The Toxic Impact of Honey Adulteration: A Review
Abstract
1. Introduction
1.1. Honey
1.2. Type of Honey
- (1)
- Blossom honey: the main source of this honey is the nectar of flowers such as linden, clover, citrus, cotton, thyme, and acacia honey.
- (2)
- Honeydew honey: the source of this honey is the “honeydew” (Rhynchota genus insects pierce plant cells, ingest plant sap, and then secrete it again) collected by bees. A typical example of honeydew honey is pine, oak, fir, and leaf honey.
- (3)
- Monofloral honey: named according to the plant that the bees that have produced the honey forage predominantly.
- (4)
- Multifloral honey (polyfloral): the source of this honey is several botanical flowers, with none of them predominant. Meadow blossom honey and forest honey are classified in this category.
1.2.1. Stingless Bee Honey
1.2.2. Honeybee (Apis)
1.3. Worldwide Honey Production and Consumption
1.4. Regulation Related to Honey
1.5. Nutritional Value of Honey
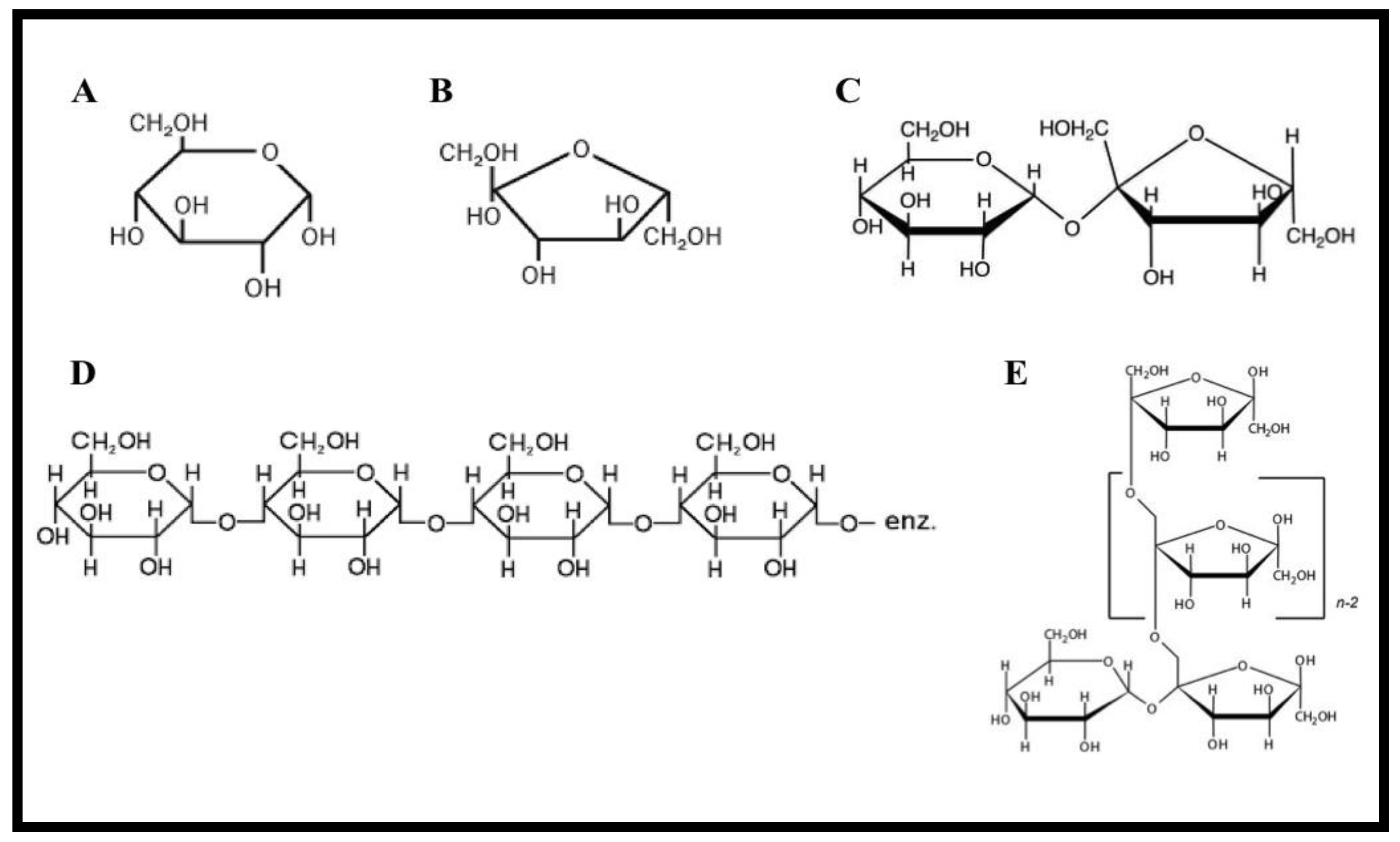
2. Honey Adulterants
2.1. Cane Sugar
2.2. Corn Syrup
2.3. Palm Sugar
2.4. Invert Sugar
2.5. Rice Syrup
2.6. Inulin Syrup
3. Adulteration Method
3.1. Direct Adulteration
3.2. Indirect Adulteration
3.3. Blending
4. Detection Method in Honey Adulteration
5. Adverse Health Impact of Honey Adulteration
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO); World Health Organization (WHO). General Standard for Food Additives, CODEX STAN 192-1995. In Codex Alimentarius Commission; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2018; Available online: http://www.fao.org/gsfaonline/docs/CXS_192e.pdf (accessed on 1 August 2020).
