D. Functionality and Application of Dietary Fiber in Meat Products. Korean J. Food Sci.. Anim. Resour. 2012, 32, 695–705. [Google Scholar] [CrossRef]
- Talukder, S. Effect of dietary fiber on properties and acceptance of meat products: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1005–1011. [Google Scholar] [CrossRef]
- Hu, G.; Yu, W. Effect of hemicellulose from rice bran on low fat meatballs chemical and functional properties. Food Chem. 2015, 186, 239–243. [Google Scholar] [CrossRef]
- Schmiele, M.; Nucci Mascarenhas, M.C.C.; da Silva Barretto, A.C.; Rodrigues Pollonio, M.A. Dietary fiber as fat substitute in emulsified and cooked meat model system. LWT Food Sci. Technol. 2015, 61, 105–111. [Google Scholar] [CrossRef]
- Barros, J.C.; Munekata, P.E.S.; Pires, M.A.; Rodrigues, I.; Andaloussi, O.S.; Rodrigues, C.E.d.C.; Trindade, M.A. Omega-3- and fibre-enriched chicken nuggets by replacement of chicken skin with chia (Salvia hispanica L.) flour. LWT 2018, 90, 283–289. [Google Scholar] [CrossRef]
- Berizi, E.; Shekarforoush, S.S.; Mohammadinezhad, S.; Hosseinzadeh, S.; Farahnaki, A. The use of inulin as fat replacer and its effect on texture and sensory properties of emulsion type sausages. Iran. J. Vet. Res. 2017, 18, 253–257. [Google Scholar]
- Yılmaz, I. Effects of rye bran addition on fatty acid composition and quality characteristics of low-fat meatballs. Meat Sci. 2004, 67, 245–249. [Google Scholar] [CrossRef]
- Petridis, D.; Raizi, P.; Ritzoulis, C. Influence of Citrus Fiber, Rice Bran and Collagen on the Texture and Organoleptic Properties of Low-Fat Frankfurters. J. Food Process. Preserv. 2014, 38, 1759–1771. [Google Scholar] [CrossRef]
- Powell, M.J.; Sebranek, J.G.; Prusa, K.J.; Tarté, R. Evaluation of citrus fiber as a natural replacer of sodium phosphate in alternatively-cured all-pork Bologna sausage. Meat Sci. 2019, 157, 107883. [Google Scholar] [CrossRef]
- Fernández-López, J.; Fernández-Ginés, J.M.; Aleson-Carbonell, L.; Sendra, E.; Sayas-Barberá, E.; Pérez-Alvarez, J.A. Application of functional citrus by-products to meat products. Trends Food Sci. Technol. 2004, 15, 176–185. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Santos, M.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva Lannes, S.C.; da Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef]
- Sohaib, M.; Anjum, F.M.; Sahar, A.; Arshad, M.S.; Rahman, U.U.; Imran, A.; Hussain, S. Antioxidant proteins and peptides to enhance the oxidative stability of meat and meat products: A comprehensive review. Int. J. Food Prop. 2017, 20, 2581–2593. [Google Scholar] [CrossRef]
- Kausar, T.; Hanan, E.; Ayob, O.; Praween, B.; Azad, Z. A review on functional ingredients in red meat products. Bioinformation 2019, 15, 358–363. [Google Scholar] [CrossRef]
- Manhani, M.R.; Nicoletti, M.A.; Barretto, A.C.D.S.; Jesus, G.R.D.; Camila Munhoz, C.; Abreu, G.R.D.; Zaccarelli-Magalhães, J.; Fukushima, A.R. Antioxidant Action of Rosemary and Oregano Extract in Pre-Cooked Meat Hamburger. Food Nutr. Sci. 2018, 9, 806–817. [Google Scholar] [CrossRef]
- Armenteros, M.; Morcuende, D.; Ventanas, S.; Estévez, M. Application of Natural Antioxidants from Strawberry Tree (Arbutus unedo L.) and Dog Rose (Rosa canina L.) to Frankfurters Subjected to Refrigerated Storage. J. Integr. Agric. 2013, 12, 1972–1981. [Google Scholar] [CrossRef]
- Pateiro, M.; Bermudez, R.; Lorenzo, J.M.; Franco, D. Effect of Addition of Natural Antioxidants on the Shelf-Life of “Chorizo”, a Spanish Dry-Cured Sausage. Antioxidants 2015, 4, 42–67. [Google Scholar] [CrossRef]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A.I.A.; Sant’Ana, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; do Amaral Sobral, P.J.; et al. Guarana seed extracts as a useful strategy to extend the shelf life of pork patties: UHPLC-ESI/QTOF phenolic profile and impact on microbial inactivation, lipid and protein oxidation and antioxidant capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Kubota, E.H.; da Silva, C.G.; Dos Santos Alves, J.; Hautrive, T.P.; Rodrigues, G.S.; Campagnol, P.C.B. Banana inflorescences: A cheap raw material with great potential to be used as a natural antioxidant in meat products. Meat Sci. 2020, 161, 107991. [Google Scholar] [CrossRef]
- Przybylski, R.; Firdaous, L.; Châtaigné, G.; Dhulster, P.; Nedjar, N. Production of an antimicrobial peptide derived from slaughterhouse by-product and its potential application on meat as preservative. Food Chem. 2016, 211, 306–313. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.; Huang, Y. Antioxidant function of tea dregs protein hydrolysates in liposome–meat system and its possible action mechanism. Int. J. Food Sci. Technol. 2014, 49, 2299–2306. [Google Scholar] [CrossRef]
- Sakanaka, S.; Tachibana, Y.; Ishihara, N.; Juneja, L.R. Antioxidant Properties of Casein Calcium Peptides and Their Effects on Lipid Oxidation in Beef Homogenates. J. Agric. Food Chem. 2005, 53, 464–468. [Google Scholar] [CrossRef]
- Peña-Ramos, E.A.; Xiong, Y.L. Whey and soy protein hydrolysates inhibit lipid oxidation in cooked pork patties. Meat Sci. 2003, 64, 259–263. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Antioxidant dietary fiber product: A new concept and a potential food ingredient. J. Agric. Food Chem. 1998, 46, 4303–4306. [Google Scholar] [CrossRef]
- Madane, P.; Das, A.K.; Pateiro, M.; Nanda, P.K.; Bandyopadhyay, S.; Jagtap, P.; Barba, F.J.; Shewalkar, A.; Maity, B.; Lorenzo, J.M. Drumstick (Moringa oleifera) Flower as an Antioxidant Dietary Fibre in Chicken Meat Nuggets. Foods 2019, 8, 307. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Madane, P.; Biswas, S.; Das, A.; Zhang, W.; Lorenzo, J.M. A comprehensive review on antioxidant dietary fibre enriched meat-based functional foods. Trends Food Sci. Technol. 2020, 99, 323–336. [Google Scholar] [CrossRef]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.-M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef]
