Water-Based Extraction of Bioactive Principles from Blackcurrant Leaves and Chrysanthellum americanum: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ground Plant, Granulometry and Density
2.3. Infusion Extraction
2.4. Maceration Extraction
2.5. Ultrasound-Assisted Extraction
2.6. Microwave-Assisted Extraction
2.7. Percolation Extraction
2.8. Optimized Easy-To-Use Infusion Extraction
2.9. Kinetic Monitoring
2.10. Preparation of the Extracts and Global Extraction Yields
2.11. Total Polyphenols Content (TPC)
2.12. Total Flavonoids Content (TFC)
2.13. Total Proanthocyanidin Oligomers Content (OPC)
2.14. UHPLC-DAD and UHPLC-ESI-MS Analysis
2.15. ESI(-) FT-ICR-MS Analysis
2.16. Enzymatic Activities Assays
2.16.1. Hyaluronidase Capillary Electrophoresis Inhibition Assay
2.16.2. Angiotensin-Converting Enzyme (ACE) Inhibition Assay
2.17. ABTS Antioxidant Assay
3. Results and Discussion
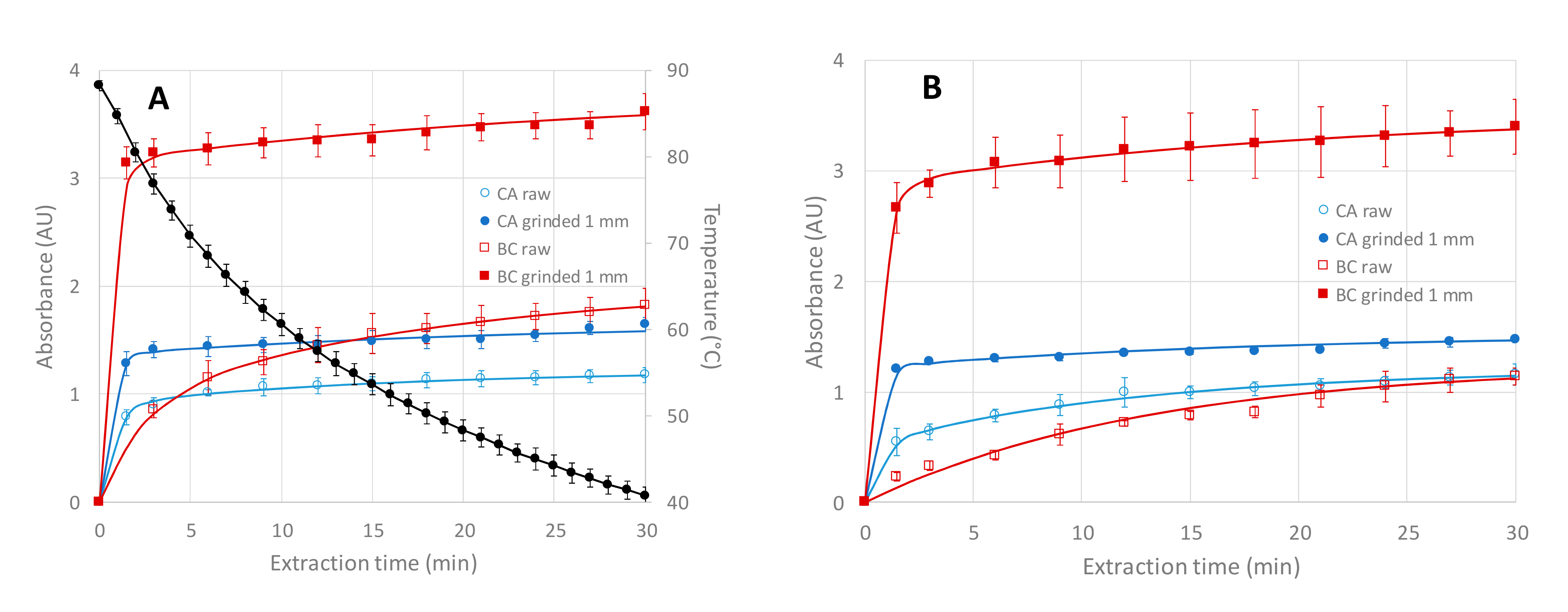