- Akhmazillah, M.; Farid, M.; Silva, F. High-pressure processing (HPP) of honey for the improvement of nutritional value. Innov. Food Sci. Emerg. Technol. 2013, 20, 59–63. [Google Scholar] [CrossRef]
- Saiful Yazan, L.; Zali, M.; Shyfiq, M.F.; Mohd Ali, R.; Zainal, N.A.; Esa, N.; Sapuan, S.; Ong, Y.S.; Tor, Y.S.; Gopalsamy, B.; et al. Chemopreventive properties and toxicity of Kelulut honey in Sprague Dawley rats induced with Azoxymethane. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Kassim, M.; Yusoff, K.M.; Ong, G.; Sekaran, S.; Yusof, M.Y.B.M.; Mansor, M. Gelam honey inhibits lipopolysaccharide-induced endotoxemia in rats through the induction of heme oxygenase-1 and the inhibition of cytokines, nitric oxide, and high-mobility group protein B1. Fitoterapia 2012, 83, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Krishnan, K.T.; Salleh, N.; Gan, S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Rev. Bras. Farmacogn. 2016, 26, 657–664. [Google Scholar] [CrossRef]
- Fauzi, A.N.; Norazmi, M.N.; Yaacob, N.S. Tualang honey induces apoptosis and disrupts the mitochondrial membrane potential of human breast and cervical cancer cell lines. Food Chem. Toxicol. 2011, 49, 871–878. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and health: A review of recent clinical research. Pharmacogn. Res. 2017, 9, 121. [Google Scholar]
- Ghashm, A.A.; Othman, N.H.; Khattak, M.N.; Ismail, N.M.; Saini, R. Antiproliferative effect of Tualang honey on oral squamous cell carcinoma and osteosarcoma cell lines. BMC Complement. Altern. Med. 2010, 10, 49. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Ghojoghi, A.; Ghajarbeygi, P. Honey safety hazards and public health. J. Chem. Health Risks 2016, 6, 249–267. [Google Scholar]
- Mijanur Rahman, M.; Gan, S.H.; Khalil, M. Neurological effects of honey: Current and future prospects. Evid. Based Complement. Altern. Med. 2014. [Google Scholar] [CrossRef]
- Fuhrman, J. The hidden dangers of fast and processed food. Am. J. Lifestyle Med. 2018, 12, 375–381. [Google Scholar] [CrossRef]
- Johnson, R.J.; Fuggle, S.V.; Mumford, L.; Bradley, J.A.; Forsythe, J.L.; Rudge, C.J.; Kidney Advisory Group of NHS Blood and Transplant. A New UK 2006 National Kidney Allocation Scheme for deceased heart-beating donor kidneys. Transplantation 2010, 89, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.; Mafra, I. A comprehensive review on the main honey authentication issues: Production and origin. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1072–1100. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO); World Health Organization (WHO). Joint FAO/WHO Food Standard Programme Codex Alimentarius Commission Twenty-Fourth Session Geneva, 2–7 July 2001. In Codex Alimentarius Commission; ALINORM 01/34A; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Samat, S.; Kanyan Enchang, F.; Nor Hussein, F.; Wan Ismail, W.I. Four-week consumption of Malaysian honey reduces excess weight gain and improves obesity-related parameters in high fat diet induced obese rats. Evid. Based Complement. Altern. Med 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Se, K.W.; Ghoshal, S.K.; Wahab, R.A.; Ibrahim, R.K.R.; Lani, M.N. A simple approach for rapid detection and quantification of adulterants in stingless bees (Heterotrigona itama) honey. Food Res. Int. 2018, 105, 453–460. [Google Scholar] [CrossRef]
- Morales, V.; Corzo, N.; Sanz, M. HPAEC-PAD oligosaccharide analysis to detect adulterations of honey with sugar syrups. Food Chem. 2008, 107, 922–928. [Google Scholar] [CrossRef]
- Damto, T. A Review on Effect of Adulteration on Honey Properties. SSRN 2019. [Google Scholar] [CrossRef]
- Jaafar, M.; Othman, M.; Yaacob, M.; Talip, B.; Ilyas, M.; Ngajikin, N.; Fauzi, N. A Review on Honey Adulteration and the Available Detection Approaches. Int. J. Integr. Eng. 2020, 12, 125–131. [Google Scholar]
- Lawal, R.; Lawal, A.; Adekalu, J. Physico-chemical studies on adulteration of honey in Nigeria. Pak. J. Biol. Sci. PJBS 2009, 12, 1080–1084. [Google Scholar] [CrossRef][Green Version]
- De Almeida-Muradian, L.B.; Stramm, K.M.; Horita, A.; Barth, O.M.; da Silva de Freitas, A.; Estevinho, L.M. Comparative study of the physicochemical and palynological characteristics of honey from Melipona subnitida and Apis mellifera. Int. J. Food Sci. Technol. 2013, 48, 1698–1706. [Google Scholar] [CrossRef]
