Fatty Acid Profiling and Chemometric Analyses for Zanthoxylum Pericarps from Different Geographic Origin and Genotype
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Determination of Environmental Factors
2.3. Fatty Acid Extraction and Analysis
2.4. Data Analyses
3. Results and Discussion
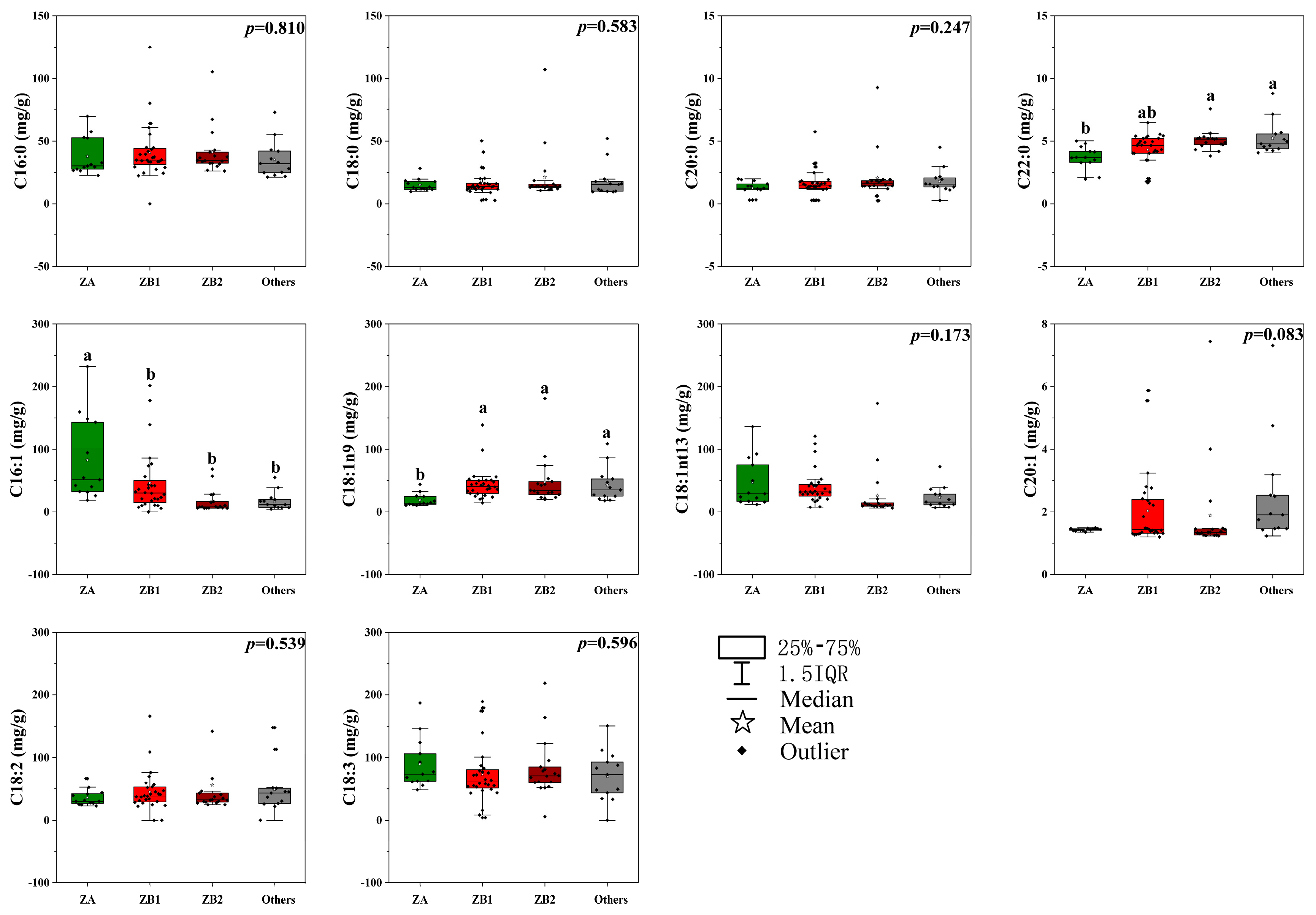
3.1. The Fatty Acid Profiling in Pericarps among Different Zanthoxylum Species
3.2. Chemometric Analyses for Zanthoxylum Pericarps Based on Fatty Acid Data
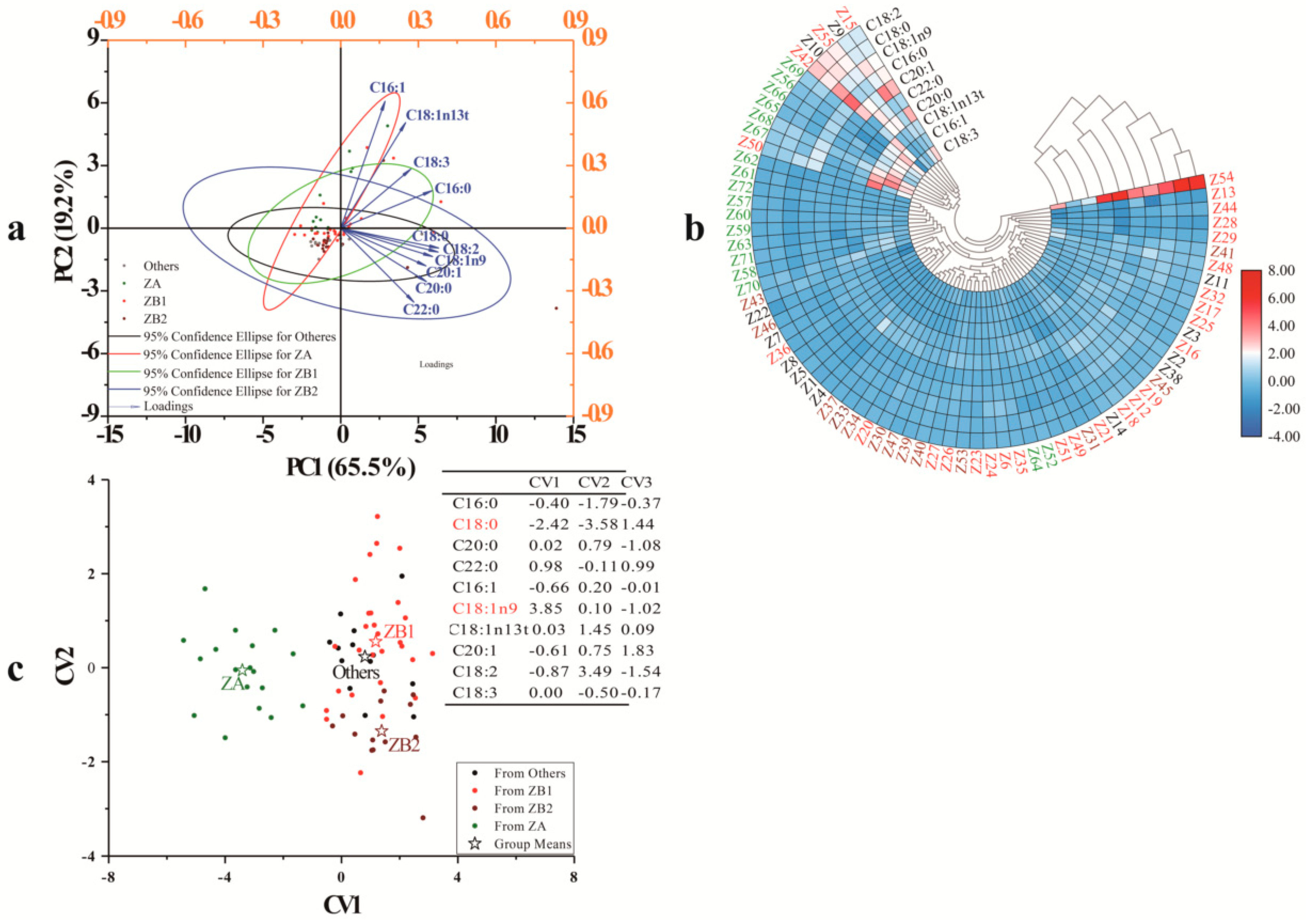
3.2.1. Principal Component Analysis
3.2.2. Cluster Heat Map (CHM)
3.2.3. Discriminant Analysis (DA)
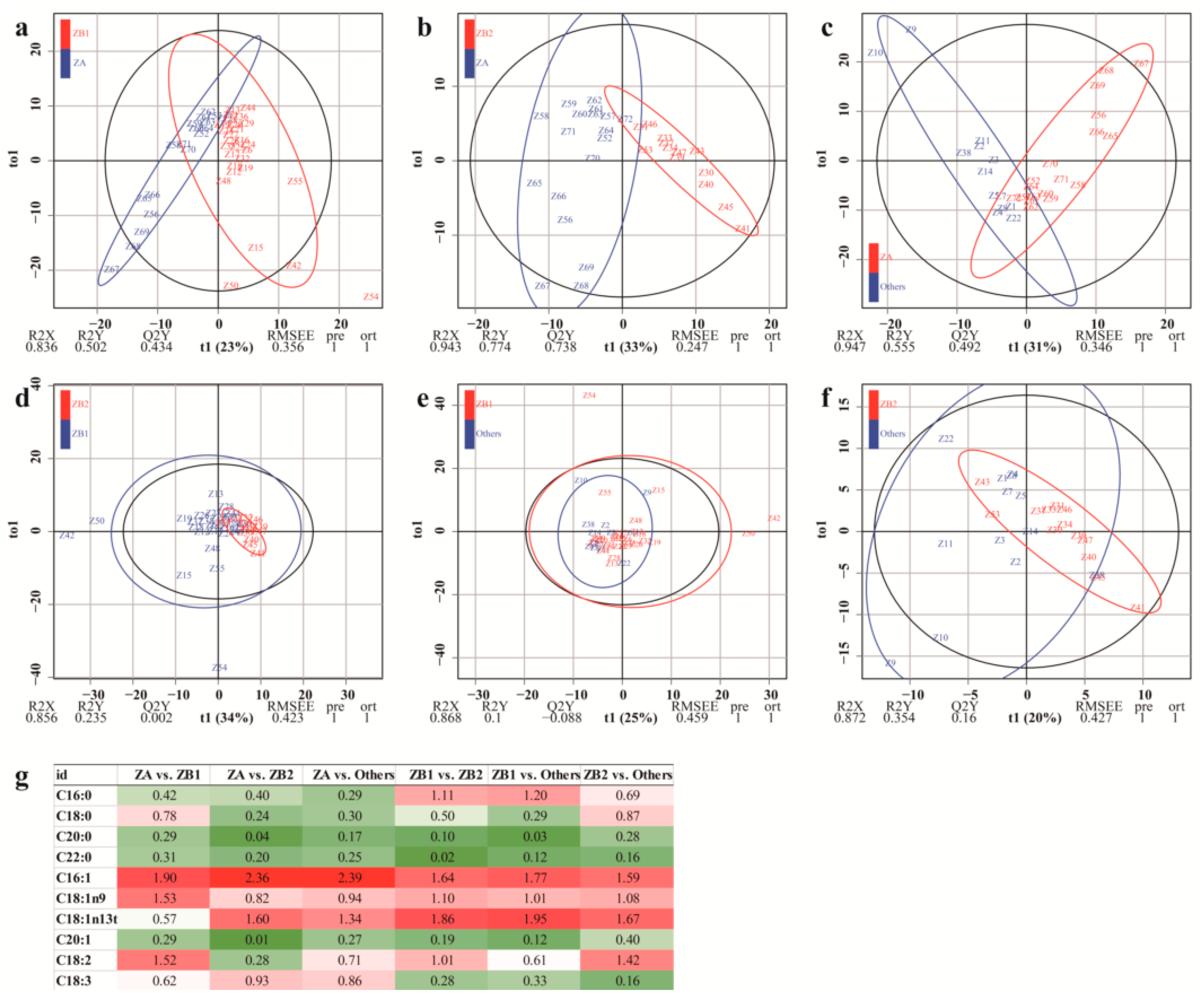
3.2.4. Orthogonal Partial Least Squares Discriminant Analysis
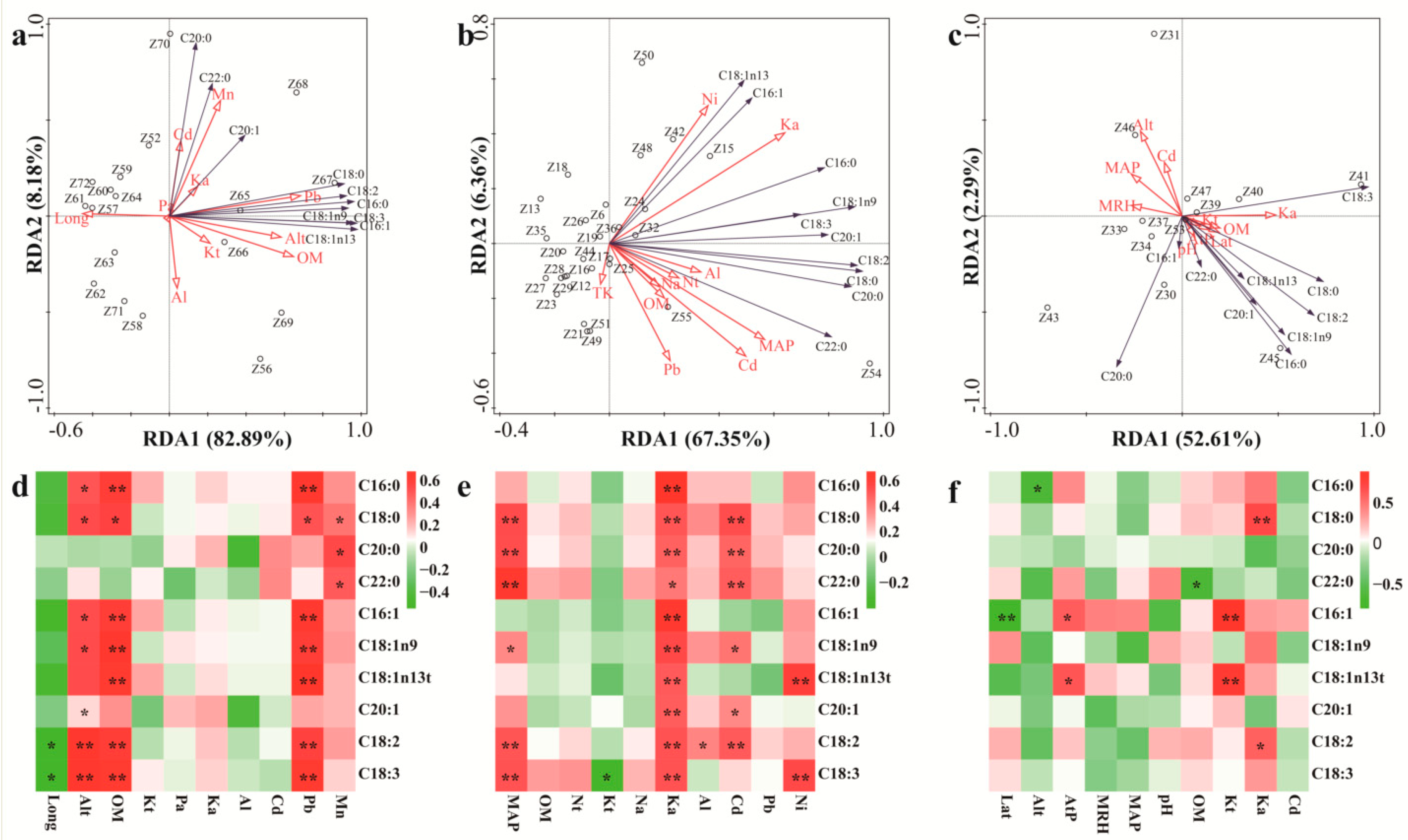
3.3. The Influences of Environmental Factors on Fatty Acid Content and Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Wang, W.; Wang, C.; Liu, Z.; Sun, Q.; Wang, D. Quality evaluation and chemometric discrimination of Zanthoxylum bungeanum Maxim leaves based on flavonoids profiles, bioactivity and HPLC-fingerprint in a common garden experiment. Ind. Crop. Prod. 2019, 134, 225–233. [Google Scholar]
- Lee, J.; da Silva, R.R.; Jang, H.S.; Kim, H.W.; Kwon, Y.S.; Kim, J.H.; Yang, H. In silico annotation of discriminative markers of three Zanthoxylum species using molecular network derived annotation propagation. Food Chem. 2019, 295, 368–376. [Google Scholar] [PubMed]
