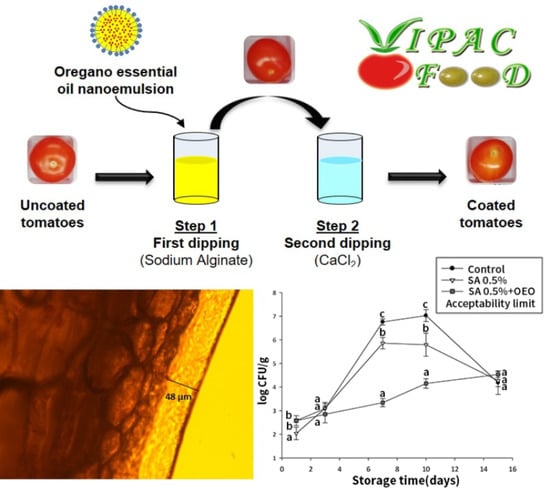
Edible Coatings Containing Oregano Essential Oil Nanoemulsion for Improving Postharvest Quality and Shelf Life of Tomatoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oregano Essential Oil Nanoemulsion Preparation
2.3. Nanoemulsion Characterization
2.3.1. Determination of Hydrodynamic Diameter, Polydispersity Index, and Zeta Potential
2.3.2. In Vitro OEO Nanoemulsions Release
2.4. Preparation of Coating Solutions
2.5. Coating Application
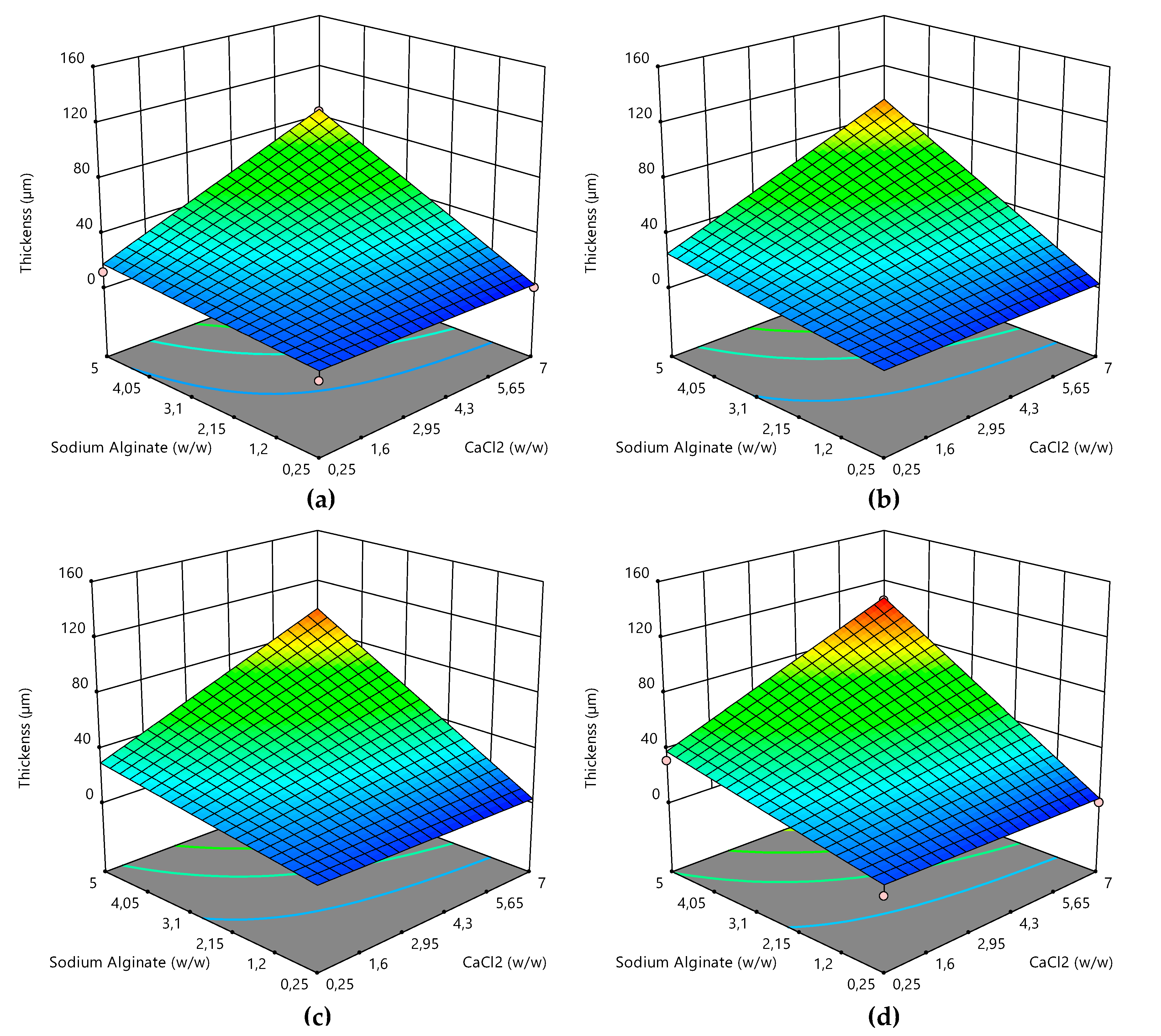
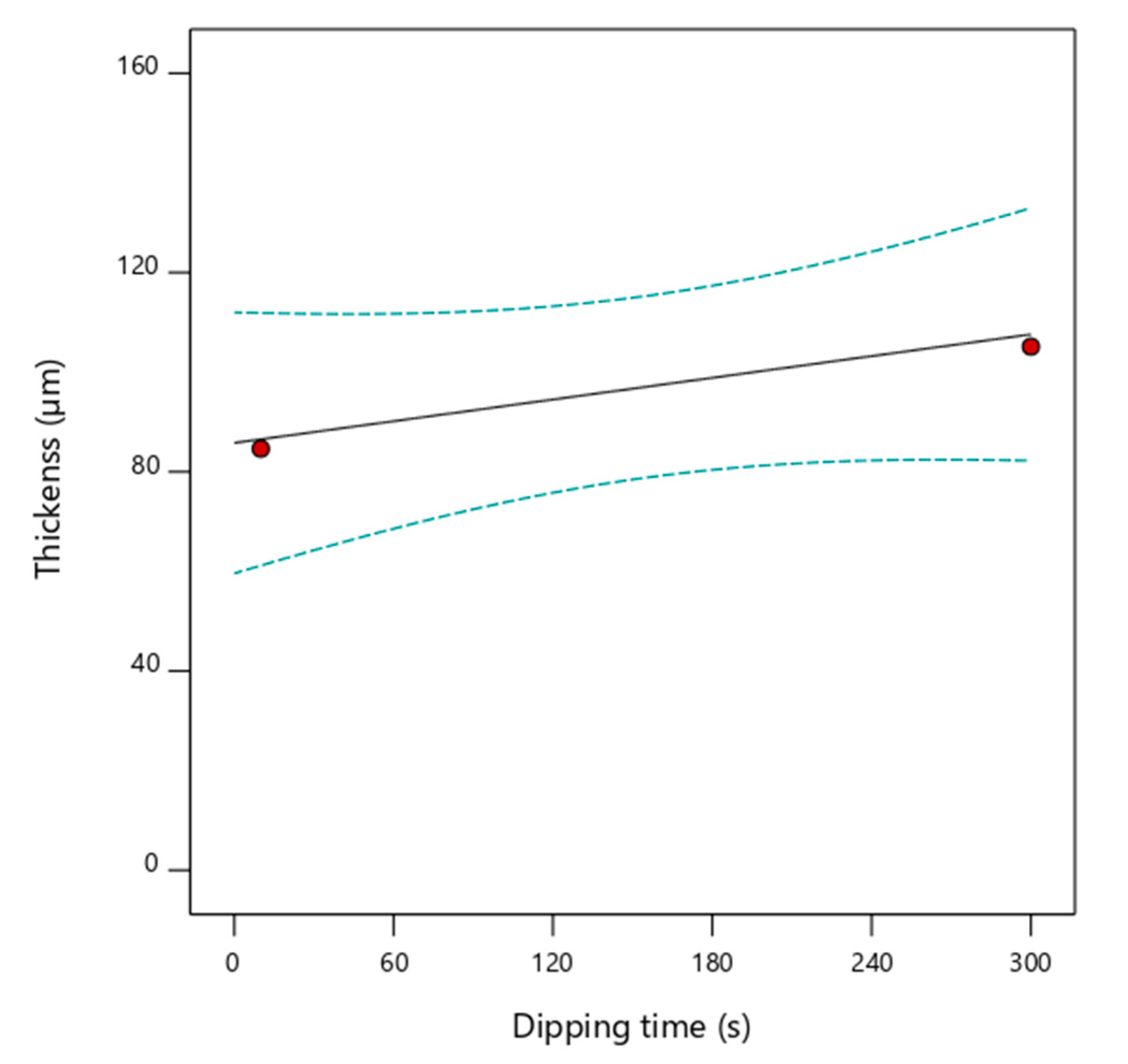
2.6. Response Surface Methodology for Coating Thickness Optimization