- Abdel-Aal, E.M.; Ziena, H.; Youssef, M. Adulteration of honey with high-fructose corn syrup: Detection by different methods. Food Chem. 1993, 48, 209–212. [Google Scholar] [CrossRef]
- Nisbet, C.; Kazak, F.; Ardalı, Y. Determination of quality criteria that allow differentiation between honey adulterated with sugar and pure honey. Biol. Trace Elem. Res. 2018, 186, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Cotte, J.-F.; Casabianca, H.; Lhéritier, J.; Perrucchietti, C.; Sanglar, C.; Waton, H.; Grenier-Loustalot, M.-F. Study and validity of 13C stable carbon isotopic ratio analysis by mass spectrometry and 2H site-specific natural isotopic fractionation by nuclear magnetic resonance isotopic measurements to characterize and control the authenticity of honey. Anal. Chim. Acta 2007, 582, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Çinar, S.B.; Ekşi, A.; Coşkun, İ. Carbon isotope ratio (13C/12C) of pine honey and detection of HFCS adulteration. Food Chem. 2014, 157, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Förstel, H. The natural fingerprint of stable isotopes—Use of IRMS to test food authenticity. Anal. Bioanal. Chem. 2007, 388, 541–544. [Google Scholar] [CrossRef]
- Wu, L.; Du, B.; Vander Heyden, Y.; Chen, L.; Zhao, L.; Wang, M.; Xue, X. Recent advancements in detecting sugar-based adulterants in honey—A challenge. TrAC Trends Anal. Chem. 2017, 86, 25–38. [Google Scholar] [CrossRef]
- Gallardo-Velázquez, T.; Osorio-Revilla, G.; Zuñiga-de Loa, M.; Rivera-Espinoza, Y. Application of FTIR-HATR spectroscopy and multivariate analysis to the quantification of adulterants in Mexican honeys. Food Res. Int. 2009, 42, 313–318. [Google Scholar] [CrossRef]
- Cordella, C.; Militao, J.S.; Clément, M.-C.; Drajnudel, P.; Cabrol-Bass, D. Detection and quantification of honey adulteration via direct incorporation of sugar syrups or bee-feeding: Preliminary study using high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) and chemometrics. Anal. Chim. Acta 2005, 531, 239–248. [Google Scholar] [CrossRef]
- Guler, A.; Kocaokutgen, H.; Garipoglu, A.V.; Onder, H.; Ekinci, D.; Biyik, S. Detection of adulterated honey produced by honeybee (Apis mellifera L.) colonies fed with different levels of commercial industrial sugar (C3 and C4 plants) syrups by the carbon isotope ratio analysis. Food Chem. 2014, 155, 155–160. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Q.; Wang, L.; Lin, L.; Shi, H.; Cao, H.; Cao, B. Detection of honey adulteration with starch syrup by high performance liquid chromatography. Food Chem. 2015, 172, 669–674. [Google Scholar] [CrossRef]
- Shapiro, A.; Mu, W.; Roncal, C.; Cheng, K.-Y.; Johnson, R.J.; Scarpace, P.J. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am. J. Physiol. Regulat. Integr. Compar. Physiol. 2008, 295, R1370–R1375. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, A.; Chamunorwa, J.P.; Erlwanger, K.H. Dietary supplementation with natural honey promotes growth and health of male and female rats compared to cane syrup. Sci. Res. Essays 2013, 8, 543–553. [Google Scholar]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The composition and biological activity of honey: A focus on Manuka honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Ratiu, I.A.; Al-Suod, H.; Bukowska, M.; Ligor, M.; Buszewski, B. Correlation Study of Honey Regarding their Physicochemical Properties and Sugars and Cyclitols Content. Molecules 2020, 25, 34. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K. Taxonomy and distribution of different honeybee species. In Beekeeping for Poverty Alleviation and Livelihood Security; Springer: Dordrecht, The Netherlands, 2014; pp. 63–103. [Google Scholar]
- Bakar, N.A.; Sata, N.S.A.M.; Ramlan, N.F.; Ibrahim, W.N.W.; Zulkifli, S.Z.; Abdullah, C.A.C.; Ahmad, S.; Amal, M.N.A. Evaluation of the neurotoxic effects of chronic embryonic exposure with inorganic mercury on motor and anxiety-like responses in zebrafish (Danio rerio) larvae. Neurotoxicol. Teratol. 2017, 59, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Machado De-Melo, A.A.; Almeida-Muradian, L.B.d.; Sancho, M.T.; Pascual-Maté, A. Composition and properties of Apis mellifera honey: A review. J. Apic. Res. 2018, 57, 5–37. [Google Scholar] [CrossRef]
- García, N.L. The current situation on the international honey market. Bee World 2018, 95, 89–94. [Google Scholar] [CrossRef]