- Lan, Y.; Li, H.; Chen, Y.Y.; Zhang, Y.W.; Liu, N.; Zhang, Q.; Wu, Q. Essential oil from Zanthoxylum bungeanum Maxim. and its main components used as transdermal penetration enhancers: A comparative study. J. Zhejiang Univ.-SCI. B 2014, 15, 940–952. [Google Scholar]
- Sriwichai, T.; Sookwong, P.; Siddiqui, M.W.; Sommano, S.R. Aromatic profiling of Zanthoxylum myriacanthum (makwhaen) essential oils from dried fruits using different initial drying techniques. Ind. Crop. Prod. 2019, 133, 284–291. [Google Scholar]
- Wang, L.; Wang, Z.; Li, X.; Zhang, H.; Zhou, X.; Zhang, H. Analysis of Volatile Compounds in the Pericarp of Zanthoxylum bungeanum Maxim. by Ultrasonic Nebulization Extraction Coupled with Headspace Single-Drop Microextraction and GC–MS. Chromatographia 2010, 71, 455–459. [Google Scholar]
- Nooreen, Z.; Singh, S.; Singh, D.K.; Tandon, S.; Ahmad, A.; Luqman, S. Characterization and evaluation of bioactive polyphenolic constituents from Zanthoxylum armatum DC., a traditionally used plant. Biomed. Pharmacother. 2017, 89, 366–375. [Google Scholar]
- Bader, M.; Stark, T.D.; Dawid, C.; Losch, S.; Hofmann, T. All-trans-configuration in Zanthoxylum alkylamides swaps the tingling with a numbing sensation and diminishes salivation. J. Agric. Food Chem. 2014, 62, 2479–2488. [Google Scholar]
- Ke, J.; Qu, Y.; Li, S.; Shen, G.; Chen, A.; Luo, Q.; Liu, X.; Wu, H.; Li, M.; Pu, B.; et al. Application of HPLC fingerprint based on acid amide components in Chinese prickly ash (Zanthoxylum). Ind. Crop. Prod. 2018, 119, 267–276. [Google Scholar]