2.7. Edible Coatings Characterization
2.7.1. Thickness Determination
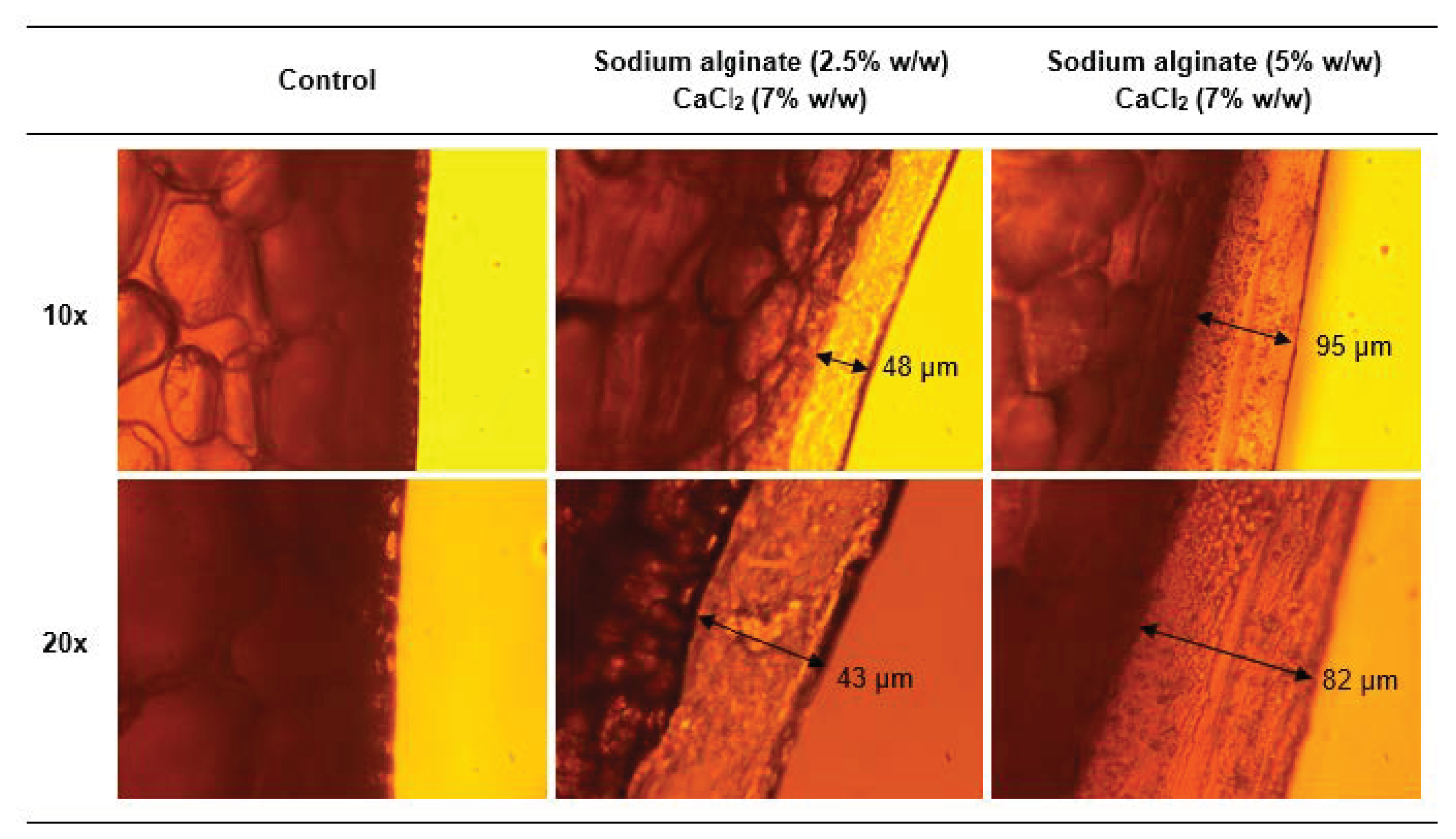
2.7.2. Light Microscopy Analysis
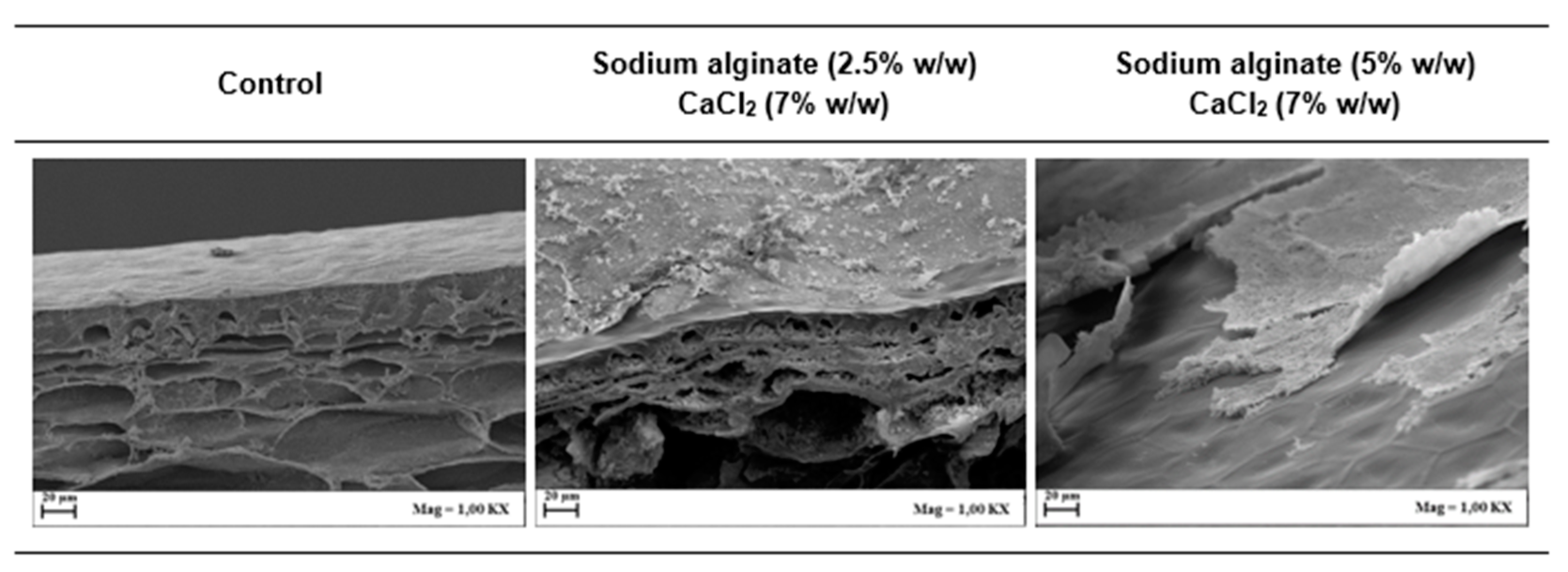
2.7.3. Scanning Electron Microscopy (SEM) Analysis
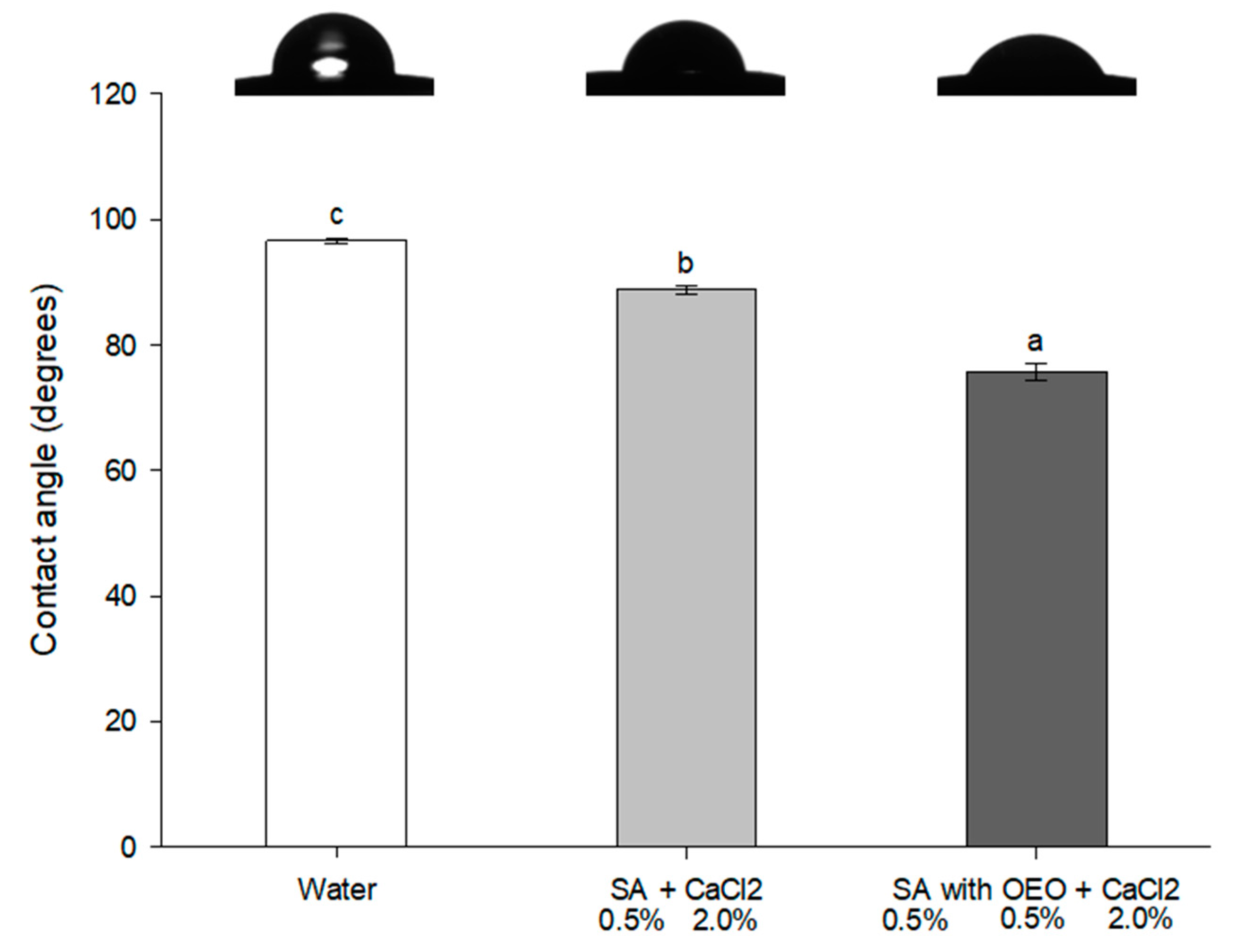
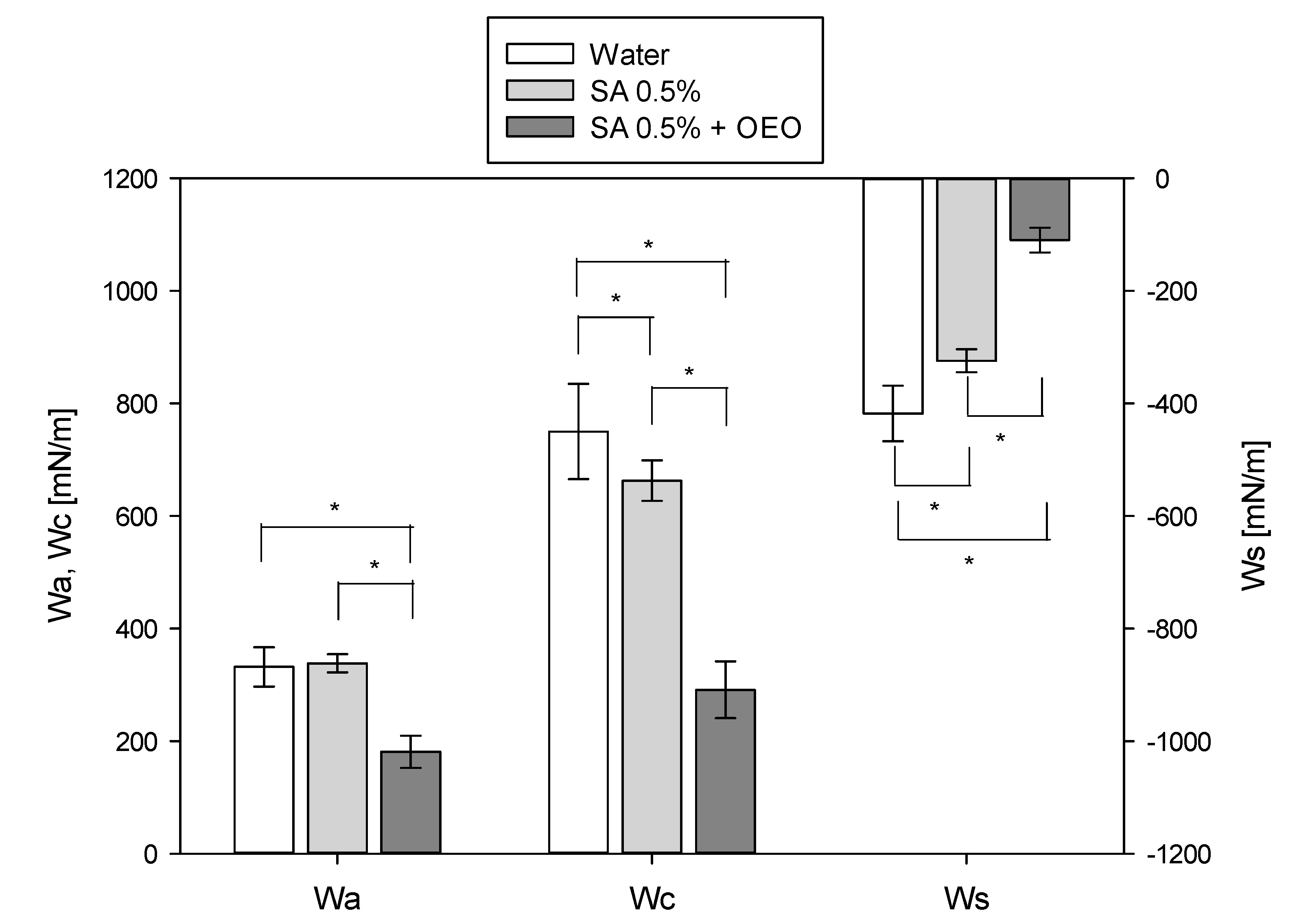
2.8. Contact Angle Measurement and Wetting Properties
2.9. Microbial Population during Storage
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Stability of Nanoemulsions Encapsulating OEO
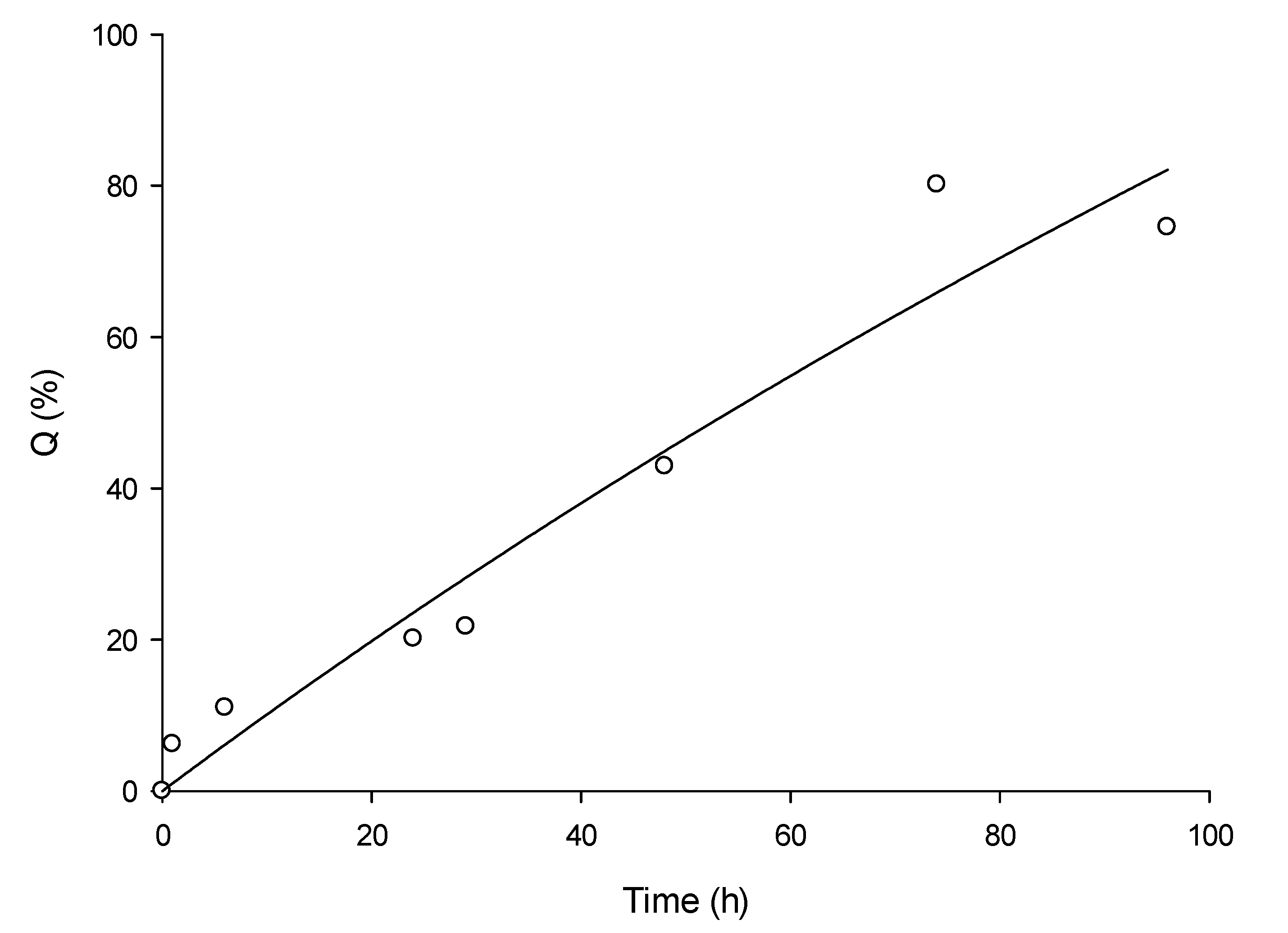
3.2. In Vitro Permeation OEO Nanoemulsion Formulation
