RSM Optimization for the Recovery of Technofunctional Protein Extracts from Porcine Hearts
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Experimental Design
2.3. Microbiological Analysis
2.4. Protein Extraction
2.5. Physicochemical Characterization
2.5.1. Proximate Analysis
2.5.2. SDS–Polyacrylamide Gel Electrophoresis (SDS–PAGE)
2.6. Technofunctional Properties
2.6.1. Protein Solubility
2.6.2. Foaming Properties
2.6.3. Emulsifying Properties
2.6.4. Heat-Induced Gelation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical and Microbiological Characterization of Porcine Hearts
3.2. Extractability and Compositional Characteristics of Heart Protein Fractions
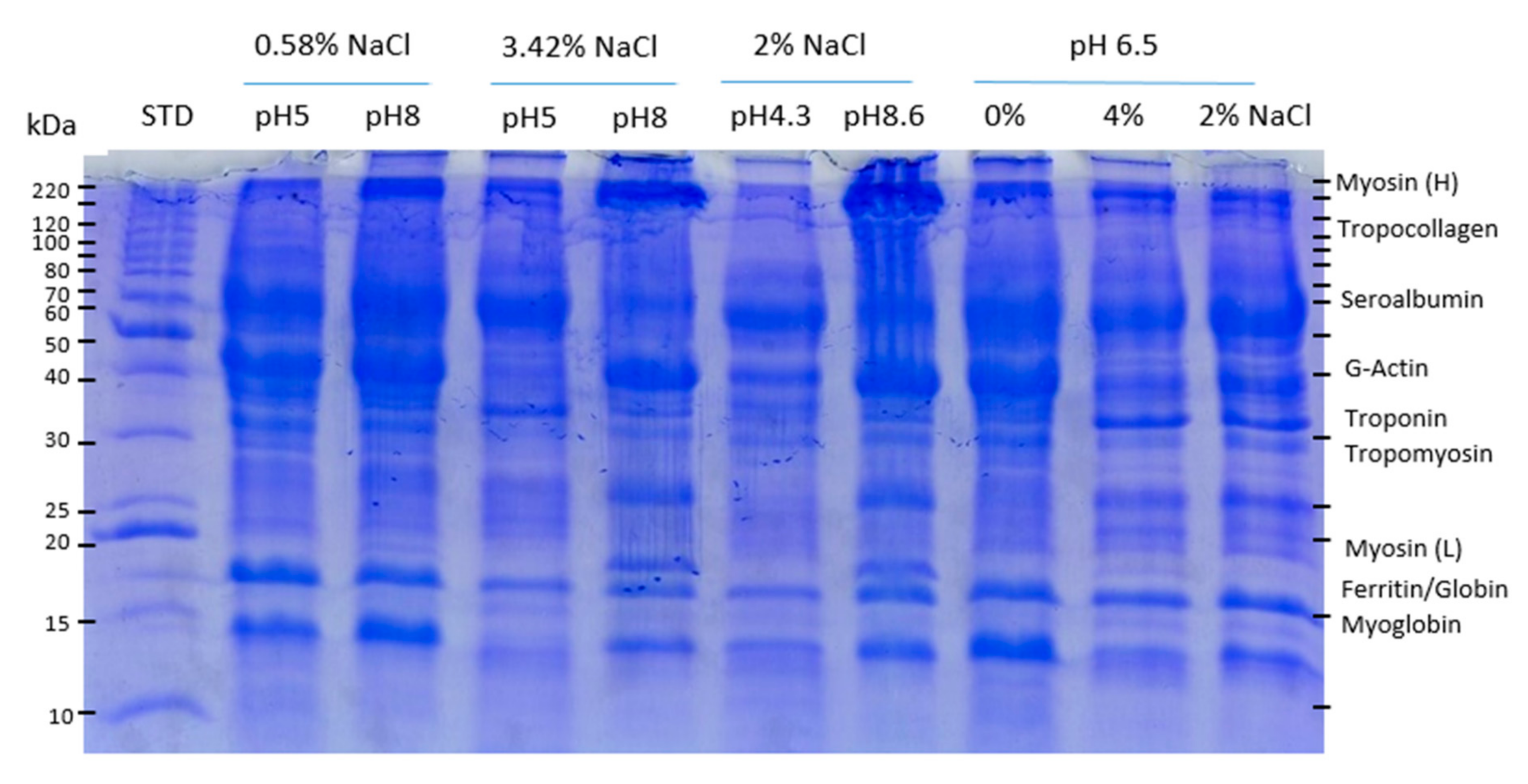
3.3. SDS-PAGE Profiles
3.4. Effects of the Extraction Conditions on the Functional Properties of Soluble Protein Extracts
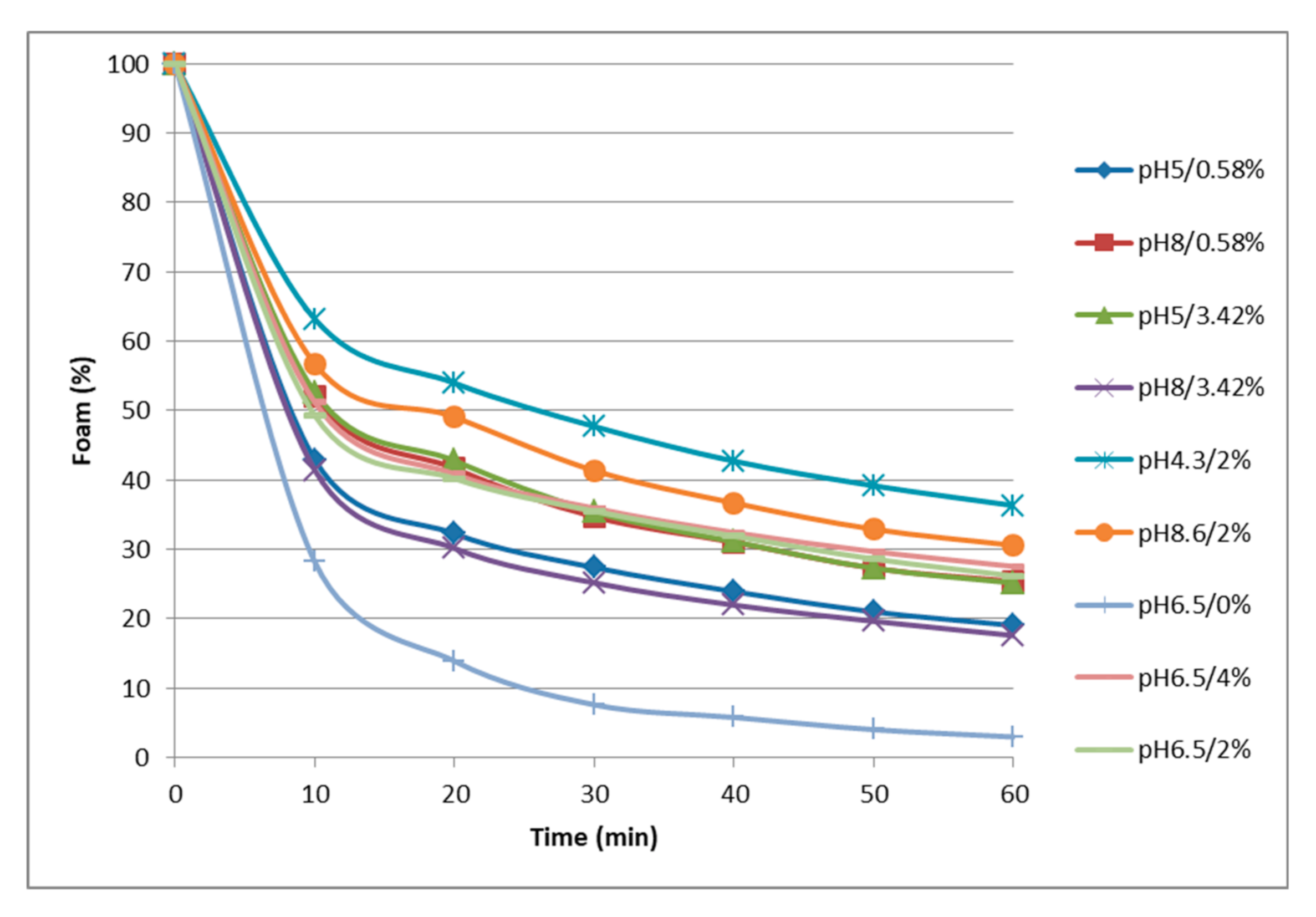
3.4.1. Foaming Properties
3.4.2. Emulsifying Properties
3.4.3. Gelling Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef]
- Rivera, J.A.; Sebranek, J.G.; Rust, R.E. Functional properties of meat by-products and mechanically separated chicken (MSC) in a high-moisture model petfood system. Meat Sci. 2000, 55, 61–66. [Google Scholar] [CrossRef]
- Toldrá, F.; Aristoy, M.C.; Mora, L.; Reig, M. Innovations in value-addition of edible meat by-products. Meat Sci. 2012, 92, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F.; Mora, L.; Reig, M. New insights into meat by-product utilization. Meat Sci. 2016, 120, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F.; Reig, M. Innovations for healthier processed meats. Trends Food Sci. Technol. 2011, 22, 517–522. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, S.; Samaraweera, H.; Lee, E.J.; Ahn, D.U. Improving functional value of meat products. Meat Sci. 2010, 86, 15–31. [Google Scholar] [CrossRef]
- Lynch, S.A.; Mullen, A.M.; O’Neill, E.; Drummond, L.; Álvarez, C. Opportunities and perspectives for utilisation of co-products in the meat industry. Meat Sci. 2018, 144, 62–73. [Google Scholar] [CrossRef]
- Matak, K.E.; Tahergorabi, R.; Jaczynski, J. A review: Protein isolates recovered by isoelectric solubilization/precipitation processing from muscle food by-products as a component of nutraceutical foods. Food Res. Int. 2015, 77, 697–703. [Google Scholar] [CrossRef]