- Tian, J.M.; Wang, Y.; Xu, Y.Z.; Yu, Z.C.; Wei, A.Z.; Zhang, W.M.; Gao, J.M. Characterization of isobutylhydroxyamides with NGF-potentiating activity from Zanthoxylum bungeanum. Bioorg. Med. Chem. Lett. 2016, 26, 338–342. [Google Scholar]
- Zhang, M.; Xie, M.; Wei, D.; Wang, L.; Hu, M.; Zhang, Q.; He, Z.; Peng, W.; Wu, C. Hydroxy-alpha-sanshool isolated from Zanthoxylum bungeanum attenuates learning and memory impairments in scopolamine-treated mice. Food Funct. 2019, 10, 7315–7324. [Google Scholar]
- Ma, Y.; Fei, X.; Li, J.; Liu, Y.; Wei, A. Effects of location, climate, soil conditions and plant species on levels of potentially toxic elements in Chinese Prickly Ash pericarps from the main cultivation regions in China. Chemosphere 2020, 244, 125501. [Google Scholar] [PubMed]
- Xiang, L.; Liu, Y.; Xie, C.; Li, X.; Yu, Y.; Ye, M.; Chen, S. The Chemical and Genetic Characteristics of Szechuan Pepper (Zanthoxylum bungeanum and Z. armatum) Cultivars and Their Suitable Habitat. Front. Plant Sci. 2016, 7, 467. [Google Scholar] [PubMed] [Green Version]
- Medeiros Melo, D.; Ferreira Roseno, T.; Barros, W.M.; de Faria, R.A.P.G.; de Souza Paglarini, C.; Bitencourt Faria, P.; Mariotto, S.; de Souza, X.R. Fatty acid profiles and cholesterol content of Five species of pacu-pevas from the pantanal region of Mato Grosso, Brazil. J. Food Compos. Anal. 2019, 83, 103283. [Google Scholar]
- He, Y.; Li, Z.; Tan, F.; Liu, H.; Zhu, M.; Yang, H.; Bi, G.; Wan, H.; Wang, J.; Xu, R.; et al. Fatty acid metabolic flux and lipid peroxidation homeostasis maintain the biomembrane stability to improve citrus fruit storage performance. Food Chem. 2019, 292, 314–324. [Google Scholar] [PubMed]