3.1. Influence of the Extraction Mode and of the Plant Grinding on the Global Extraction Yields and on the Kinetics of Extraction
3.2. Quantification of Total Flavonoid, Polyphenol, and Proanthocyanidin Contents
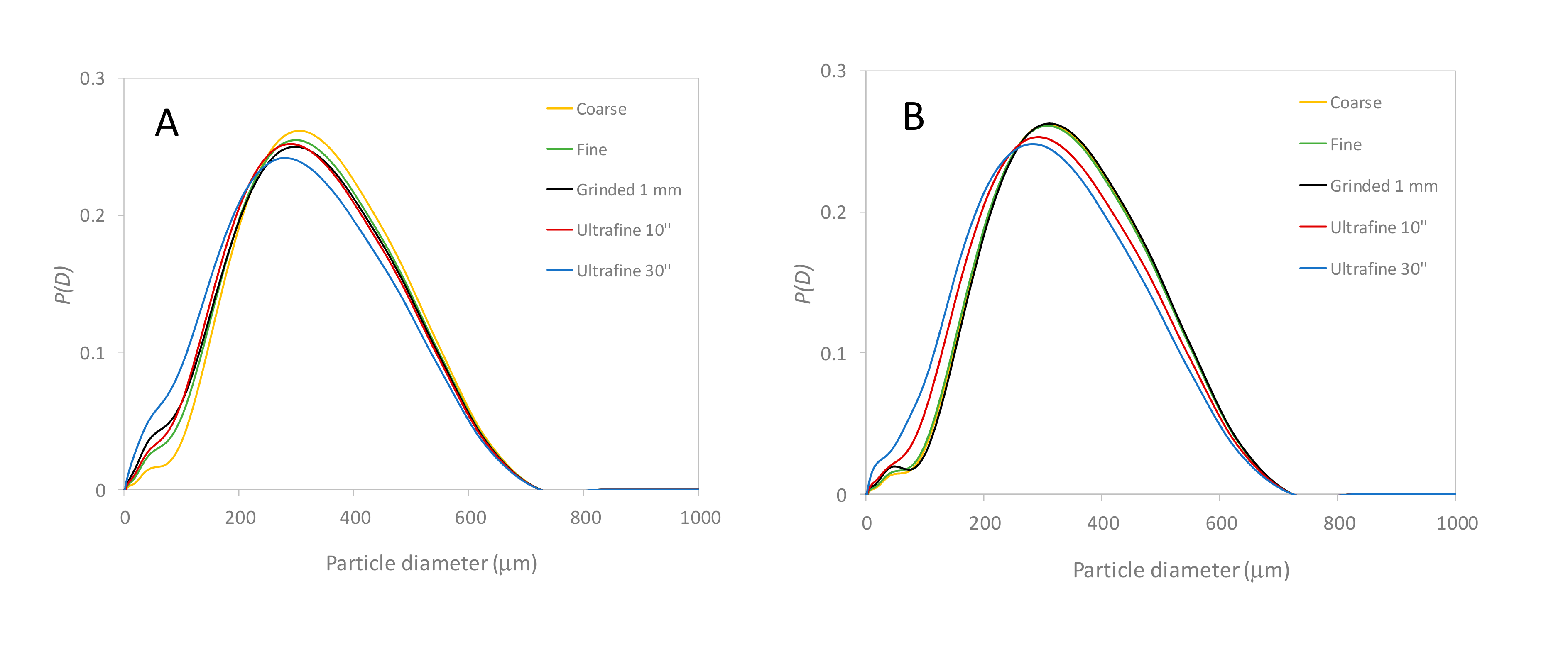
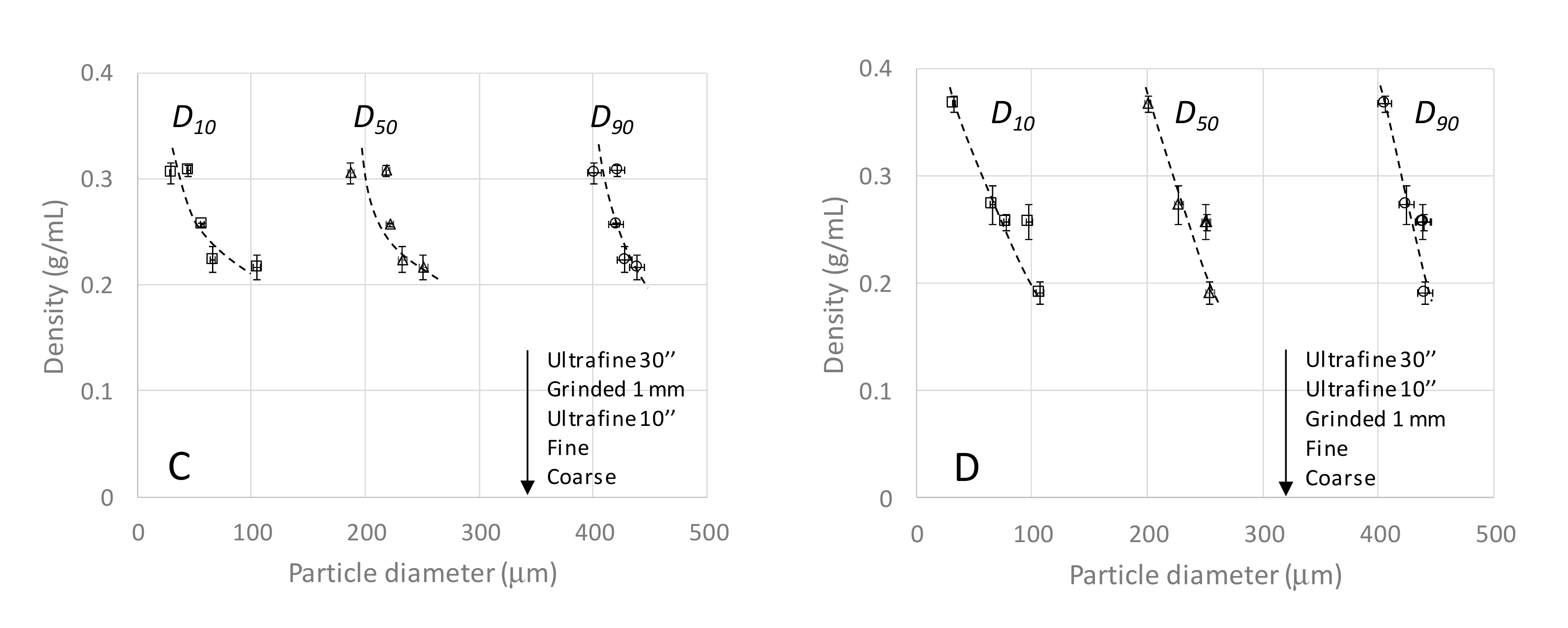
3.3. Characterization of the Plant Granulometry and Optimized Easy-To-Use Infusion Protocol
3.4. Chemical Composition of the CA and BC Plant Extracts Investigated by UHPLC and Its Coupling with MS
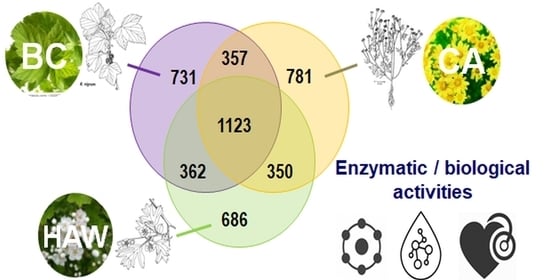
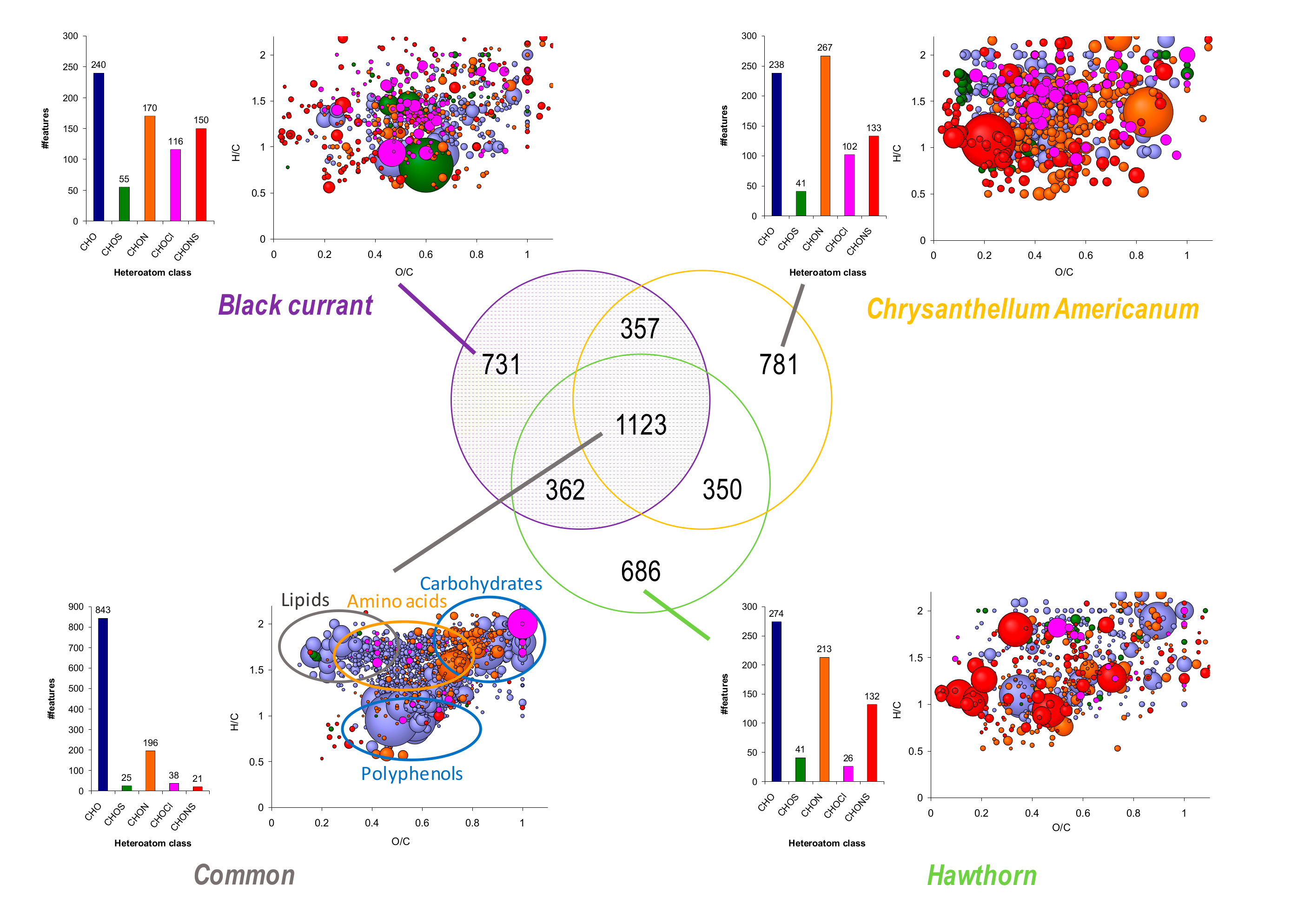
3.5. Global Composition and Differences in Chemical Composition between Plants Achieved by ESI FT-ICR-MS in Negative Mode
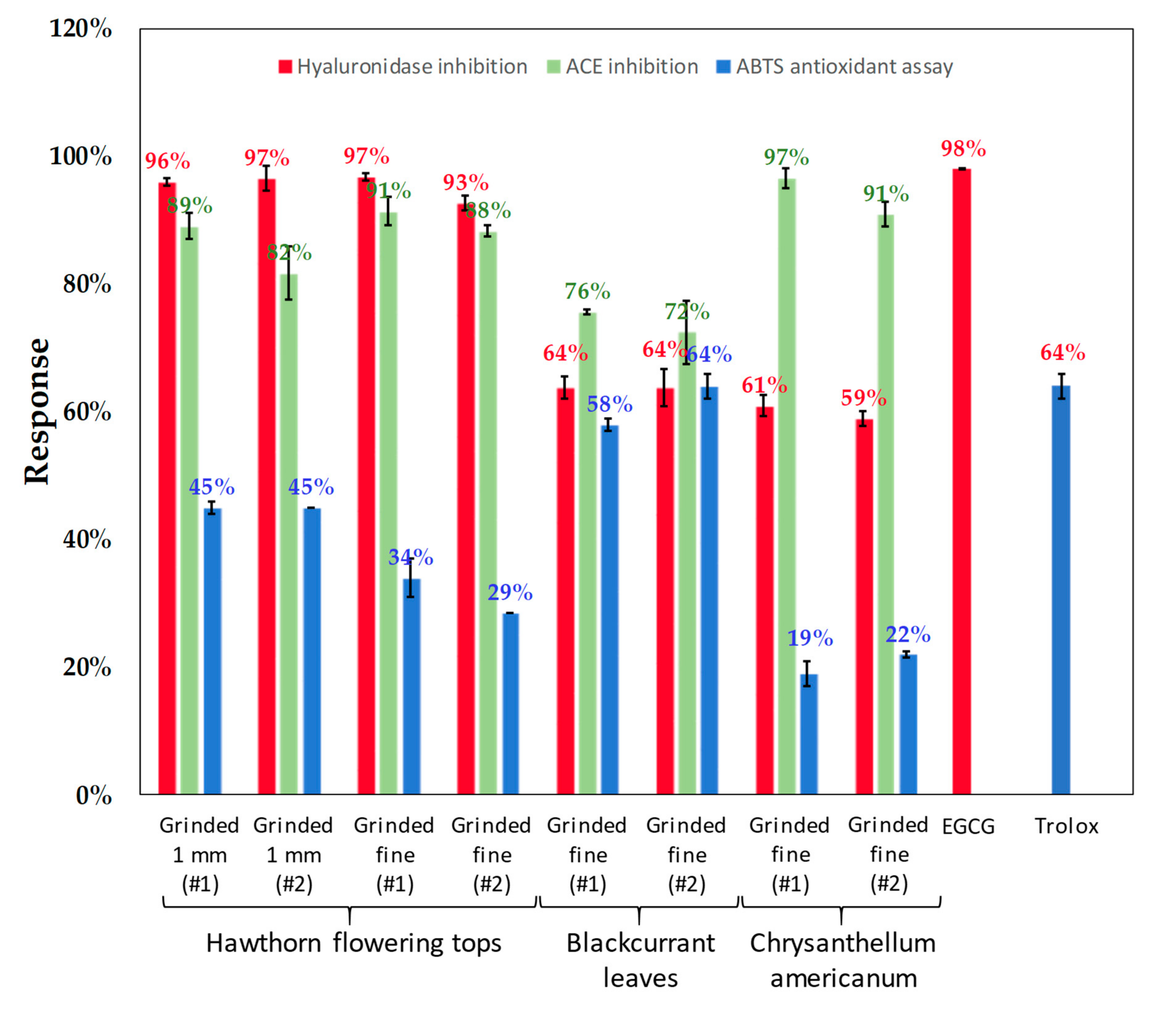
3.6. Enzymatic Activities
3.6.1. Hyaluronidase CE Inhibition Assay
3.6.2. ACE Inhibition Assay
3.7. ABTS Antioxidant Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Chikezie, P.C.; Ibegbulem, C.O.; Mbagwu, F.N. Bioactive principles from medicinal plants. Res. J. Phytochem. 2015, 9, 88–115. [Google Scholar] [CrossRef]