- Rivera, K.; Salas-Perez, F.; Echeverria, G.; Urquiaga, I.; Dicenta, S.; Perez, D.; de la Cerda, P.; Gonzalez, L.; Andia, M.E.; Uribe, S.; et al. Red Wine Grape Pomace Attenuates Atherosclerosis and Myocardial Damage and Increases Survival in Association with Improved Plasma Antioxidant Activity in a Murine Model of Lethal Ischemic Heart Disease. Nutrients 2019, 11, 2135. [Google Scholar] [CrossRef]
- Malav Om, P.; Sharma, B.D.; Kumar, R.R.; Talukder, S.; Ahmed, S.R.; Irshad, A. Antioxidant potential and quality characteristics of functional mutton patties incorporated with cabbage powder. Nutr. Food Sci. 2015, 45, 542–563. [Google Scholar] [CrossRef]
- Noor, S.A.A.; Siti, N.M.; Mahmad, N.J. Chemical Composition, Antioxidant Activity and Functional Properties of Mango (Mangifera indica L. var Perlis Sunshine) Peel Flour (MPF). Appl. Mech. Mater. 2015, 754–755, 1065–1070. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chem. 2012, 135, 1520–1526. [Google Scholar] [CrossRef]
- Tagliani, C.; Perez, C.; Curutchet, A.; Arcia, P.; Cozzano, S. Blueberry pomace, valorization of an industry by-product source of fibre with antioxidant capacity. Food Sci. Technol. 2019, 39, 644–651. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Zeppa, G.; Stevigny, C. Cocoa Bean Shell-A By-Product with Nutritional Properties and Biofunctional Potential. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef]
- Montalvo-González, E.; Aguilar-Hernández, G.; Hernández-Cázares, A.S.; Ruiz-López, I.I.; Pérez-Silva, A.; Hernández-Torres, J.; Vivar-Vera, M.D.L.Á. Production, chemical, physical and technological properties of antioxidant dietary fiber from pineapple pomace and effect as ingredient in sausages. CyTA J. Food 2018, 16, 831–839. [Google Scholar] [CrossRef]
- Benitez, V.; Rebollo-Hernanz, M.; Hernanz, S.; Chantres, S.; Aguilera, Y.; Martin-Cabrejas, M.A. Coffee parchment as a new dietary fiber ingredient: Functional and physiological characterization. Food Res. Int. 2019, 122, 105–113. [Google Scholar] [CrossRef]
- Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Angeloni, S.; Mustafa, A.M.; Vittori, S.; Maggi, F.; Caprioli, G. Chemical Composition, Antioxidant and Enzyme Inhibitory Properties of Different Extracts Obtained from Spent Coffee Ground and Coffee Silverskin. Foods 2020, 9, 713. [Google Scholar] [CrossRef]
- Perța-Crișan, S.; Ursachi, C.; Munteanu, F.D. Trends in valorisation of spent cofee grounds: A review. Sci. Tech. Bull. Ser. Chem. Food Sci. Eng. 2019, 16, 31–42. [Google Scholar]
- Sáyago-Ayerdi, S.G.; Brenes, A.; Goñi, I. Effect of grape antioxidant dietary fiber on the lipid oxidation of raw and cooked chicken hamburgers. LWT Food Sci. Technol. 2009, 42, 971–976. [Google Scholar] [CrossRef]
- Verma, A.K.; Rajkumar, V.; Banerjee, R.; Biswas, S.; Das, A.K. Guava (Psidium guajava L.) Powder as an Antioxidant Dietary Fibre in Sheep Meat Nuggets. Asian-Australas J. Anim. Sci. 2013, 26, 886–895. [Google Scholar] [CrossRef]
- Hegazy, A.E.; Ibrahium, M.I. Antioxidant activities of orange peel extracts. World Appl. Sci. J. 2012, 18, 684–688. [Google Scholar]
- Goswami, M.; Prajapati, B.; Solanki, B.; Nalwaya, S.; Shendurse, A. Shelf life evaluation of chicken meat nuggets incorporated with gooseberry (pulp and seed coat) powder as natural preservatives at refrigerated storage (4 ± 1 °C). Int. J. Livest. Res. 2019, 9, 53–63. [Google Scholar]
- Cofrades, S.; Benedí, J.; Garcimartin, A.; Sánchez-Muniz, F.J.; Jimenez-Colmenero, F. A comprehensive approach to formulation of seaweed-enriched meat products: From technological development to assessment of healthy properties. Food Res. Int. 2017, 99, 1084–1094. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A Comparative Review on the Extraction, Antioxidant Content and Antioxidant Potential of Different Parts of Walnut (Juglans regia L.) Fruit and Tree. Molecules 2019, 24, 2133. [Google Scholar] [CrossRef]
- Madane, P.; Das, A.K.; Nanda, P.K.; Bandyopadhyay, S.; Jagtap, P.; Shewalkar, A.; Maity, B. Dragon fruit (Hylocereus undatus) peel as antioxidant dietary fibre on quality and lipid oxidation of chicken nuggets. J. Food Sci. Technol. 2020, 57, 1449–1461. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Sabrina, M.; Boeira, C.; Copetti, C.; Polli, V.A.; Rosa, C.; Terra, N.N. Development and Quality of Ham Pâté with Added Natural Antioxidant Kiwi Fruit (Actinidia deliciosa) Skin. J. Nutr. Food Sci. 2017, 7, 1000624. [Google Scholar]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Probiotcs in Food—Health and Nutritional Properties and Guidelines for Evaluation; FAO: Rome, Italy, 2006. [Google Scholar]
- Ashaolu, T.J. Immune boosting functional foods and their mechanisms: A critical evaluation of probiotics and prebiotics. Biomed. Pharmacother. 2020, 130, 110625. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Scourboutakos, M.J.; Franco-Arellano, B.; Murphy, S.A.; Norsen, S.; Comelli, E.M.; L’Abbe, M.R. Mismatch between Probiotic Benefits in Trials versus Food Products. Nutrients 2017, 9, 400. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.; Merrifield, C.A.; Hutkins, R. Probiotics for human use. Nutr. Bull. 2018, 43, 212–225. [Google Scholar] [CrossRef]
- Didari, T.; Solki, S.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. A systematic review of the safety of probiotics. Expert Opin. Drug Saf. 2014, 13, 227–239. [Google Scholar] [CrossRef]
- Silva, K.C.G.; Cezarino, E.C.; Michelon, M.; Kawazoe Sato, A.C. Symbiotic microencapsulation to enhance Lactobacillus acidophilus survival. LWT Food Sci. Technol. 2018, 89, 503–509. [Google Scholar] [CrossRef]