3.3. Coating Characterization: Microstructure of Films
3.4. Coating Thickness Optimization
3.5. Wettability of Tomato Peel with the Film-Forming Solutions
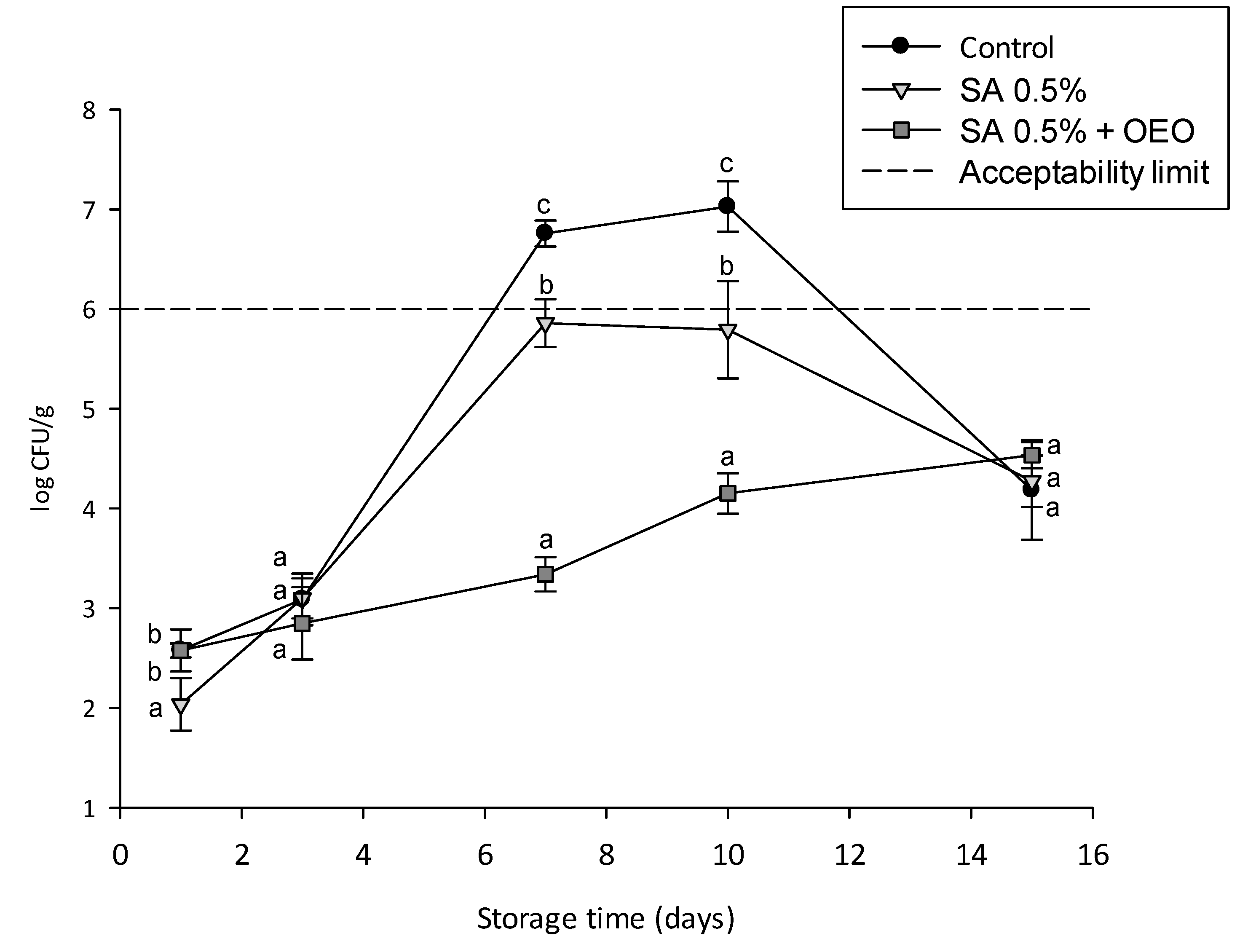
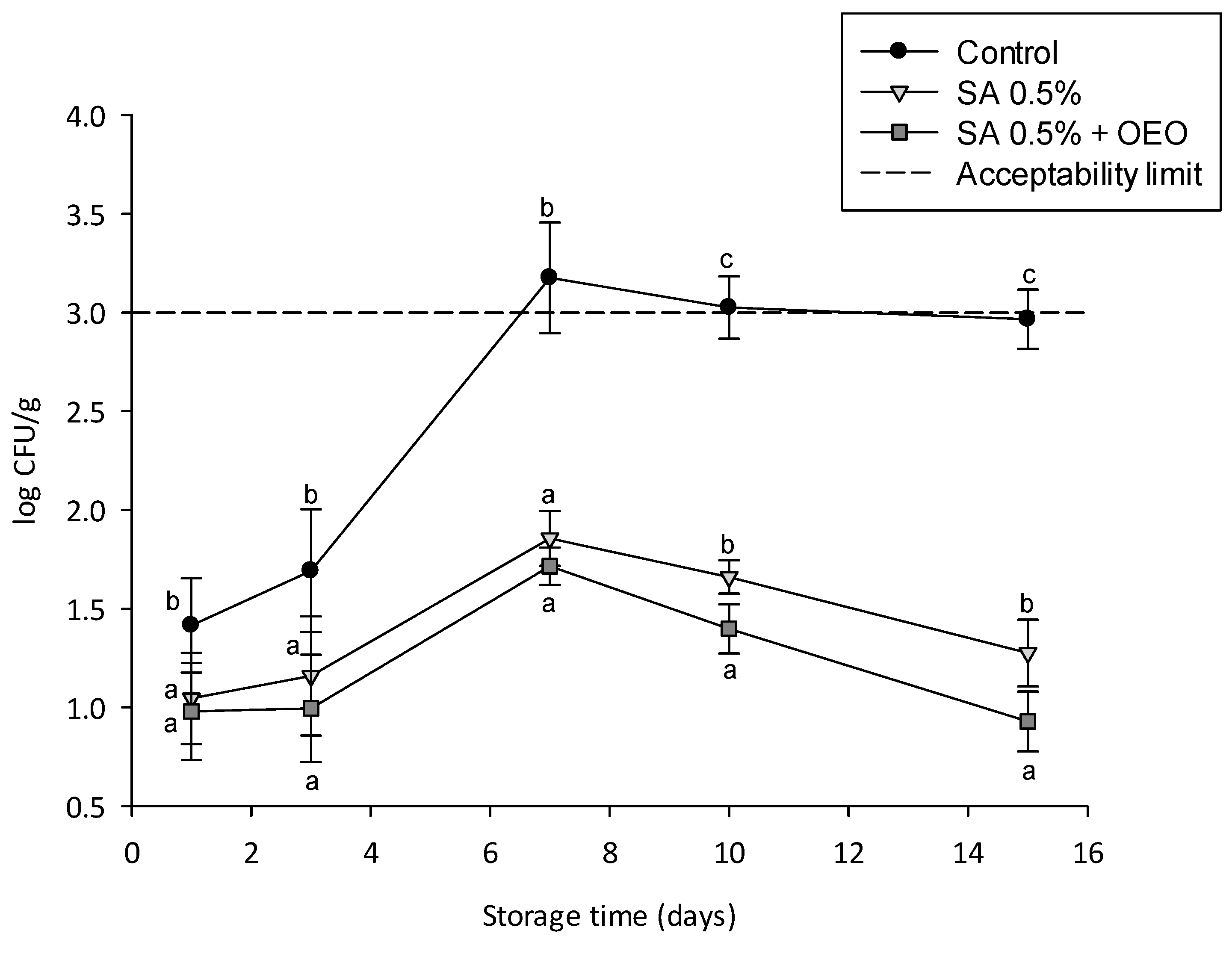
3.6. Effect of Coatings on Microbial Growth and Shelf Life of Tomatoes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, Y. Edible coatings for extending shelf-life of fresh produce during postharvest-storage. In Encyclopedia of Food Security and Sustainability; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128126882. [Google