- Aries, E.; Burton, J.; Carrasco, L.; De Rudder, O.; Maquet, A. Scientific Support to the Implementation of a Coordinated Control Plan with a View to Establishing the Prevalence of Fraudulent Practices in the Marketing of Honey; European Commission: Brussels, Belgium, 2016; pp. 1–38. [Google Scholar]
- Bogdanov, S.; Lüllmann, C.; Martin, P.; von der Ohe, W.; Russmann, H.; Vorwohl, G.; Oddo, L.P.; Sabatini, A.-G.; Marcazzan, G.L.; Piro, R. Honey quality and international regulatory standards: Review by the International Honey Commission. Bee World 1999, 80, 61–69. [Google Scholar] [CrossRef]
- Wei, G.-X.; Huang, J.-K.; Jun, Y. Honey safety standards and its impacts on China’s honey export. J. Integr. Agric. 2012, 11, 684–693. [Google Scholar] [CrossRef]
- Schievano, E.; Stocchero, M.; Morelato, E.; Facchin, C.; Mammi, S. An NMR-based metabolomic approach to identify the botanical origin of honey. Metabolomics 2012, 8, 679–690. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Honey: A novel antioxidant. Molecules 2012, 17, 4400–4423. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kim, Y.J.; Kim, M.K. Effect of fructose or sucrose feeding with different levels on oral glucose tolerance test in normal and type 2 diabetic rats. Nutr. Res. Pract. 2008, 2, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Kellett, G.L.; Brot-Laroche, E.; Mace, O.J.; Leturque, A. Sugar absorption in the intestine: The role of GLUT2. Annu. Rev. Nutr. 2008, 28, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Bobiş, O.; Dezmirean, D.S.; Moise, A.R. Honey and diabetes: The importance of natural simple sugars in diet for preventing and treating different type of diabetes. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Meireles, M.; Martel, F.; Araújo, J.; Santos-Buelga, C.; Gonzalez-Manzano, S.; Duenas, M.; de Freitas, V.; Mateus, N.; Calhau, C.; Faria, A. Characterization and modulation of glucose uptake in a human blood—Brain barrier model. J. Membr. Biol. 2013, 246, 669–677. [Google Scholar] [CrossRef]
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.; Ansari, M.J. Antibiotic, pesticide, and microbial contaminants of honey: Human health hazards. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef]
- Ismail, M.M.; Ismail, W.I.W. Development of stingless beekeeping projects in Malaysia. In Proceedings of the CSSPO International Conference 2018: Towards Inclusive & Sustainable Agriculture—Harmonizing Environmental, Social and Economic Dimensions: Is it Possible? Sarawak, Malaysia, 9–11 July 2018; p. 5. [Google Scholar] [CrossRef]
- Tosun, M. Detection of adulteration in honey samples added various sugar syrups with 13C/12C isotope ratio analysis method. Food Chem. 2013, 138, 1629–1632. [Google Scholar] [CrossRef]
- Spiteri, M.; Dubin, E.; Cotton, J.; Poirel, M.; Corman, B.; Jamin, E.; Lees, M.; Rutledge, D. Data fusion between high resolution 1 H-NMR and mass spectrometry: A synergetic approach to honey botanical origin characterization. Anal. Bioanal. Chem. 2016, 408, 4389–4401. [Google Scholar] [CrossRef]
- Corradini, C.; Cavazza, A.; Bignardi, C. High-performance anion-exchange chromatography coupled with pulsed electrochemical detection as a powerful tool to evaluate carbohydrates of food interest: Principles and applications. Int. J. Carbohydr. Chem. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Low, N.; Hammond, D. Detection of high fructose syrup from inulin in apple juice by capillary gas chromatography with flame ionization detection. Fruit Process. 1996, 4, 135–141. [Google Scholar]
- Ruiz-Matute, A.I.; Soria, A.C.; Martínez-Castro, I.; Sanz, M.L. A new methodology based on GC-MS to detect honey adulteration with commercial syrups. J. Agric. Food Chem. 2007, 55, 7264–7269. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, X.; Shan, Y.; Su, D.; Ma, Q.; Wen, R.; Li, J. Qualitative and quantitative detection of honey adulterated with high-fructose corn syrup and maltose syrup by using near-infrared spectroscopy. Food Chem. 2017, 218, 231–236. [Google Scholar] [CrossRef] [PubMed]
- European Chemical Agency. Evaluation of New Scientific Evidence Concerning the Restrictions Contained in Annex XVII to Regulation (EC) No. 1907/2006 (REACH): Review of New Available Information for Di-’isononyl’Phthalate (DIN); European Chemical Agency: Helsinki, Finland, 2010.