- Mullen, A.M.; Álvarez, C.; Zeugolis, D.I.; Henchion, M.; O’Neill, E.; Drummond, L. Alternative uses for co-products: Harnessing the potential of valuable compounds from meat processing chains. Meat Sci. 2017, 132, 90–98. [Google Scholar] [CrossRef]
- Papier, K.; Ahmed, F.; Lee, P.; Wiseman, J. Stress and dietary behaviour among first-year university students in Australia: Sex differences. Nutrition 2015, 31, 324–330. [Google Scholar] [CrossRef]
- Toldrà, M.; Parés, D.; Saguer, E.; Carretero, C. Recovery and Extraction of Technofunctional Proteins from Porcine Spleen Using Response Surface Methodology. Food Bioprocess Technol. 2019, 12, 298–312. [Google Scholar] [CrossRef]
- Zouari, N.; Fakhfakh, N.; Amara-Dali, W.B.; Sellami, M.; Msaddak, L.; Ayadi, M.A. Turkey liver: Physicochemical characteristics and functional properties of protein fractions. Food Bioprod. Process. 2011, 89, 142–148. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cheong, J.K.; Yang Sungnam, S.Y.; Lee Suwon, M.H. Functional properties of the porcine variety meats. Korean J. Anim. Sci. 1991, 33, 507–514. [Google Scholar]
- Nuckles, R.O.; Smith, D.M.; Merkel, R.A. Meat By-product protein composition and functional properties in model systems. J. Food Sci. 1990, 55, 640–643. [Google Scholar] [CrossRef]
- Kim, H.K.; Ha, S.J.; Kim, Y.H.; Hong, S.P.; Kim, Y.U.; Song, K.M.; Lee, N.H.; Jung, S.K. Protein extraction from porcine myocardium using ultrasonication. J. Food Sci. 2017, 82, 1059–1065. [Google Scholar] [CrossRef]
- Tsermoula, P.; Virgili, C.; Ortega, R.G.; Mullen, A.M.; Álvarez, C.; O’Brien, N.M.; O’Flaherty, E.A.A.; O’Neill, E.E. Functional protein rich extracts from bovine and porcine hearts using acid or alkali solubilisation and isoelectric precipitation. Int. J. Food Sci. Technol. 2019, 54, 1292–1298. [Google Scholar] [CrossRef]
- International Organization for Standardization. Microbiology of the Food Chain. Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique; Standard No. 4833-1:2013; International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC; Association of Analytical Communities: Gaithersburg, MD, USA, 2000. [Google Scholar]
- International Organization for Standardization. Determination of Nitrogen Content. International Standards Meat and Meat Products; Standard No. 93:1978; International Organization for Standardization: Geneva, Switzerland, 1978. [Google Scholar]
- Kolar, K. Colorimetric determination of hydroxyproline as measure of collagen content in meat and meat products: NMKL collaborative study. J. AOAC 1990, 73, 54–57. [Google Scholar] [CrossRef]