Scholar]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Edible coatings and antimicrobial nanoemulsions for enhancing shelf life and reducing foodborne pathogens of fruits and vegetables: A review. Sustain. Mater. Technol. 2020, 26, e00215. [Google Scholar]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Mohamed Amin Tawakkal, I.S.; Muda Mohamed, M.T. Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Maria Leena, M.; Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C. Edible coating with resveratrol loaded electrospun zein nanofibers with enhanced bioaccessibility. Food Biosci. 2020, 36, 100669. [Google Scholar] [CrossRef]
- Pirozzi, A.; Pataro, G.; Donsì, F.; Ferrari, G. Edible Coating and Pulsed Light to Increase the Shelf Life of Food Products. Food Eng. Rev. 2020, 1–26. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of edible coating with essential oil in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 2467–2480. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Amylose-lipid complex as a measure of variations in physical, mechanical and barrier attributes of rice starch-ι-carrageenan biodegradable edible film. Food Packag. Shelf Life 2017, 14, 108–115. [Google Scholar] [CrossRef]
- Krochta, J.M. Edible protein films and coatings. In Food Proteins and Their Applications; Damodaran, S., Ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9780367401047. [Google Scholar]
- Paul, S.K. Edible Films and Coatings for Fruits and Vegetables. In Encyclopedia of Renewable and Sustainable Materials; Springer: New York, NY, USA, 2020. [Google Scholar]
- U.S. Fda Food Additives Permitted for Direct Addition to Food for Human Consumption. In CFR-Code Fed. Regul. Title 21, Part 172. 2019. Volume 3. 21CFR172. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=172 (accessed on 10 October 2019).