- Olivares-Pérez, A.; Mejias-Brizuela, N.; Grande-Grande, A.; Fuentes-Tapia, I. Corn syrup holograms. Optik 2012, 123, 447–450. [Google Scholar] [CrossRef]
- Zábrodská, B.; Vorlová, L. Adulteration of honey and available methods for detection—A review. Acta Vet. Brno 2015, 83, 85–102. [Google Scholar] [CrossRef]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.-P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef]
- Yamada, T.; Iida, T.; Takamine, S.; Hayashi, N.; Okuma, K. Safety evaluation of rare sugar syrup: Single-dose oral toxicity in rats, reverse mutation assay, chromosome aberration assay, and acute non-effect level for diarrhea of a single dose in humans. Shokuhin Eiseigaku Zasshi. J. Food Hyg. Soc. Jpn. 2015, 56, 211–216. [Google Scholar] [CrossRef][Green Version]
- Luis, G.; Rubio, C.; Gutiérrez, A.; Hernández, C.; González-Weller, D.; Revert, C.; Castilla, A.; Abreu, P.; Hardisson, A. Palm tree syrup; nutritional composition of a natural edulcorant. Nutr. Hosp. 2012, 27, 548–552. [Google Scholar]
- Mishra, S.; Kamboj, U.; Kaur, H.; Kapur, P. Detection of jaggery syrup in honey using near-infrared spectroscopy. Int. J. Food Sci. Nutr. 2010, 61, 306–315. [Google Scholar] [CrossRef]
- Gehlawat, J. New Technology for Invert Sugar and High Fructose Syrups from Sugarcane. Indian J. Chem. Technol. 2001, 8, 28–32. [Google Scholar]
- Veana, F.; Flores-Gallegos, A.C.; Gonzalez-Montemayor, A.M.; Michel-Michel, M.; Lopez-Lopez, L.; Aguilar-Zarate, P.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R. Invertase: An Enzyme with Importance in Confectionery Food Industry. In Enzymes in Food Technology; Springer: Singapore, 2018; pp. 187–212. [Google Scholar]
- Paradkar, M.; Irudayaraj, J. Discrimination and classification of beet and cane inverts in honey by FT-Raman spectroscopy. Food Chem. 2002, 76, 231–239. [Google Scholar] [CrossRef]
- White, J.S.; Foreyt, J.P.; Melanson, K.J.; Angelopoulos, T.J. High-fructose corn syrup: Controversies and common sense. Am. J. Lifestyle Med. 2010, 4, 515–520. [Google Scholar] [CrossRef]
- Du, B.; Wu, L.; Xue, X.; Chen, L.; Li, Y.; Zhao, J.; Cao, W. Rapid screening of multiclass syrup adulterants in honey by ultrahigh-performance liquid chromatography/quadrupole time of flight mass spectrometry. J. Agric. Food Chem. 2015, 63, 6614–6623. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I. Application of analytical methods in authentication and adulteration of honey. Food Chem. 2017, 217, 687–698. [Google Scholar] [CrossRef]
- Lai, T.N.H.; André, C.; Rogez, H.; Mignolet, E.; Nguyen, T.B.T.; Larondelle, Y. Nutritional composition and antioxidant properties of the sim fruit (Rhodomyrtus tomentosa). Food Chem. 2015, 168, 410–416. [Google Scholar] [CrossRef]
- Jackson, B.P.; Taylor, V.F.; Karagas, M.R.; Punshon, T.; Cottingham, K.L. Arsenic, organic foods, and brown rice syrup. Environ. Health Perspect. 2012, 120, 623–626. [Google Scholar] [CrossRef]
- Drabińska, N.; Jarocka-Cyrta, E.; Markiewicz, L.H.; Krupa-Kozak, U. The effect of oligofructose-enriched inulin on faecal bacterial counts and microbiota-associated characteristics in celiac disease children following a gluten-free diet: Results of a randomized, placebo-controlled trial. Nutrients 2018, 10, 201. [Google Scholar] [CrossRef]
- Mehryar, L.; Esmaiili, M. Honey and honey adulteration detection: A review. In Proceedings of the 11th International Congress on Engineering and Food, Athens, Greece, 22–26 May 2011; pp. 1713–1714. [Google Scholar]
- Amiry, S.; Esmaiili, M.; Alizadeh, M. Classification of honeys adulterated with date and invert syrups. Food Chem. 2017, 1, 390–397. [Google Scholar] [CrossRef]