- Sönmez, Ş.; Önal Darilmaz, D.; Beyatli, Y. Determination of the relationship between oxalate degradation and exopolysaccharide production by different Lactobacillus probiotic strains. Int. J. Dairy Technol. 2018, 71, 741–752. [Google Scholar] [CrossRef]
- Bai, M.; Huang, T.; Guo, S.; Wang, Y.; Wang, J.; Kwok, L.-Y.; Dan, T.; Zhang, H.; Bilige, M. Probiotic Lactobacillus casei Zhang improved the properties of stirred yogurt. Food Biosci. 2020, 37, 100718. [Google Scholar] [CrossRef]
- Karimi, R.; Mortazavian, A.M.; Amiri-Rigi, A. Selective enumeration of probiotic microorganisms in cheese. Food Microbiol. 2012, 29, 1–9. [Google Scholar] [CrossRef]
- Huang, C.-H.; Lin, Y.-C.; Jan, T.-R. Lactobacillus reuteri induces intestinal immune tolerance against food allergy in mice. J. Funct. Foods 2017, 31, 44–51. [Google Scholar] [CrossRef]
- Coelho, S.R.; Lima, Í.A.; Martins, M.L.; Benevenuto Júnior, A.A.; Torres Filho, R.D.A.; Ramos, A.D.L.S.; Ramos, E.M. Application of Lactobacillus paracasei LPC02 and lactulose as a potential symbiotic system in the manufacture of dry-fermented sausage. LWT 2019, 102, 254–259. [Google Scholar] [CrossRef]
- Bis-Souza, C.V.; Pateiro, M.; Dominguez, R.; Lorenzo, J.M.; Penna, A.L.B.; da Silva Barretto, A.C. Volatile profile of fermented sausages with commercial probiotic strains and fructooligosaccharides. J. Food Sci. Technol. 2019, 56, 5465–5473. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Pastoriza, S.; Gironés, A.; Avellaneda, A.; Pilar Francino, M.; Rufián-Henares, J.A. Potential probiotic salami with dietary fiber modulates metabolism and gut microbiota in a human intervention study. J. Funct. Foods 2020, 66, 103790. [Google Scholar] [CrossRef]
- Khan, M.I.; Arshad, M.S.; Anjum, F.M.; Sameen, A.; Aneeq ur, R.; Gill, W.T. Meat as a functional food with special reference to probiotic sausages. Food Res. Int. 2011, 44, 3125–3133. [Google Scholar] [CrossRef]
- Ayyash, M.; Liu, S.-Q.; Al Mheiri, A.; Aldhaheri, M.; Raeisi, B.; Al-Nabulsi, A.; Osaili, T.; Olaimat, A. In vitro investigation of health-promoting benefits of fermented camel sausage by novel probiotic Lactobacillus plantarum: A comparative study with beef sausages. LWT 2019, 99, 346–354. [Google Scholar] [CrossRef]
- Campaniello, D.; Speranza, B.; Bevilacqua, A.; Altieri, C.; Rosaria Corbo, M.; Sinigaglia, M. Industrial Validation of a Promising Functional Strain of Lactobacillus plantarum to Improve the Quality of Italian Sausages. Microorganisms 2020, 8, 116. [Google Scholar] [CrossRef]
- Bagdatli, A.; Kundakci, A. Optimization of compositional and structural properties in probiotic sausage production. J. Food Sci. Technol. 2016, 53, 1679–1689. [Google Scholar] [CrossRef]
- Lewis, Z.T.; Shani, G.; Masarweh, C.F.; Popovic, M.; Frese, S.A.; Sela, D.A.; Underwood, M.A.; Mills, D.A. Validating bifidobacterial species and subspecies identity in commercial probiotic products. Pediatr. Res. 2016, 79, 445–452. [Google Scholar] [CrossRef]
- Niamah, A.K. Physicochemical and Microbial Characteristics of Yogurt with Added Saccharomyces Boulardii. Curr. Res. Nutr. Food Sci. J. 2017, 5, 300–307. [Google Scholar] [CrossRef]
- Behnsen, J.; Deriu, E.; Sassone-Corsi, M.; Raffatellu, M. Probiotics: Properties, examples, and specific applications. Cold Spring Harb. Perspect. Med. 2013, 3, a010074. [Google Scholar] [CrossRef]
- Akpinar, A.; Saygili, D.; Yerlikaya, O. Production of set-type yoghurt using Enterococcus faecium and Enterococcus durans strains with probiotic potential as starter adjuncts. Int. J. Dairy Technol. 2020, 73, 726–736. [Google Scholar] [CrossRef]
- Uriot, O.; Denis, S.; Junjua, M.; Roussel, Y.; Dary-Mourot, A.; Blanquet-Diot, S. Streptococcus thermophilus: From yogurt starter to a new promising probiotic candidate? J. Funct. Foods 2017, 37, 74–89. [Google Scholar] [CrossRef]
- Halim, M.; Mohd Mustafa, N.A.; Othman, M.; Wasoh, H.; Kapri, M.R.; Ariff, A.B. Effect of encapsulant and cryoprotectant on the viability of probiotic Pediococcus acidilactici ATCC 8042 during freeze-drying and exposure to high acidity, bile salts and heat. LWT Food Sci. Technol. 2017, 81, 210–216. [Google Scholar] [CrossRef]
- Yi, Y.-J.; Lim, J.-M.; Gu, S.; Lee, W.-K.; Oh, E.; Lee, S.-M.; Oh, B.-T. Potential use of lactic acid bacteria Leuconostoc mesenteroides as a probiotic for the removal of Pb(II) toxicity. J. Microbiol. 2017, 55, 296–303. [Google Scholar] [CrossRef]
- Konuray, G.; Erginkaya, Z. Potential Use of Bacillus coagulans in the Food Industry. Foods 2018, 7, 92. [Google Scholar] [CrossRef]
- Jeon, H.-L.; Lee, N.-K.; Yang, S.-J.; Kim, W.-S.; Paik, H.-D. Probiotic characterization of Bacillus subtilis P223 isolated from kimchi. Food Sci. Biotechnol. 2017, 26, 1641–1648. [Google Scholar] [CrossRef]
- Rubio, R.; Jofre, A.; Aymerich, T.; Guardia, M.D.; Garriga, M. Nutritionally enhanced fermented sausages as a vehicle for potential probiotic lactobacilli delivery. Meat Sci. 2014, 96, 937–942. [Google Scholar] [CrossRef]
- Gandhi, A.; Shah, N.P. Effect of salt on cell viability and membrane integrity of Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium longum as observed by flow cytometry. Food Microbiol. 2015, 49, 197–202. [Google Scholar] [CrossRef]
- Jofré, A.; Aymerich, T.; Garriga, M. Probiotic Fermented Sausages: Myth or Reality? Procedia Food Sci. 2015, 5, 133–136. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, Q.; Li, F.; Zheng, D.; Kong, B. Biogenic amine inhibition and quality protection of Harbin dry sausages by inoculation with Staphylococcus xylosus and Lactobacillus plantarum. Food Control. 2016, 68, 358–366. [Google Scholar] [CrossRef]
- Vuyst, L.D.; Falony, G.; Leroy, F. Probiotics in fermented sausages. Meat Sci. 2008, 80, 75–78. [Google Scholar] [CrossRef]