- U.S. FDA Direct Food Substances Affirmed as Generally Recognized as Safe. In Code for Federal Regulations Title 21 Part 184. 2019. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=184 (accessed on 10 October 2019).
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Re-evaluation of alginic acid and its sodium, potassium, ammonium and calcium salts (E 400–E 404) as food additives. EFSA J. 2017, 15, e05049. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 1–38. [Google Scholar]
- Parreidt, T.S.; Lindner, M.; Rothkopf, I.; Schmid, M.; Müller, K. The development of a uniform alginate-based coating for cantaloupe and strawberries and the characterization of water barrier properties. Foods 2019, 8, 203. [Google Scholar] [CrossRef]
- Kalyan, B.; Swati, S.; Mohammed, W.S. Emerging Postharvest Treatment of Fruits and Vegetables, Postharvest Biology and Technology, Apple Academic Press; Kalyan, B., Swati, S., Mohammed, W.S., Eds.; Apple Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Fathi, M.; Vinceković, M.; Jurić, S.; Viskić, M.; Režek Jambrak, A.; Donsì, F. Food-Grade Colloidal Systems for the Delivery of Essential Oils. Food Rev. Int. 2019, 1–45. [Google Scholar] [CrossRef]
- Das, S.; Vishakha, K.; Banerjee, S.; Mondal, S.; Ganguli, A. Sodium alginate-based edible coating containing nanoemulsion of Citrus sinensis essential oil eradicates planktonic and sessile cells of food-borne pathogens and increased quality attributes of tomatoes. Int. J. Biol. Macromol. 2020, 162, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Raei, P.; Alizadeh, N.; Aghdam, M.A.; Kafil, H.S. Carvacrol and thymol: Strong antimicrobial agents against resistant isolates. Rev. Med. Microbiol. 2017, 28, 63–68. [Google Scholar] [CrossRef]
- Gutierrez, E.E.V. An Overview of Recent Studies of Tomato; Department of Plant Breeding, SLU: Alnarp, Sweden, 2018. [Google Scholar]
- USDA. United States Standards for Grades of Fresh Tomatoes. 1991. Available online: https://www.ams.usda.gov/sites/default/files/media/Tomato_Standard%5B1%5D.pdf (accessed on 19 September 2019).
- Ribes, S.; Fuentes, A.; Talens, P.; Barat, J.M.; Ferrari, G.; Donsì, F. Influence of emulsifier type on the antifungal activity of cinnamon leaf, lemon and bergamot oil nanoemulsions against Aspergillus niger. Food Control 2017, 73, 784–795. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G. Effect of nanoemulsion formulation on permeation of essential oils through biological membranes. Chem. Eng. Trans. 2019, 75, 247–252. [Google Scholar]
- Mauriello, E.; Ferrari, G.; Donsì, F. Effect of formulation on properties, stability, carvacrol release and antimicrobial activity of carvacrol emulsions. Colloids Surf. B Biointerfaces 2020, 197, 111424. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology-Oxidants and Antioxidants Part A.; Academic Press: Cambridge, MA, USA, 1999; Volume 299. [Google Scholar]
- Sessa, M.; Balestrieri, M.L.; Ferrari, G.; Servillo, L.; Castaldo, D.; D’Onofrio, N.; Donsì, F.; Tsao, R. Bioavailability of encapsulated resveratrol into nanoemulsion-based delivery systems. Food Chem. 2014, 147, 42–50. [Google Scholar] [CrossRef]
- Fernández, K.; Roeckel, M.; Canales, E.; Dumont, J. Modeling of the Nanoparticles Absorption Under a Gastrointestinal Simulated Ambient Condition. AAPS PharmSciTech 2017, 18, 2691–2701. [Google Scholar] [CrossRef]
- Hamedi, H.; Kargozari, M.; Shotorbani, P.M.; Mogadam, N.B.; Fahimdanesh, M. A novel bioactive edible coating based on sodium alginate and galbanum gum incorporated with essential oil of Ziziphora persica: The antioxidant and antimicrobial activity, and application in food model. Food Hydrocoll. 2017, 72, 35–46. [Google Scholar] [CrossRef]
- Rangel-Marrónab, L.; Mani-Lópeza, E.; Paloua, E.; López-Malo, A. Effects of alginate-glycerol-citric acid concentrations on selected physical, mechanical, and barrier properties of papaya puree-based edible films and coatings, as evaluated by response surface methodology. LWT 2019, 101, 83–91. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Quintero-Pimiento, C.; Gómez-Hoyos, C.; Zuluaga-Gallego, R.; López-Córdoba, A. Alginate-edible coatings for application on wild andean blueberries (Vaccinium meridionale swartz): Effect of the addition of nanofibrils isolated from cocoa by-products. Polymers 2020, 12, 824. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Alcântara, L.O.; de Martins, M.E.O.; Sousa, J.R.; Cerqueira, M.Â.; Silva, A.L.C.; de Souza Filho, M.S.M.; Souza, B.W.S. Wettability of edible coatings on Nile tilapia fillets (Oreochromis niloticus). J. Food Eng. 2019, 247, 152–159. [Google Scholar]
- Johnson, R.E.; Dettre, R.H. The wettability of low-energy liquid surfaces. J. Colloid Interface Sci. 1966, 21, 610–622. [Google Scholar] [CrossRef]