- International Organization for Standardization. Determination of Total Fat Content. International Standards Meat and Meat Products; Standard No. 1443:1973; International Organization for Standardization: Geneva, Switzerland, 1973. [Google Scholar]
- Fort, N.; Kerry, J.P.; Carretero, C.; Kelly, A.L.; Saguer, E. Cold storage of porcine plasma treated with microbial transglutaminase under high pressure. Effects on its heat-induced gel properties. Food Chem. 2009, 115, 602–608. [Google Scholar] [CrossRef]
- Dàvila, E.; Saguer, E.; Toldrà, M.; Carretero, C.; Parés, D. Surface functional properties of blood plasma protein fractions. Eur. Food Res. Technol. 2007, 226, 207–214. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins—Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Parés, D.; Ledward, D.A. Emulsifying and gelling properties of porcine blood plasma as influenced by high-pressure processing. Food Chem. 2001, 74, 139–145. [Google Scholar] [CrossRef]
- Seong, P.N.; Park, K.M.; Cho, S.H.; Kang, S.M.; Kang, G.H.; Park, B.Y.; Moon, S.S.; Van Ba, H. Characterization of edible pork by-products by means of yield and nutritional composition. Korean J. Food Sci. Anim. Res. 2014, 34, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.; Seong, P.; Moon, S.; Cho, S.; Ham, H.; Park, K.; Kang, S.; Park, B. Distribution Channel and Microbial Characteristics of Pig By-products in Korea. Korean J. Food Sci. 2014, 34, 792–798. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Commission regulation (EC) no 2073/2005 of 15th November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 338, 1–26. [Google Scholar]
- Steen, L.; Glorieux, S.; Goemaere, O.; Brijs, K.; Paelinck, H.; Foubert, I.; Fraeye, I. Functional properties of pork liver protein fractions. Food Bioprocess Technol. 2016, 9, 970–980. [Google Scholar] [CrossRef]
- Selmane, D.; Christophe, V.; Gholamreza, D. Extraction of proteins from slaughterhouse by-products: Influence of operating conditions on functional properties. Meat Sci. 2008, 79, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Hrynets, Y.; Omana, D.A.; Xu, Y.; Betti, M. Effect of acid- and alkaline-aided extractions on functional and rheological properties of proteins recovered from mechanically separated turkey meat (MSTM). J. Food Sci. 2010, 75, 477–486. [Google Scholar] [CrossRef]