- Pasqualin Cavalheiro, C.; Ruiz-Capillas, C.; Herrero, A.M.; Jiménez-Colmenero, F.; Ragagnin de Menezes, C.; Martins Fries, L.L. Application of probiotic delivery systems in meat products. Trends Food Sci. Technol. 2015, 46, 120–131. [Google Scholar] [CrossRef]
- Erkkilä, S.; Petäjä, E. Screening of commercial meat starter cultures at low pH and in the presence of bile salts for potential probiotic use. Meat Sci. 2000, 55, 297–300. [Google Scholar] [CrossRef]
- Sameshima, T.; Magome, C.; Takeshita, K.; Arihara, K.; Itoh, M.; Kondo, Y. Effect of intestinal Lactobacillus starter cultures on the behaviour of Staphylococcus aureus in fermented sausage. Int. J. Food Microbiol. 1998, 41, 1–7. [Google Scholar] [CrossRef]
- Ba, H.V.; Seo, H.W.; Seong, P.N.; Kang, S.M.; Kim, Y.S.; Cho, S.H.; Park, B.Y.; Ham, J.S.; Kim, J.H. Lactobacillus plantarum (KACC 92189) as a Potential Probiotic Starter Culture for Quality Improvement of Fermented Sausages. Korean J. Food Sci. Anim. Resour. 2018, 38, 189–202. [Google Scholar] [CrossRef]
- Ba, H.V.; Seo, H.-W.; Cho, S.-H.; Kim, Y.-S.; Kim, J.-H.; Park, B.-Y.; Kim, H.-W.; Ham, J.-S.; Seong, P.-N. Utilisation possibility of Enterococcus faecalis isolates from neonate’s faeces for production of fermented sausages as starter cultures. Int. J. Food Sci. Technol. 2017, 52, 1660–1669. [Google Scholar] [CrossRef]
- Sidira, M.; Karapetsas, A.; Galanis, A.; Kanellaki, M.; Kourkoutas, Y. Effective survival of immobilized Lactobacillus casei during ripening and heat treatment of probiotic dry-fermented sausages and investigation of the microbial dynamics. Meat Sci. 2014, 96, 948–955. [Google Scholar] [CrossRef]
- Fernández-Ginés, J.M.; Fernández-López, J.; Sayas-Barberá, E.; Pérez-Alvarez, J.A. Meat Products as Functional Foods: A Review. J. Food Sci. 2005, 70, R37–R43. [Google Scholar] [CrossRef]
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; Villarán, M.d.C. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef]
- Ramos, P.E.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Physiological protection of probiotic microcapsules by coatings. Crit. Rev. Food Sci. Nutr. 2018, 58, 1864–1877. [Google Scholar] [CrossRef]
- Călinoiu, L.-F.; Ştefănescu, B.; Pop, I.; Muntean, L.; Vodnar, D. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef]
- Song, M.Y.; Van-Ba, H.; Park, W.S.; Yoo, J.Y.; Kang, H.B.; Kim, J.H.; Kang, S.M.; Kim, B.M.; Oh, M.H.; Ham, J.S. Quality Characteristics of Functional Fermented Sausages Added with Encapsulated Probiotic Bifidobacterium longum KACC 91563. Korean J. Food Sci. Anim. Resour. 2018, 38, 981–994. [Google Scholar] [CrossRef]
- Cavalheiro, C.P.; Ruiz-Capillas, C.; Herrero, A.M.; Jiménez-Colmenero, F.; Pintado, T.; de Menezes, C.R.; Fries, L.L.M. Effect of encapsulated Lactobacillus plantarum as probiotic on dry-sausages during chilled storage. Int. J. Food Sci. Technol. 2020, 55, 3613–3621. [Google Scholar] [CrossRef]
- Sidira, M.; Galanis, A.; Nikolaou, A.; Kanellaki, M.; Kourkoutas, Y. Evaluation of Lactobacillus casei ATCC 393 protective effect against spoilage of probiotic dry-fermented sausages. Food Control. 2014, 42, 315–320. [Google Scholar] [CrossRef]
- Sparo, M.D.; Confalonieri, A.; Urbizu, L.; Ceci, M.; Sánchez Bruni, S.F. Bio-preservation of ground beef meat by Enterococcus faecalis CECT7121. Braz. J. Microbiol. 2013, 44, 43–49. [Google Scholar] [CrossRef]
- Muthukumarasamy, P.; Holley, R.A. Survival of Escherichia coli O157:H7 in dry fermented sausages containing micro-encapsulated probiotic lactic acid bacteria. Food Microbiol. 2007, 24, 82–88. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- He, F.J.; Brown, M.; Tan, M.; MacGregor, G.A. Reducing population salt intake—An update on latest evidence and global action. J. Clin. Hypertens. 2019, 21, 1596–1601. [Google Scholar] [CrossRef]
- Petit, G.; Jury, V.; Lamballerie, M.; Duranton, F.; Pottier, L.; Martin, J.L. Salt Intake from Processed Meat Products: Benefits, Risks and Evolving Practices. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1453–1473. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Wen, R.; Liu, Q.; Chen, Q.; Kong, B. Effect of NaCl substitutes on the physical, microbial and sensory characteristics of Harbin dry sausage. Meat Sci. 2019, 156, 205–213. [Google Scholar] [CrossRef]
- Yotsuyanagi, S.E.; Contreras-Castillo, C.J.; Haguiwara, M.M.H.; Cipolli, K.M.V.A.B.; Lemos, A.L.S.C.; Morgano, M.A.; Yamada, E.A. Technological, sensory and microbiological impacts of sodium reduction in frankfurters. Meat Sci. 2016, 115, 50–59. [Google Scholar] [CrossRef]
- Kloss, L.; Meyer, J.D.; Graeve, L.; Vetter, W. Sodium intake and its reduction by food reformulation in the European Union—A review. NFS J. 2015, 1, 9–19. [Google Scholar] [CrossRef]
- Pinton, M.B.; dos Santos, B.A.; Lorenzo, J.M.; Cichoski, A.J.; Boeira, C.P.; Campagnol, P.C.B. Green technologies as a strategy to reduce NaCl and phosphate in meat products: An overview. Curr. Opin. Food Sci. 2021, 40, 1–5. [Google Scholar] [CrossRef]
- Delgado-Pando, G.; Fischer, E.; Allen, P.; Kerry, J.P.; O’Sullivan, M.G.; Hamill, R.M. Salt content and minimum acceptable levels in whole-muscle cured meat products. Meat Sci. 2018, 139, 179–186. [Google Scholar] [CrossRef]
- Aaslyng, M.D.; Vestergaard, C.; Koch, A.G. The effect of salt reduction on sensory quality and microbial growth in hotdog sausages, bacon, ham and salami. Meat Sci. 2014, 96, 47–55. [Google Scholar] [CrossRef]
- Fellendorf, S.; Kerry, J.P.; Hamill, R.M.; O’Sullivan, M.G. Impact on the physicochemical and sensory properties of salt reduced corned beef formulated with and without the use of salt replacers. LWT 2018, 92, 584–592. [Google Scholar] [CrossRef]