- Sanchez Granel, M.L.; Canepa, C.; Cid, N.G.; Navarro, J.C.; Monroig, O.; Verstraeten, S.V.; Nudel, C.B.; Nusblat, A.D. Gene identification and functional characterization of a Delta12 fatty acid desaturase in Tetrahymena thermophila and its influence in homeoviscous adaptation to low temperature. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1644–1655. [Google Scholar]
- Shi, F.; Zhou, X.; Yao, M.M.; Zhou, Q.; Ji, S.J.; Wang, Y. Low-temperature stress-induced aroma loss by regulating fatty acid metabolism pathway in ’Nanguo’ pear. Food Chem. 2019, 297, 124927. [Google Scholar]
- Kumar, N.; Gautam, A.; Dubey, A.K.; Ranjan, R.; Pandey, A.; Kumari, B.; Singh, G.; Mandotra, S.; Chauhan, P.S.; Srikrishna, S.; et al. GABA mediated reduction of arsenite toxicity in rice seedling through modulation of fatty acids, stress responsive amino acids and polyamines biosynthesis. Ecotoxicol. Environ. Saf. 2019, 173, 15–27. [Google Scholar]
- Zaman, S.; Hu, S.; Alam, M.A.; Du, H.; Che, S. The accumulation of fatty acids in different organs of purslane under salt stress. Sci. Hortic. 2019, 250, 236–242. [Google Scholar]
- Hosseini, S.S.; Rezadoost, H.; Nadjafi, F.; Hassan Asareh, M. Comparative essential oil composition and fatty acid profiling of some Iranian black cumin landraces. Ind. Crop. Prod. 2019, 140, 111628. [Google Scholar]
- Ma, T.; Zheng, Z.; Guo, H.; Lian, X.; Rane, M.J.; Cai, L.; Kim, K.S.; Kim, K.T.; Zhang, Z.; Bi, L. 4-O-methylhonokiol ameliorates type 2 diabetes-induced nephropathy in mice likely by activation of AMPK-mediated fatty acid oxidation and Nrf2-mediated anti-oxidative stress. Toxicol. Appl. Pharmacol. 2019, 370, 93–105. [Google Scholar]