- Krasnowska, G.; Gorska, I.; Gergont, J. Evaluation of functional properties of offal proteins. Meat Sci. 1995, 39, 149–155. [Google Scholar] [CrossRef]
- Pérez-Chabela, M.L.; Soriano-Santos, J.; Ponce-Alquicira, E.; Díaz-Tenorio, L.M. Electroforesis en gel de poliacrilamida-SDS como herramienta en el estudio de las proteínas miofibrilares. Nacameh 2015, 9, 77–96. [Google Scholar]
- Howell, N.K.; Lawrie, R.A. Functional aspects of blood plasma proteins I. Separation and characterization. J. Food Technol. 1983, 18, 747–762. [Google Scholar] [CrossRef]
- Luna, E.J.; Hitt, A.L. Cytoskeleton-plasma membrane interactions. Science 1992, 258, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Grujić, R.; Savanović, D. Analysis of myofibrillar and sarcoplasmic proteins in pork meat by capillary gel electrophoresis. Foods Raw Mater. 2018, 6, 421–428. [Google Scholar] [CrossRef]
- Toldrà, M.; Parés, D.; Saguer, E.; Carretero, C. Hemoglobin hydrolysates from porcine blood obtained through enzymatic hydrolysis assisted by high hydrostatic pressure processing. Innov. Food Sci. Emerg. Technol. 2011, 12, 435–442. [Google Scholar] [CrossRef]
- Aluko, R.E.; McIntosh, T. Polypeptide profile and functional properties of defatted meals and protein isolates of canola seeds. J. Sci. Food Agric. 2001, 81, 391–396. [Google Scholar] [CrossRef]
- Indrawati, L.; Wang, Z.; Narsimhan, G.; Gonzalez, J. Effect of processing parameters on foam formation using a continuous system with a mechanical whipper. J. Food Eng. 2008, 88, 65–74. [Google Scholar] [CrossRef]
- Yang, Q.L.; Lou, X.W.; Wang, Y.; Pan, D.D.; Sun, Y.Y.; Cao, J.X. Effect of pH on the interaction of volatile compounds with the myofibrillar proteins of duck meat. Poultry Sci. 2017, 96, 1963–1969. [Google Scholar] [CrossRef]
- Lynch, S.A.; Álvarez, C.; O’Neill, E.E.; Keenan, D.F.; Mullen, A.M. Optimization of protein recovery from bovine lung by pH shift process using response surface methodology. J. Sci. Food Agric. 2018, 98, 1951–1960. [Google Scholar] [CrossRef]
- Akasha, I.; Campbell, L.; Lonchamp, J.; Euston, S.R. The major proteins of the seed of the fruit of the date palm (Phoenix dactylifera L.): Characterisation and emulsifying properties. Food Chem. 2016, 197, 799–806. [Google Scholar] [CrossRef]
- Díaz, O.; Pereira, C.D.; Cobos, A. Functional properties of ovine whey protein concentrates produced by membrane technology after clarification of cheese manufacture by-products. Food Hydrocoll. 2004, 18, 601–610. [Google Scholar] [CrossRef]