- Gaudette, N.J.; Pietrasik, Z. The sensory impact of salt replacers and flavor enhancer in reduced sodium processed meats is matrix dependent. J. Sens. Stud. 2017, 32, e12247. [Google Scholar] [CrossRef]
- Nachtigall, F.M.; Vidal, V.A.S.; Pyarasani, R.D.; Dominguez, R.; Lorenzo, J.M.; Pollonio, M.A.R.; Santos, L.S. Substitution effects of NaCl by KCl and CaCl2 on Lipolysis of Salted Meat. Foods 2019, 8, 595. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Zhang, Z.; Tiwari, B.K.; Kerry, J.P.; Burgess, C.M. Salt reduction strategies in processed meat products—A review. Trends Food Sci. Technol. 2017, 59, 70–78. [Google Scholar] [CrossRef]
- Armenteros, M.; Aristoy, M.-C.; Barat, J.M.; Toldrá, F. Biochemical and sensory changes in dry-cured ham salted with partial replacements of NaCl by other chloride salts. Meat Sci. 2012, 90, 361–367. [Google Scholar] [CrossRef]
- Dos Santos, B.A.; Campagnol, P.C.B.; Morgano, M.A.; Pollonio, M.A.R. Monosodium glutamate, disodium inosinate, disodium guanylate, lysine and taurine improve the sensory quality of fermented cooked sausages with 50% and 75% replacement of NaCl with KCl. Meat Sci. 2014, 96, 509–513. [Google Scholar] [CrossRef]
- Da Silva, S.L.; Lorenzo, J.M.; Machado, J.M.; Manfio, M.; Cichoski, A.J.; Fries, L.L.M.; Morgano, M.A.; Campagnol, P.C.B. Application of arginine and histidine to improve the technological and sensory properties of low-fat and low-sodium bologna-type sausages produced with high levels of KCl. Meat Sci. 2020, 159, 107939. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Kum, J.-S.; Jeon, K.-H.; Park, J.-D.; Choi, H.-W.; Hwang, K.-E.; Jeong, T.-J.; Kim, Y.-B.; Kim, C.-J. Effects of Edible Seaweed on Physicochemical and Sensory Characteristics of Reduced-salt Frankfurters. Korean J. Food Sci. Anim. Resour. 2015, 35, 748–756. [Google Scholar] [CrossRef]
- Fellendorf, S.; O’Sullivan, M.G.; Kerry, J.P. Impact of ingredient replacers on the physicochemical properties and sensory quality of reduced salt and fat black puddings. Meat Sci. 2016, 113, 17–25. [Google Scholar] [CrossRef]
- Vilar, E.G.; Ouyang, H.; O’Sullivan, M.G.; Kerry, J.P.; Hamill, R.M.; O’Grady, M.N.; Mohammed, H.O.; Kilcawley, K.N. Effect of salt reduction and inclusion of 1% edible seaweeds on the chemical, sensory and volatile component profile of reformulated frankfurters. Meat Sci. 2020, 161, 108001. [Google Scholar] [CrossRef]
- Jin, S.-K.; Choi, J.S.; Yang, H.-S.; Park, T.-S.; Yim, D.-G. Natural curing agents as nitrite alternatives and their effects on the physicochemical, microbiological properties and sensory evaluation of sausages during storage. Meat Sci. 2018, 146, 34–40. [Google Scholar] [CrossRef]
- Gassara, F.; Kouassi, A.P.; Brar, S.K.; Belkacemi, K. Green Alternatives to Nitrates and Nitrites in Meat-based Products–A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2133–2148. [Google Scholar] [CrossRef]
- Herrmann, S.S.; Granby, K.; Duedahl-Olesen, L. Formation and mitigation of N-nitrosamines in nitrite preserved cooked sausages. Food Chem. 2015, 174, 516–526. [Google Scholar] [CrossRef]
- Alahakoon, A.U.; Jayasena, D.D.; Ramachandra, S.; Jo, C. Alternatives to nitrite in processed meat: Up to date. Trends Food Sci. Technol. 2015, 45, 37–49. [Google Scholar] [CrossRef]
- Flores, M.; Toldra, F. Chemistry, safety, and regulatory considerations in the use of nitrite and nitrate from natural origin in meat products—Invited review. Meat Sci. 2021, 171, 108272. [Google Scholar] [CrossRef]
- Safa, H.; Portanguen, S.; Mirade, P.-S. Reducing the Levels of Sodium, Saturated Animal Fat, and Nitrite in Dry-Cured Pork Meat Products: A Major Challenge. Food Nutr. Sci. 2017, 8, 419–443. [Google Scholar] [CrossRef]
- Villaverde, A.; Ventanas, J.; Estévez, M. Nitrite promotes protein carbonylation and Strecker aldehyde formation in experimental fermented sausages: Are both events connected? Meat Sci. 2014, 98, 665–672. [Google Scholar] [CrossRef]
- Hung, Y.; de Kok, T.M.; Verbeke, W. Consumer attitude and purchase intention towards processed meat products with natural compounds and a reduced level of nitrite. Meat Sci. 2016, 121, 119–126. [Google Scholar] [CrossRef]
- Laranjo, M.; Potes, M.E.; Elias, M. Role of Starter Cultures on the Safety of Fermented Meat Products. Front. Microbiol. 2019, 10, 853. [Google Scholar] [CrossRef]
- Ferysiuk, K.; Wojciak, K.M. Reduction of Nitrite in Meat Products through the Application of Various Plant-Based Ingredients. Antioxidants 2020, 9, 711. [Google Scholar] [CrossRef]
- Bryan, N.S.; Ivy, J.L. Inorganic nitrite and nitrate: Evidence to support consideration as dietary nutrients. Nutr. Res. 2015, 35, 643–654. [Google Scholar] [CrossRef]
- Raubenheimer, K.; Bondonno, C.; Blekkenhorst, L.; Wagner, K.-H.; Peake, J.M.; Neubauer, O. Effects of dietary nitrate on inflammation and immune function, and implications for cardiovascular health. Nutr. Rev. 2019, 77, 584–599. [Google Scholar] [CrossRef]
- Correia, M.; Barroso, Â.; Barroso, M.F.; Soares, D.; Oliveira, M.B.P.P.; Delerue-Matos, C. Contribution of different vegetable types to exogenous nitrate and nitrite exposure. Food Chem. 2010, 120, 960–966. [Google Scholar] [CrossRef]
- Colla, G.; Kim, H.-J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, T.K.; Jeon, K.H.; Park, J.D.; Kim, H.W.; Hwang, K.E.; Kim, Y.B. Effects of Pre-Converted Nitrite from Red Beet and Ascorbic Acid on Quality Characteristics in Meat Emulsions. Korean J. Food Sci. Anim. Resour. 2017, 37, 288–296. [Google Scholar] [CrossRef]
- Sebranek, J.G.; Jackson-Davis, A.L.; Myers, K.L.; Lavieri, N.A. Beyond celery and starter culture: Advances in natural/organic curing processes in the United States. Meat Sci. 2012, 92, 267–273. [Google Scholar] [CrossRef]
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R1169 (accessed on 16 November 2020).