- Irawati, N.; Isa, N.M.; Mohamed, A.F.; Rahman, H.A.; Harun, S.W.; Ahmad, H. Optical microfiber sensing of adulterated honey. IEEE Sens. J. 2017, 17, 5510–5514. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, S.; Li, H.; Han, X.; Ouyang, Q.; Zhao, J. Determination of rice syrup adulterant concentration in honey using three-dimensional fluorescence spectra and multivariate calibrations. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2014, 131, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Oddo, L.P.; Piana, L.; Bogdanov, S.; Bentabol, A.; Gotsiou, P.; Kerkvliet, J.; Martin, P.; Morlot, M.; Valbuena, A.O.; Ruoff, K. Botanical species giving unifloral honey in Europe. Apidologie 2004, 35, S82–S93. [Google Scholar] [CrossRef]
- Sammataro, D.; Avitabile, A. The Beekeeper’s Handbook; Cornell University Press: Ithaca, NY, USA, 1998. [Google Scholar]
- Guler, A. The effects of the shook swarm technique on honey bee (Apis mellifera L.) colony productivity and honey quality. J. Apic. Res. 2008, 47, 27–34. [Google Scholar] [CrossRef]
- Guler, A.; Bakan, A.; Nisbet, C.; Yavuz, O. Determination of important biochemical properties of honey to discriminate pure and adulterated honey with sucrose (Saccharum officinarum L.) syrup. Food Chem. 2007, 105, 1119–1125. [Google Scholar] [CrossRef]
- Dumté, M.E.J. Development of a Method for the Quantitative Detection of Honey in Imported Products. University of Waikato. Ph.D. Thesis, University of Waikato, Hamilton, New Zealand, 2010. [Google Scholar]
- Kenjerić, D.; Mandić, M.L.; Primorac, L.; Bubalo, D.; Perl, A. Flavonoid profile of Robinia honeys produced in Croatia. Food Chem. 2007, 102, 683–690. [Google Scholar] [CrossRef]
- Vit, P.; Pedro, S.R.; Roubik, D. Pot-Honey: A Legacy of Stingless Bees; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Başar, B.; Özdemir, D. Determination of honey adulteration with beet sugar and corn syrup using infrared spectroscopy and genetic-algorithm-based multivariate calibration. J. Sci. Food Agric. 2018, 98, 5616–5624. [Google Scholar] [CrossRef]
- Hošťálková, A.; Klingelhöfer, I.; Morlock, G.E. Comparison of an HPTLC method with the Reflectoquant assay for rapid determination of 5-hydroxymethylfurfural in honey. Anal. Bioanal. Chem. 2013, 405, 9207–9218. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Tatlisu, N.B.; Toker, O.S.; Karaman, S.; Dertli, E.; Sagdic, O.; Arici, M. Steady, dynamic and creep rheological analysis as a novel approach to detect honey adulteration by fructose and saccharose syrups: Correlations with HPLC-RID results. Food Res. Int. 2014, 64, 634–646. [Google Scholar] [CrossRef]
- Baglio, E. Overheating Indexes and Honey Quality. In Chemistry and Technology of Honey Production; Springer: Cham, Switzerland, 2018; pp. 23–40. [Google Scholar]
- Sobrino-Gregorio, L.; Vargas, M.; Chiralt, A.; Escriche, I. Thermal properties of honey as affected by the addition of sugar syrup. J. Food Eng. 2017, 213, 69–75. [Google Scholar] [CrossRef]
- Sobrino-Gregorio, L.; Vilanova, S.; Prohens, J.; Escriche, I. Detection of honey adulteration by conventional and real-time PCR. Food Control. 2019, 95, 57–62. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Rodríguez-Sánchez, S.; Sanz, M.L.; Martínez-Castro, I. Detection of adulterations of honey with high fructose syrups from inulin by GC analysis. J. Food Compos. Anal. 2010, 23, 273–276. [Google Scholar] [CrossRef]
- Mantha, M.; Urban, J.R.; Mark, W.A.; Chernyshev, A.; Kubachka, K.M. Direct comparison of cavity ring down spectrometry and isotope ratio mass spectrometry for detection of sugar adulteration in honey samples. J. AOAC Int. 2018, 101, 1857–1863. [Google Scholar] [CrossRef]
- Li, S.; Shan, Y.; Zhu, X.; Zhang, X.; Ling, G. Detection of honey adulteration by high fructose corn syrup and maltose syrup using Raman spectroscopy. J. Food Compos. Anal. 2012, 28, 69–74. [Google Scholar] [CrossRef]