- Rai, N.; Sjoberg, V.; Forsberg, G.; Karlsson, S.; Olsson, P.E.; Jass, J. Metal contaminated soil leachates from an art glass factory elicit stress response, alter fatty acid metabolism and reduce lifespan in Caenorhabditis elegans. Sci. Total Environ. 2019, 651, 2218–2227. [Google Scholar] [PubMed]
- Church, M.W.; Jen, K.L.; Anumba, J.I.; Jackson, D.A.; Adams, B.R.; Hotra, J.W. Excess omega-3 fatty acid consumption by mothers during pregnancy and lactation caused shorter life span and abnormal ABRs in old adult offspring. Neurotoxicol. Teratol. 2010, 32, 171–181. [Google Scholar] [PubMed] [Green Version]
- Krzyżaniak, M.; Stolarski, M.J.; Tworkowski, J.; Puttick, D.; Eynck, C.; Załuski, D.; Kwiatkowski, J. Yield and seed composition of 10 spring camelina genotypes cultivated in the temperate climate of Central Europe. Ind. Crop. Prod. 2019, 138, 111443. [Google Scholar]
- Gatzias, I.S.; Karabagias, I.K.; Kontominas, M.G.; Badeka, A.V. Geographical differentiation of feta cheese from northern Greece based on physicochemical parameters, volatile compounds and fatty acids. LWT Food Sci. Technol. 2020, 138, 109615. [Google Scholar]
- Ma, Y.; Li, J.; Tian, M.; Liu, Y.; Wei, A. Authentication of Chinese prickly ash by ITS2 sequencing and the influence of environmental factors on pericarp quality traits. Ind. Crop. Prod. 2020, 155, 112770. [Google Scholar]