- Kim, T.-K.; Hwang, K.-E.; Lee, M.-A.; Paik, H.-D.; Kim, Y.-B.; Choi, Y.-S. Quality characteristics of pork loin cured with green nitrite source and some organic acids. Meat Sci. 2019, 152, 141–145. [Google Scholar] [CrossRef]
- Ozaki, M.M.; Munekata, P.E.S.; Jacinto-Valderrama, R.A.; Efraim, P.; Pateiro, M.; Lorenzo, J.M.; Pollonio, M.A.R. Beetroot and radish powders as natural nitrite source for fermented dry sausages. Meat Sci. 2020, 171, 108275. [Google Scholar] [CrossRef]
- Leroy, S.; Vermassen, A.; Ras, G.; Talon, R. Insight into the Genome of Staphylococcus xylosus, a Ubiquitous Species Well Adapted to Meat Products. Microorganisms 2017, 5, 52. [Google Scholar] [CrossRef]
- Löfblom, J.; Rosenstein, R.; Nguyen, M.-T.; Ståhl, S.; Götz, F. Staphylococcus carnosus: From starter culture to protein engineering platform. Appl. Microbiol. Biotechnol. 2017, 101, 8293–8307. [Google Scholar] [CrossRef]
- Ko, Y.M.; Park, J.H.; Yoon, K.S. Nitrite formation from vegetable sources and its use as a preservative in cooked sausage. J. Sci. Food Agric. 2017, 97, 1774–1783. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Bae, S.M.; Yoon, J.; Jeong, D.H.; Gwak, S.H. Effect of Using Vegetable Powders as Nitrite/Nitrate Sources on the Physicochemical Characteristics of Cooked Pork Products. Food Sci. Anim. Resour. 2020, 40, 831–843. [Google Scholar] [CrossRef]
- Shin, D.M.; Hwang, K.E.; Lee, C.W.; Kim, T.K.; Park, Y.S.; Han, S.G. Effect of Swiss Chard (Beta vulgaris var. cicla) as Nitrite Replacement on Color Stability and Shelf-Life of Cooked Pork Patties during Refrigerated Storage. Korean J. Food Sci. Anim. Resour. 2017, 37, 418–428. [Google Scholar] [CrossRef]
- Sucu, C.; Turp, G.Y. The investigation of the use of beetroot powder in Turkish fermented beef sausage (sucuk) as nitrite alternative. Meat Sci. 2018, 140, 158–166. [Google Scholar] [CrossRef]
- De Mey, E.; de Maere, H.; Paelinck, H.; Fraeye, I. Volatile N-nitrosamines in meat products: Potential precursors, influence of processing, and mitigation strategies. Crit. Rev. Food Sci. Nutr. 2017, 57, 2909–2923. [Google Scholar] [CrossRef]
- Flores, M.; Mora, L.; Reig, M.; Toldrá, F. Risk assessment of chemical substances of safety concern generated in processed meats. Food Sci. Hum. Wellness 2019, 8, 244–251. [Google Scholar] [CrossRef]
- Sallan, S.; Kaban, G.; Şişik Oğraş, Ş.; Çelik, M.; Kaya, M. Nitrosamine formation in a semi-dry fermented sausage: Effects of nitrite, ascorbate and starter culture and role of cooking. Meat Sci. 2020, 159, 107917. [Google Scholar] [CrossRef]
- Cantwell, M.; Elliott, C. Nitrates, Nitrites and Nitrosamines from Processed Meat Intake and ColorectalCancer Risk. J. Clin. Nutr. Diet. 2017, 3, 27–30. [Google Scholar] [CrossRef]
- Walters, C.L.; Edwards, M.W.; Elsey, T.S.; Martin, M. The effect of antioxidants on the production, of volatile nitrosamines during the frying of bacon. Zeitschrift für Lebensmittel-Untersuchung und Forschung 1976, 162, 377–385. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q.; Wang, S. Effects of rosemary extract, grape seed extract and green tea polyphenol on the formation of N-nitrosamines and quality of western-style smoked sausage. J. Food Process. Preserv. 2020, 44, e14459. [Google Scholar] [CrossRef]
- Pinton, M.B.; Correa, L.P.; Facchi, M.M.X.; Heck, R.T.; Leaes, Y.S.V.; Cichoski, A.J.; Lorenzo, J.M.; Dos Santos, M.; Pollonio, M.A.R.; Campagnol, P.C.B. Ultrasound: A new approach to reduce phosphate content of meat emulsions. Meat Sci. 2019, 152, 88–95. [Google Scholar] [CrossRef]
- O’Neill, C. High Pressure Processing as a Hurdle Technology for Development of Consumer-Accepted, Low-Salt Processed Meat Products with Enhanced Safety and Shelf-Life; University College Cork: Cork, Ireland, 2018. [Google Scholar]
- Huang, H.-W.; Wu, S.-J.; Lu, J.-K.; Shyu, Y.-T.; Wang, C.-Y. Current status and future trends of high-pressure processing in food industry. Food Control. 2017, 72, 1–8. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.-D.A. Applied and Emerging Methods for Meat Tenderization: A Comparative Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 841–859. [Google Scholar] [CrossRef]
- Sikes, A.L.; Warner, R. 10—Application of High Hydrostatic Pressure for Meat Tenderization. In Woodhead Publishing Series in Food Science, Technology and Nutrition. Innovative Food Processing Technologies; Knoerzer, K.J.P., Smithers, G., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 259–290. [Google Scholar]
- Warner, R.D.; McDonnell, C.K.; Bekhit, A.E.D.; Claus, J.; Vaskoska, R.; Sikes, A.; Dunshea, F.R.; Ha, M. Systematic review of emerging and innovative technologies for meat tenderisation. Meat Sci. 2017, 132, 72–89. [Google Scholar] [CrossRef]
- Yang, H.-J.; Han, M.-Y.; Wang, H.-F.; Cao, G.-T.; Tao, F.; Xu, X.-L.; Zhou, G.-H.; Shen, Q. HPP improves the emulsion properties of reduced fat and salt meat batters by promoting the adsorption of proteins at fat droplets/water interface. LWT 2020, 137, 110394. [Google Scholar] [CrossRef]