- Liceaga-Gesualdo, A.M.; Li-Chan, E.C.-Y. Functional Properties of Fish Protein Hydrolysate from Herring (Clupea harengus). J. Food Sci. 1999, 64, 1000–1004. [Google Scholar] [CrossRef]
- Ziegler, G.R.; Foegeding, E.A. The gelation of proteins. Adv. Food Nutr. Res. 1990, 34, 203–298. [Google Scholar]
- Zayas, J.F. Gelling Properties of proteins. In Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997; pp. 310–366. [Google Scholar]
- James, J.M.; Mireles DeWitt, C.A. Gel Attributes of Beef Heart When Treated by Acid Solubilization Isoelectric Precipitation. J. Food Sci. 2004, 69, 473–480. [Google Scholar] [CrossRef]
- Parés, D.; Saguer, E.; Carretero, C. Blood by-products as ingredients in processed meat. In Processed Meats: Improving Safety, Nutrition and Quality; Kerry, J.P., Kerry, J.F., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2011; pp. 218–242. [Google Scholar]

| Run | Coded Variables | Order | Experimental Variables | ||
|---|---|---|---|---|---|
| pH | NaCl (%) | ||||
| 1 | −1 | −1 | 13 | 5 | 0.58 |
| 2 | +1 | −1 | 5 | 8 | 0.58 |
| 3 | −1 | +1 | 7 | 5 | 3.42 |
| 4 | +1 | +1 | 12 | 8 | 3.42 |
| 5 | −α | 0 | 6 | 4.3 | 2 |
| 6 | +α | 0 | 1 | 8.6 | 2 |
| 7 | 0 | −α | 3 | 6.5 | 0 |
| 8 | 0 | +α | 10 | 6.5 | 4 |
| 9 | 0 | 0 | 4 | 6.5 | 2 |
| 10 | 0 | 0 | 9 | 6.5 | 2 |
| 11 | 0 | 0 | 11 | 6.5 | 2 |
| 12 | 0 | 0 | 8 | 6.5 | 2 |
| 13 | 0 | 0 | 2 | 6.5 | 2 |
| Weight (g) | 407.67 ± 36.14 |
| pH | 5.89 ± 0.10 |
| Moisture (%) | 79.31 ± 0.34 |
| Protein (%) | 16.70 ± 0.18 |
| Collagen (%) | 1.13 ± 0.30 |
| Fat (%) | 2.46 ± 0.54 |
| Ashes (%) | 1.04 ± 0.07 |
| Iron (ppm) | 60.33 ± 20.19 |
| Color properties | |
| Chroma | 19.79 ± 0.59 |
| Hue (°) | 25.60 ± 1.39 |
| Lightness (L*) | 32.55 ± 1.16 |
| Bacterial counts (log cfu g−1) | 3.49 ± 0.46 |
| pH | NaCl | Yield (%) | Proximate Composition (%) | ||
|---|---|---|---|---|---|
| Moisture | Protein | Ash | |||
| 5 | 0.58 | 67.2 | 96.63 | 2.33 | 0.89 |
| 8 | 0.58 | 63.2 | 95.57 | 2.85 | 1.11 |
| 5 | 3.42 | 59.5 | 93.78 | 3.03 | 2.74 |
| 8 | 3.42 | 68.6 | 94.21 | 3.35 | 2.00 |
| 4.3 | 2 | 71.3 | 95.63 | 1.79 | 2.01 |
| 8.6 | 2 | 72.8 | 94.61 | 3.59 | 1.62 |
| 6.5 | 0 | 68.6 | 96.82 | 2.24 | 0.37 |
| 6.5 | 4 | 67.7 | 92.84 | 3.35 | 3.30 |
| 6.5 | 2 | 68.0 ± 1.7 | 94.64 ± 0.39 | 1.88 ± 0.20 | 3.05 ± 0.01 |
| pH | NaCl | Solubility (%) | Foaming | Emulsifying | Gelling | ||