- Tilburt, J.C.; Kaptchuk, T.J. Herbal medicine research and global health: An ethical analysis. Bull. World Health Organ. 2008, 86, 594–599. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Neurol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Cao-Ngoc, P.; Leclercq, L.; Rossi, J.C.; Desvignes, I.; Hertzog, J.; Fabiano-Tixier, A.S.; Chemat, F.; Schmitt-Kopplin, P.; Cottet, H. Optimizing water-based extraction of bioactive principles of hawthorn: From experimental laboratory research to homemade preparations. Molecules 2019, 24, 4420. [Google Scholar] [CrossRef] [PubMed]
- Vagari, M. Black currant (Ribes nigrum L.)—An insight into the crop. In Introductory Paper at the Faculty of Landscape Planning, Horticulture and Agricultural Science; Swedish University of Agricultural Sciences: Alnarp, Sweden, 2012. [Google Scholar]
- Vagiri, M.; Johansson, E.; Rumpunen, K. Health promoting compounds in black currants—The start of a study concerning ontogenetic and genetic effects. Acta Hortic. 2012, 946, 427–432. [Google Scholar] [CrossRef]
- Karjalainen, R.; Anttonen, M.; Saviranta, N.; Hilz, H.; Törrönen, R.; Stewart, D.; McDougall, G.J.; Mattila, P. A review on bioactive compounds in black currants (Ribes nigrum L.) and their potential health-promoting properties. Acta Hortic. 2009, 839, 301–307. [Google Scholar] [CrossRef]
- Stević, T.; Šavikin, K.; Ristić, M.; Zdunić, G.; Janković, T.; Krivokuća-dokić, D.; Vulić, T. Composition and antimicrobial activity of the essential oil of the leaves of black currant (Ribes nigrum L.) cultivar Čačanska crna. J. Serbian Chem. Soc. 2010, 75, 35–43. [Google Scholar] [CrossRef]
- Vagiri, M.; Conner, S.; Stewart, D.; Andersson, S.C.; Verrall, S.; Johansson, E.; Rumpunen, K. Phenolic compounds in blackcurrant (Ribes nigrum L.) leaves relative to leaf position and harvest date. Food Chem. 2015, 172, 135–142. [Google Scholar] [CrossRef]