- Pietrasik, Z.; Gaudette, N.J.; Johnston, S.P. The impact of high hydrostatic pressure on the functionality and consumer acceptability of reduced sodium naturally cured wieners. Meat Sci. 2017, 129, 127–134. [Google Scholar] [CrossRef]
- Misra, N.N.; Jo, C. Applications of cold plasma technology for microbiological safety in meat industry. Trends Food Sci. Technol. 2017, 64, 74–86. [Google Scholar] [CrossRef]
- Varilla, C.; Marcone, M.; Annor, G.A. Potential of Cold Plasma Technology in Ensuring the Safety of Foods and Agricultural Produce: A Review. Foods 2020, 9, 1435. [Google Scholar] [CrossRef]
- Rudy, M.; Kucharyk, S.; Duma-Kocan, P.; Stanisławczyk, R.; Gil, M. Unconventional Methods of Preserving Meat Products and Their Impact on Health and the Environment. Sustainability 2020, 12, 5948. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, E.-J.; Choi, E.H.; Kim, Y.-J. Inactivation of Staphylococcus aureus on the beef jerky by radio-frequency atmospheric pressure plasma discharge treatment. Innov. Food Sci. Emerg. Technol. 2014, 22, 124–130. [Google Scholar] [CrossRef]
- Dirks, B.P.; Dobrynin, D.; Fridman, G.; Mukhin, Y.; Fridman, A.; Quinlan, J.J. Treatment of Raw Poultry with Nonthermal Dielectric Barrier Discharge Plasma To Reduce Campylobacter jejuni and Salmonella enterica. J. Food Prot. 2012, 75, 22–28. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yong, H.I.; Park, S.; Choe, W.; Jo, C. Corrigendum to “Effects of dielectric barrier discharge plasma on pathogen inactivation and the physicochemical and sensory characteristics of pork loin” [Curr. Appl. Phys. 13 (7) (2013) 1420–1425]. Curr. Appl. Phys. 2013, 13, 1953. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Kim, H.J.; Yong, H.I.; Park, S.; Kim, K.; Choe, W.; Jo, C. Flexible thin-layer dielectric barrier discharge plasma treatment of pork butt and beef loin: Effects on pathogen inactivation and meat-quality attributes. Food Microbiol. 2015, 46, 51–57. [Google Scholar] [CrossRef]
- Jung, S.; Lee, J.; Lim, Y.; Choe, W.; Yong, H.I.; Jo, C. Direct infusion of nitrite into meat batter by atmospheric pressure plasma treatment. Innov. Food Sci. Emerg. Technol. 2017, 39, 113–118. [Google Scholar] [CrossRef]
- Jung, S.; Kim, H.J.; Park, S.; Yong, H.I.; Choe, J.H.; Jeon, H.J.; Choe, W.; Jo, C. Color Developing Capacity of Plasma-treated Water as a Source of Nitrite for Meat Curing. Korean J. Food Sci. Anim. Resour. 2015, 35, 703–706. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2020, 70, 105293. [Google Scholar] [CrossRef]
- Alarcon-Rojo, A.D.; Carrillo-Lopez, L.M.; Reyes-Villagrana, R.; Huerta-Jimenez, M.; Garcia-Galicia, I.A. Ultrasound and meat quality: A review. Ultrason. Sonochem. 2019, 55, 369–382. [Google Scholar] [CrossRef]
- Cichoski, A.J.; Silva, M.S.; Leaes, Y.S.V.; Brasil, C.C.B.; de Menezes, C.R.; Barin, J.S.; Wagner, R.; Campagnol, P.C.B. Ultrasound: A promising technology to improve the technological quality of meat emulsions. Meat Sci. 2019, 148, 150–155. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, Y.; Zhou, Y.; Leng, Y. Effect of ultrasonic-assisted brining on mass transfer of beef. J. Food Process. Eng. 2019, 42, e13257. [Google Scholar] [CrossRef]
- Barretto, T.L.; Pollonio, M.A.R.; Telis-Romero, J.; da Silva Barretto, A.C. Improving sensory acceptance and physicochemical properties by ultrasound application to restructured cooked ham with salt (NaCl) reduction. Meat Sci. 2018, 145, 55–62. [Google Scholar] [CrossRef]
- Sena Vaz Leães, Y.; Basso Pinton, M.; Terezinha de Aguiar Rosa, C.; Sasso Robalo, S.; Wagner, R.; Ragagnin de Menezes, C.; Smanioto Barin, J.; Cezar Bastianello Campagnol, P.; José Cichoski, A. Ultrasound and basic electrolyzed water: A green approach to reduce the technological defects caused by NaCl reduction in meat emulsions. Ultrason. Sonochem. 2020, 61, 104830. [Google Scholar] [CrossRef]
| Developed Product | Fiber Source | Recommended Dose (%) | Effect on Meat Product Quality | Reference |
|---|---|---|---|---|
| Beef patties | Rice bran | 2; 4; 6 | Substitution of fat and total trans fatty acids. | [24] |
| Pork and beef sausage | Amorphous cellulose fibers from the husk of oat, soy and rice grains | 1;3 | 50% fat reduction; Increasing emulsion stability and consistency. | [25] |
| Chicken nuggets | Chia flour | 10 | Decreasing the moisture, saturated and monounsaturated fatty acids contents; Increasing the total amount of dietary fibers. | [26] |
| Emulsion type sausages | Inulin | 6 | Reduction of fat and energy content; Sensory acceptance is comparable with the one of a traditional product | [27] |
| Meatballs | Rye bran | 20 | Reduction of total trans fatty acids; Reduction of weight losses, improving nutritional value, health benefits and color. | [28] |
| Frankfurter sausages | Citrus fibers Rice bran | 1.5 0.5 | Positive effect on the acceptability; Adequate hardness and cohesivity; Acceptable decrease of color intensity. | [29] |
| Cured Bologna sausage | Citrus fibers Cherry powder | 1 0.3 | Replacement of fat and sodium tripolyphosphate; Hardness improvement. | [30] |