- Jordi Folch, M.L.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1956, 226, 497–509. [Google Scholar]
- Christie, W.W. Preparation of ester derivatives of fatty acids for chromatographic analysis. In Advanced in Lipid Methodology-Two; Oily Press LTD.: Dundee, Scotland, 1993; pp. 69–111. [Google Scholar]
- Nengroo, Z.R.; Rauf, A. Fatty acid composition and antioxidant activities of five medicinal plants from Kashmir. Ind. Crop. Prod. 2019, 140, 111596. [Google Scholar]
- Hou, L.; Liu, Y.; Wei, A. Geographical variations in the fatty acids of Zanthoxylum seed oils: A chemometric classification based on the random forest algorithm. Ind. Crop. Prod. 2019, 134, 146–153. [Google Scholar]
- Boschin, G.; D’Agostina, A.; Annicchiarico, P.; Arnoldi, A. Effect of genotype and environment on fatty acid composition of Lupinus albus L. seed. Food Chem. 2008, 108, 600–606. [Google Scholar]
- Gresta, F.; Ceravolo, G.; Presti, V.L.; D’Agata, A.; Rao, R.; Chiofalo, B. Seed yield, galactomannan content and quality traits of different guar (Cyamopsis tetragonoloba L.) genotypes. Ind. Crop. Prod. 2017, 107, 122–129. [Google Scholar]
- HMSO, U.K. Nutritional Aspects of Cardiovascular Disease. In Report on Health and Social Subjects; HMSO: London, UK, 1994; p. 46. [Google Scholar]
- Jia, G.; Sha, K.; Feng, X.; Liu, H. Post-thawing metabolite profile and amino acid oxidation of thawed pork tenderloin by HVEF-A short communication. Food Chem. 2019, 291, 16–21. [Google Scholar] [PubMed]