|---|---|---|---|---|---|---|---|
| FC | RFS | EAI | ESI | GS | |||
| 5 | 0.58 | 26.9 | 596.5 | 8.53 | 236.44 | 20.13 | 1.31 |
| 8 | 0.58 | 29.9 | 375.9 | 12.37 | 297.32 | 170.62 | 2.18 |
| 5 | 3.42 | 31.5 | 572.0 | 12.89 | 278.89 | 41.10 | 1.19 |
| 8 | 3.42 | 38.9 | 343.2 | 8.03 | 150.39 | 22.47 | 11.47 |
| 4.3 | 2 | 22.4 | 738.1 | 22.04 | 259.09 | 25.65 | 0.82 |
| 8.6 | 2 | 44.3 | 283.3 | 16.17 | 251.79 | 19.94 | 2.42 |
| 6.5 | 0 | 26.3 | 365.0 | 5.55 | 354.74 | 35.19 | 2.15 |
| 6.5 | 4 | 39.2 | 324.1 | 11.64 | 206.35 | 26.15 | 18.77 |
| 6.5 | 2 | 35.8 ± 1.1 | 326.8 ± 41.6 | 11.15 ± 1.78 | 308.00 ± 39.01 | 25.32 ± 6.50 | 5.38 ± 0.89 |
| Protein Solubility (%) | Foaming | Emulsifying | Gelling | |||
|---|---|---|---|---|---|---|
| FC | RFS | EAI | ESI | GS | ||
| Coefficients | ||||||
| Constant | 14.762 | 2732.309 | 64.900 | −385.954 | −110.123 | 1.629 |
| pH | 6.090 | −647.477 | −18.880 | 201.037 | ns | ns |
| NaCl | −16.740 | ns | 4.396 | ns | 55.987 | ns |
| pH*NaCl | ns | ns | ns | ns | ns | ns |
| pH2 | −0.669 | 43.023 | 1.502 | −13.923 | 3.971 | ns |
| NaCl2 | ns | ns | ns | 28.200 | ns | −11.310 |
| pH2*NaCl | 0.550 | ns | ns | ns | −1.576 | ns |
| pH*NaCl2 | 1.408 | ns | ns | −5.456 | ns | 3.544 |
| pH2*NaCl2 | −0.236 | ns | −0.021 | ns | ns | −0.253 |
| Adjusted R2 | 0.978 | 0.901 | 0.614 | 0.655 | 0.490 | 0.946 |
| Significance | 0.000 | 0.000 | 0.017 | 0.011 | 0.028 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parés, D.; Toldrà, M.; Camps, E.; Geli, J.; Saguer, E.; Carretero, C. RSM Optimization for the Recovery of Technofunctional Protein Extracts from Porcine Hearts. Foods 2020, 9, 1733. https://doi.org/10.3390/foods9121733
Parés D, Toldrà M, Camps E, Geli J, Saguer E, Carretero C. RSM Optimization for the Recovery of Technofunctional Protein Extracts from Porcine Hearts. Foods. 2020; 9(12):1733. https://doi.org/10.3390/foods9121733
Chicago/Turabian StyleParés, Dolors, Mònica Toldrà, Estel Camps, Juan Geli, Elena Saguer, and Carmen Carretero. 2020. "RSM Optimization for the Recovery of Technofunctional Protein Extracts from Porcine Hearts" Foods 9, no. 12: 1733. https://doi.org/10.3390/foods9121733
APA StyleParés, D., Toldrà, M., Camps, E., Geli, J., Saguer, E., & Carretero, C. (2020). RSM Optimization for the Recovery of Technofunctional Protein Extracts from Porcine Hearts. Foods, 9(12), 1733. https://doi.org/10.3390/foods9121733