| Developed Product | Antioxidant Source | Recommended Dose | Effect on Meat Product Quality | Reference |
|---|---|---|---|---|
| Beef burgers | Rosemary extract | 0.5% | Oxidative stability and sensorial characteristics preservation of burgers stored in freezing at −18 °C for 30 days. | [35] |
| Frankfurters sausages | Strawberry extract | 130–350 mg GAE/kg | Reduced lipid oxidation during 30 days of 4 °C storage. | [36] |
| Dry-cured sausages | Grape seed | 50; 200; 1000 mg/kg | Suppression of lipids oxidation during ripening and storage periods. | [37] |
| Raw pork patties | Guarana seed | 250; 500; 1000 mg/kg | Reduction of carbonyls and TBARS formation. | [38] |
| Pork sausages | Banana inflorescences | 0.5; 1; 1.5; 2% | Positive effect on the control of lipids oxidation during storage; Sensory acceptance unaffected even when 2% dose was used. | [39] |
| Minced meat | α137–141peptide from hydrolyzed bovine hemoglobin | 0.1; 0.5% | Inhibition of lipids oxidation at the same level as BHT synthetic antioxidant. | [40] |
| Chicken products | Protein hydrolysates from tea residues | 0.1; 0.5; 1% | Strong antioxidant effect, similar to BHT synthetic antioxidant. | [41] |
| Homogenized ground beef | Casein calcium peptides | 2% | Inhibition of about 70% lipid oxidation | [42] |
| Pork patties | Whey bioactive peptide | 2% | Inhibition of oxidative deterioration during storage. | [43] |
| Developed Product | ADFs Source | ADFs Level (%) | Effect on Meat Product Quality | Reference |
|---|---|---|---|---|
| Chicken hamburgers | Red grape pomace | 0.5; 1.0; 1.5; 2.0 | Improved color; Inhibited and retarded lipid oxidation; No adverse influence on sensory attributes. | [58] |
| Sheep meat nuggets | Guava | 0.5; 1.0 | Increased dietary fibers and phenolics content; Improved oxidative stability; No change in textural properties; No adverse effect on sensory properties. | [59] |
| Cooked sausages (bolognas) and dry-cured sausages | Lemon albedo | 2.5; 5.0; 7.5; 10.0 | Increased dietary fiber amount; Decreased residual nitrite; Increased hardness; Better sensory scores. | [31] |
| Cooked sausages (bolognas) | Orange fiber powder (0.5, 1.0, 1.5 and 2.0%) | 0.5; 1.0; 1.5; 2.0 | Intensified color of product; Increased dietary fiber content; Increased hardness; Less elasticity than control product. | [60] |
| Functional mutton patties | Cabbage powder | 6.0 | Inhibition of lipid oxidation; Better sensory scores; Improved textural properties; Increased nutritive value. | [49] |
| Spent hen nuggets | Gooseberry pulp powder Seed coat | 0.5 1.5 | Improved shelf-life; Improved physico-chemical properties; Better acceptability of product. | [61] |
| Low-salt beef patties (raw and cooked) | Wakame seaweed | 3.0 | Improved water-binding properties; High antioxidant activity; Improved textural properties; No adverse effect on product acceptability. | [62] |
| Frankfurters | Walnut | 25 | Increased polyunsaturated fatty acids amount; Increased dietary fiber content; Healthier amino acid profile; Improved yield. | [63] |
| Pork and turkey sausages (Vienna type) | Pineapple pomace | 2.5; 5; 7.5; 10 | Increased dietary fiber content; Improved color; Decreased values of shrinkage and shear forces. | [54] |
| Chicken nuggets | Dragon fruit peel | 1.5; 3.0 | Improved emulsion stability; Decreased lipid oxidation; Improved redness of nuggets; Decreased hardness and gumminess compared to control product. | [64] |
| Ham pâté | Kiwi fruit skin flour | 0.5; 1.0; 2.0 | Increased dietary fiber content; Enhanced odor and flavor; Best acceptability at 1% level. | [65] |
| Genus | Species |
|---|---|
| Lactobacillus | L. acidophilus [74]; L. delbrueckii subsp. bulgaricus [66]; L. brevis, L. fermentum [75]; L. casei Zhang [76,77]; L.reuteri [78]; L. paracasei [79]; L. rhamnosus [80,81]; L. gasseri [82]; L. plantarum [83,84]; L. casei [85] |
| Bifidobacterium | B. infantis; B. animalis subsp. lactis; B. bifidum; B. breve; B. longum [82,86] |
| Saccharomyces | S. boulardii [87] |
| Lactococcus | L. lactis [88] |
| Enterocccocus | E. durans; E. faecium [89] |
| Streptococcus | S. termophilus [90] |
| Pediococcus | P. acidilactici [91] |
| Leuconostoc | L. mesenteroides [92] |
| Bacillus | B. coagulans [93]; B. subtilis [94] |
| Escherichia | E. coli Nissle 1917 [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ursachi, C.Ș.; Perța-Crișan, S.; Munteanu, F.-D. Strategies to Improve Meat Products’ Quality. Foods 2020, 9, 1883. https://doi.org/10.3390/foods9121883
Ursachi CȘ, Perța-Crișan S, Munteanu F-D. Strategies to Improve Meat Products’ Quality. Foods. 2020; 9(12):1883. https://doi.org/10.3390/foods9121883
Chicago/Turabian StyleUrsachi, Claudiu Ștefan, Simona Perța-Crișan, and Florentina-Daniela Munteanu. 2020. "Strategies to Improve Meat Products’ Quality" Foods 9, no. 12: 1883. https://doi.org/10.3390/foods9121883
APA StyleUrsachi, C. Ș., Perța-Crișan, S., & Munteanu, F.-D. (2020). Strategies to Improve Meat Products’ Quality. Foods, 9(12), 1883. https://doi.org/10.3390/foods9121883