- Qu, L.; Jiang, Y.; Huang, X.; Cui, M.; Ning, F.; Liu, T.; Gao, Y.; Wu, D.; Nie, Z.; Luo, L. High-Throughput Monitoring of Multiclass Syrup Adulterants in honey based on the oligosaccharide and polysaccharide profiles by MALDI mass spectrometry. J. Agric. Food Chem. 2019, 67, 11256–11261. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; García-Nicolás, M.; Castell, A.; Campillo, N.; Viñas, P.; López-García, I.; Hernández-Córdoba, M. Untargeted headspace gas chromatography–Ion mobility spectrometry analysis for detection of adulterated honey. Talanta 2019, 205, 120123. [Google Scholar] [CrossRef]
- Wang, J.; Xue, X.; Du, X.; Cheng, N.; Chen, L.; Zhao, J.; Zheng, J.; Cao, W. Identification of acacia honey adulteration with rape honey using liquid chromatography–electrochemical detection and chemometrics. Food Anal. Methods 2014, 7, 2003–2012. [Google Scholar] [CrossRef]
- Peng, J.; Xie, W.; Jiang, J.; Zhao, Z.; Zhou, F.; Liu, F. Fast Quantification of Honey Adulteration with Laser-Induced Breakdown Spectroscopy and Chemometric Methods. Foods 2020, 9, 341. [Google Scholar] [CrossRef]
- Awasthi, S.; Jain, K.; Das, A.; Alam, R.; Surti, G.; Kishan, N. Analysis of food quality and food adulterants from different departmental & local grocery stores by qualitative analysis for food safety. IOSR JESTFT 2014, 8, 22–26. [Google Scholar]
- Afroz, R.; Tanvir, E.; Paul, S.; Bhoumik, N.C.; Gan, S.H.; Khalil, M.I. DNA damage inhibition properties of sundarban honey and its phenolic composition. J. Food Biochem. 2016, 40, 436–445. [Google Scholar] [CrossRef]
- Muraoka-Cook, R.; Shin, I.; Yi, J.; Easterly, E.; Barcellos-Hoff, M.; Yingling, J.; Zent, R.; Arteaga, C. Activated type I TGF β receptor kinase enhances the survival of mammary epithelial cells and accelerates tumor progression. Oncogene 2006, 25, 3408–3423. [Google Scholar] [CrossRef]
- Samat, S.; Enchang, F.K.; Abd Razak, A.; Hussein, F.N.; Ismail, W.I.W. Adulterated honey consumption can induce obesity, increase blood glucose level and demonstrate toxicity effects. Sains Malays. 2018, 47, 353–365. [Google Scholar]
- Li, L.; Zhao, Z.; Xia, J.; Xin, L.; Chen, Y.; Yang, S.; Li, K. A long-term high-fat/high-sucrose diet promotes kidney lipid deposition and causes apoptosis and glomerular hypertrophy in bama minipigs. PLoS ONE 2015, 10, e0142884. [Google Scholar] [CrossRef]
- Arise, R.; Malomo, S. Effects of ivermectin and albendazole on some liver and kidney function indices in rats. Afr. J. Biochem. Res. 2009, 3, 190–197. [Google Scholar]
- Larson-Meyer, D.E.; Willis, K.S. Vitamin D and athletes. Curr. Sports Med. Rep. 2010, 9, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Marinho, J.F.U.; Correia, J.E.; de Castro Marcato, A.C.; Pedro-Escher, J.; Fontanetti, C.S. Sugar cane vinasse in water bodies: Impact assessed by liver histopathology in tilapia. Ecotoxicol. Environ. Saf. 2014, 110, 239–245. [Google Scholar] [CrossRef]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.-H. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef]
- Yaghoobi, N.; Al-Waili, N.; Ghayour-Mobarhan, M.; Parizadeh, S.; Abasalti, Z.; Yaghoobi, Z.; Yaghoobi, F.; Esmaeili, H.; Kazemi-Bajestani, S.; Aghasizadeh, R.; et al. Natural honey and cardiovascular risk factors; effects on blood glucose, cholesterol, triacylglycerole, CRP, and body weight compared with sucrose. Sci. World J. 2008, 8, 463–469. [Google Scholar] [CrossRef]
- Khambualai, O.; Yamauchi, K.-E.; Ruttanavut, J.; Incharoen, T.; Kashimura, J. Effect of sugar cane extract, commercial probiotic and their mixture on growth performance and intestinal histology in broiler chickens. Am. J. Anim. Vet. Sci. 2010, 5, 132–138. [Google Scholar] [CrossRef]

| Component | Honey | Value |
|---|---|---|
| 1. Moisture content | Honey that is not listed below | <20% |
| Heather honey (Calluna) | <23% | |
| 2. Sugar content | ||
| a. Fructose + Glucose content | Honey that is not listed below | >60 g/100 g |
| Honeydew honey and its blends with blossom honey | >45 g/100 g | |
| b. Sucrose content | Honey that is not listed below | <5 g/100 g |