- Zhao, L.; Wei, Y.; Huang, Y.; He, B.; Zhou, Y.; Fu, J. Nanoemulsion improves the oral bioavailability of baicalin in rats: In vitro and in vivo evaluation. Int. J. Nanomed. 2013, 8, 3769–3779. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.A.; Vicente, A.A. Nanoemulsions for Food Applications: Development and Characterization; Springer: Berlin, Germany, 2012; Volume 5, pp. 854–867. [Google Scholar]
- Parreidt, T.S.; Schott, M.; Schmid, M.; Müller, K. Effect of presence and concentration of plasticizers, vegetable oils, and surfactants on the properties of sodium-alginate-based edible coatings. Int. J. Mol. Sci. 2018, 19, 742. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Krochta, J.M. Dependence of coating thickness on viscosity of coating solution applied to fruits and vegetables by dipping method. J. Food Sci. 2003, 68, 503–510. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Musavi, M.; Oromiehie, A.R.; Rezayi, K.; Razmi Rad, E.; Milani, J. Effect of plasticizing sugars on water vapor permeability, surface energy and microstructure properties of zein films. LWT-Food Sci. Technol. 2007, 40, 1191–1197. [Google Scholar] [CrossRef]
- Ribeiro, C.; Vicente, A.A.; Teixeira, J.A.; Miranda, C. Optimization of edible coating composition to retard strawberry fruit senescence. Postharvest Biol. Technol. 2007, 44, 63–70. [Google Scholar] [CrossRef]
- Choi, W.Y.; Park, H.J.; Ahn, D.J.; Lee, J.; Lee, C.Y. Wettability of Chitosan Coating Solution on ‘Fuji’ Apple Skin. J. Food Sci. 2002, 67, 3–7. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Edible coatings for fresh-cut fruits. Crit. Rev. Food Sci. Nutr. 2005, 45, 657–670. [Google Scholar] [CrossRef]
- Stannard, C. Development and use of microbiological criteria for foods Guidance for those involved in using and interpreting microbiological criteria for foods. Food Sci. Technol. Today 1997, 11, 137–176. [Google Scholar]
- Luksiene, Z.; Buchovec, I.; Kairyte, K.; Paskeviciute, E.; Viskelis, P. High-power pulsed light for microbial decontamination of some fruits and vegetables with different surfaces. J. Food Agric. Environ. 2012, 10, 162–167. [Google Scholar]
- Gómez-López, V.M.; Devlieghere, F.; Bonduelle, V.; Debevere, J. Intense light pulses decontamination of minimally processed vegetables and their shelf-life. Int. J. Food Microbiol. 2005, 103, 79–89. [Google Scholar] [CrossRef] [PubMed]
| Standard Order | Run Number | Dipping Time (s) X1 | Concentration (% w/w) | |
|---|---|---|---|---|
| Calcium Chloride X2 | Sodium Alginate X3 | |||
| 1 | 1 | 10 (−1) | 0.250 (−1) | 0.250 (−1) |
| 13 | 2 | 155 (0) | 3.625 (0) | 0.250 (−1) |
| 7 | 3 | 10 (−1) | 7.000 (+1) | 5.000 (+1) |
| 10 | 4 | 300 (+1) | 3.625 (0) | 2.625 (0) |
| 11 | 5 | 155 (0) | 0.250 (−1) | 2.625 (0) |
| 18 | 6 | 155 (0) | 3.625 (0) | 2.625 (0) |
| 14 | 7 | 155 (0) | 3.625 (0) | 5.000 (+1) |
| 15 | 8 | 155 (0) | 3.625 (0) | 2.625 (0) |
| 12 | 9 | 155 (0) | 7.000 (+1) | 2.625 (0) |
| 5 | 10 | 10 (−1) | 0.250 (−1) | 5.000 (+1) |
| 2 | 11 | 300 (+1) | 0.250 (−1) | 0.250 (−1) |
| 4 | 12 | 300 (+1) | 7.000 (+1) | 0.250 (−1) |
| 8 | 13 | 300 (+1) | 7.000 (+1) | 5.000 (+1) |
| 19 | 14 | 155 (0) | 3.625 (0) | 2.625 (0) |
| 3 | 15 | 10 (−1) | 7.000 (+1) | 0.250 (−1) |
| 17 | 16 | 155 (0) | 3.625 (0) | 2.625 (0) |
| 16 | 17 | 155 (0) | 3.625 (0) | 2.625 (0) |
| 20 | 18 | 155 (0) | 3.625 (0) | 2.625 (0) |
| 9 | 19 | 10 (−1) | 3.625 (0) | 2.625 (0) |
| 6 | 20 | 300 (+1) | 0.250 (−1) | 5.000 (+1) |
| Composition | Hydrodynamic Diameter (nm) | PDI (-) | ζ-Potential (mV) | |
|---|---|---|---|---|
| Oil Phase | Emulsifier | |||
| Origanum vulgare essential oil 0.5% w/w | Lecithin 0.5% w/w | 176.8 ± 2.4 | 0.26 ± 0.01 | −23.7 ± 1.2 |
| Parameters | Value |
|---|---|
| a0 | 1.0338 |
| b | 0.0022 |
| R2 | 0.9439 |
| Adj R2 | 0.9346 |
| Source | Coefficient | Sum of Squares | p-Value |
|---|---|---|---|
| β0 | 0.0339 | 0.0003 | 0.0001 |
| β1 | 0.0054 | 0.0026 | 0.2373 |
| β2 | 0.0161 | 0.0083 | 0.0027 |
| β3 | 0.0287 | 4.88 × 10−8 | <0.0001 |
| β12 | 0.0001 | 0.0002 | 0.9875 |
| β13 | 0.0050 | 0.0027 | 0.3193 |
| β23 | 0.0184 | 0.0025 | 0.0024 |
| Ɛ | 0.0025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirozzi, A.; Del Grosso, V.; Ferrari, G.; Donsì, F. Edible Coatings Containing Oregano Essential Oil Nanoemulsion for Improving Postharvest Quality and Shelf Life of Tomatoes. Foods 2020, 9, 1605. https://doi.org/10.3390/foods9111605
Pirozzi A, Del Grosso V, Ferrari G, Donsì F. Edible Coatings Containing Oregano Essential Oil Nanoemulsion for Improving Postharvest Quality and Shelf Life of Tomatoes. Foods. 2020; 9(11):1605. https://doi.org/10.3390/foods9111605
Chicago/Turabian StylePirozzi, Annachiara, Vittoria Del Grosso, Giovanna Ferrari, and Francesco Donsì. 2020. "Edible Coatings Containing Oregano Essential Oil Nanoemulsion for Improving Postharvest Quality and Shelf Life of Tomatoes" Foods 9, no. 11: 1605. https://doi.org/10.3390/foods9111605
APA StylePirozzi, A., Del Grosso, V., Ferrari, G., & Donsì, F. (2020). Edible Coatings Containing Oregano Essential Oil Nanoemulsion for Improving Postharvest Quality and Shelf Life of Tomatoes. Foods, 9(11), 1605. https://doi.org/10.3390/foods9111605