- Zribi, I.; Bleton, J.; Moussa, F.; Abderrabba, M. GC-MS analysis of the volatile profile and the essential oil compositions of Tunisian Borago Officinalis L.: Regional locality and organ dependency. Ind. Crop. Prod. 2019, 129, 290–298. [Google Scholar]
- Berti, M.; Gesch, R.; Eynck, C.; Anderson, J.; Cermak, S. Camelina uses, genetics, genomics, production, and management. Ind. Crop. Prod. 2016, 94, 690–710. [Google Scholar]
- Vollmann, J.; Moritz, T.; Kargl, C.; Baumgartner, S.; Wagentristl, H. Agronomic evaluation of camelina genotypes selected for seed quality characteristics. Ind. Crop. Prod. 2007, 26, 270–277. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Tian, J.; Wang, X.; Huang, C.; Tian, M.; Wei, A. Fatty Acid Profiling and Chemometric Analyses for Zanthoxylum Pericarps from Different Geographic Origin and Genotype. Foods 2020, 9, 1676. https://doi.org/10.3390/foods9111676
Ma Y, Tian J, Wang X, Huang C, Tian M, Wei A. Fatty Acid Profiling and Chemometric Analyses for Zanthoxylum Pericarps from Different Geographic Origin and Genotype. Foods. 2020; 9(11):1676. https://doi.org/10.3390/foods9111676
Chicago/Turabian StyleMa, Yao, Jieyun Tian, Xiaona Wang, Chen Huang, Mingjing Tian, and Anzhi Wei. 2020. "Fatty Acid Profiling and Chemometric Analyses for Zanthoxylum Pericarps from Different Geographic Origin and Genotype" Foods 9, no. 11: 1676. https://doi.org/10.3390/foods9111676
APA StyleMa, Y., Tian, J., Wang, X., Huang, C., Tian, M., & Wei, A. (2020). Fatty Acid Profiling and Chemometric Analyses for Zanthoxylum Pericarps from Different Geographic Origin and Genotype. Foods, 9(11), 1676. https://doi.org/10.3390/foods9111676