| Alfalfa (Medicago sativa), Citrus spp., False Acacia (Robinia pseudoacacia), French Honeysuckle (Hedysarum), Menzies Banksia (Banksia menziesii), Red Gum (Eucalyptus camaldulensis), Leatherwood (Eucryphia lucida), Eucryphia milligani | <10 g/100 g | |
| Lavender (Lavandula spp.) and Borage (Borago officinalis) | <15 g/100 g | |
| 3. Water-insoluble solid content | Honey that is not listed below | <0.1 g/100 g |
| Pressed honey | <0.5 g/100 g |
| Nutrition | Blossom Honey | Honeydew Honey |
|---|---|---|
| Range | Range | |
| Water | 15–20 | |
| Total sugars | ||
| Monosaccharides | ||
| Fructose | 30–45 | 28–40 |
| Glucose | 24–40 | 19–32 |
| Disaccharides | ||
| Sucrose | 0.1–4.8 | 0.1–4.7 |
| Others | 2.0–8.0 | 1.0–6.0 |
| Trisaccharides | ||
| Erlose | 0.5–6.0 | 0.1–6.0 |
| Melezitose | NA | 0.3–22 |
| Others | 0.5–1.0 | 0.1–6.0 |
| Minerals | 0.1–0.5 | 0.6–2.0 |
| Amino acids, proteins | 0.2–0.4 | 0.4–0.7 |
| Acids | 0.2–0.8 | 0.8–1.5 |
| pH value | 3.2–4.5 | 4.5–6.5 |
| Minerals | Amount (mg/100 g) | Vitamins | Amount (mg/100 g) |
|---|---|---|---|
| Sodium (Na) | 1.6–17 | Thiamine (B1) | 0.00–0.01 |
| Calcium (Ca) | 3–31 | Riboflavin (B2) | 0.010.02 |
| Potassium (K) | 40–3500 | Niacin (B3) | 0.10–0.20 |
| Magnesium (Mg) | 0.7–13 | Pantothenic acid (B5) | 0.02–0.11 |
| Phosphorus (P) | 2–15 | Pyridoxine (B6) | 0.01–0.32 |
| Selenium (Se) | 0.002–0.01 | Folic acid (B9) | 0.002–0.01 |
| Copper (Cu) | 0.02–0.6 | Ascorbic acid (C) | 2.2–2.5 |
| Iron (Fe) | 0.03–4 | Phyllochinon (K) | 0.025 |
| Manganese (Mn) | 0.02–2 | ||
| Chromium (Cr) | 0.01–0.3 | ||
| Zink (Zn) | 0.05–2 |
| Adulterants | Targeted | Non-Targeted | Reference |
|---|---|---|---|
| Rice molasses | Conventional and real-time PCR | - | [98] |
| Sugar syrup | DSC | PCA | [91] |
| HFS | HPLC; SCIRA | - | [90] |
| CS and HFCS | HPAEC-PAD | - | [32] |
| RS | 3DFS | PCA | [17] |
| CS | HS-GC-IMS | OPLS-DA | [77] |
| HFIS | GC-MS | - | [96] |
| HFCS | RAMAN spectroscopy | PLS-LDA | [92] |
| IS and CS | MALDI/MS | - | [94] |
| CS | CRDS; IRMS | - | [95] |
| RS, HFCS, CS, IS | UHPLC/Q-TOF-MS | - | [93] |
| Rape honey | LC-ECD | PCA | [69] |
| Rape honey and HFCS | LIBS | PLS | [97] |
| Type of Sugar | Animal Type | Affected Organ | Reference |
|---|---|---|---|
| Inverted sugar | Human being | Stomach disorder | [109] |
| Commercial honey | Sparague Dawley rat | Increase body weight, serum lipid, liver, and kidney damage | [99] |
| Sucrose | Sparague Dawley rat | Increase urea and creatinine level | [15] |
| HFCS | Sparague Dawley rat | Kidney failure | [103] |
| Sugar syrup | Sparague Dawley rat | Hypercholesterolemia, hypertriglyceridemia, and hyperinsulinemia | [104] |
| Sugar cane vinasse | Oreochromis niloticus (tilapia fish) | Liver damage | [34] |
| Fructose | Rat | Renal failure | [106] |
| Sugar cane extract | Broiler chicken | Hypertrophied intestinal villi and epithelial cells | [12] |
| High fructose | Sparague Dawley rat | Hypertriglyceridemia | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakhlaei, R.; Selamat, J.; Khatib, A.; Razis, A.F.A.; Sukor, R.; Ahmad, S.; Babadi, A.A. The Toxic Impact of Honey Adulteration: A Review. Foods 2020, 9, 1538. https://doi.org/10.3390/foods9111538
Fakhlaei R, Selamat J, Khatib A, Razis AFA, Sukor R, Ahmad S, Babadi AA. The Toxic Impact of Honey Adulteration: A Review. Foods. 2020; 9(11):1538. https://doi.org/10.3390/foods9111538
Chicago/Turabian StyleFakhlaei, Rafieh, Jinap Selamat, Alfi Khatib, Ahmad Faizal Abdull Razis, Rashidah Sukor, Syahida Ahmad, and Arman Amani Babadi. 2020. "The Toxic Impact of Honey Adulteration: A Review" Foods 9, no. 11: 1538. https://doi.org/10.3390/foods9111538
APA StyleFakhlaei, R., Selamat, J., Khatib, A., Razis, A. F. A., Sukor, R., Ahmad, S., & Babadi, A. A. (2020). The Toxic Impact of Honey Adulteration: A Review. Foods, 9(11), 1538. https://doi.org/10.3390/foods9111538