- Vagiri, M.; Ekholm, A.; Öberg, E.; Johansson, E.; Andersson, S.C.; Rumpunen, K. Phenols and ascorbic acid in black currants (Ribes nigrum L.): Variation due to genotype, location, and year. J. Agric. Food Chem. 2013, 61, 9298–9306. [Google Scholar] [CrossRef]
- Lyall, K.A.; Hurst, S.M.; Cooney, J.; Jensen, D.; Lo, K.; Hurst, R.D.; Stevenson, L.M. Short-term blackcurrant extract consumption modulates exercise-induced oxidative stress and lipopolysaccharide-stimulated inflammatory responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, 70–81. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Sipel, A.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Optimisation of extraction of phenolics and antioxidants from black currant leaves and buds and of stability during storage. Food Chem. 2007, 105, 1268–1275. [Google Scholar] [CrossRef]
- Kapasakalidis, P.G.; Rastall, R.A.; Gordon, M.H. Extraction of polyphenols from processed black currant (Ribes nigrum L.) residues. J. Agric. Food Chem. 2006, 54, 4016–4021. [Google Scholar] [CrossRef] [PubMed]
- Nour, V.; Trandafir, I.; Cosmulescu, S. Antioxidant capacity, phenolic compounds and minerals content of blackcurrant (Ribes nigrum L.) leaves as influenced by harvesting date and extraction method. Ind. Crops Prod. 2014, 53, 133–139. [Google Scholar] [CrossRef]
- Staszowska-Karkut, M.; Materska, M. Phenolic composition, mineral content, and beneficial bioactivities of leaf extracts from black currant (Ribes nigrum L.), raspberry (Rubus idaeus), and aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef]
- Ryding, O.; Bremer, K. Phylogeny, Distribution, and Classification of the Coreopsideae (Asteraceae). Syst. Bot. 1992, 17, 649–659. [Google Scholar] [CrossRef]
- Chrysanthellum. Available online: https://en.wikipedia.org/wiki/Chrysanthellum (accessed on 10 September 2020).
- Honore-Thorez, D. Description, identification et usages therapeutiques de Chrysanthellum americanum. J. Pharm. Belg. 1985, 40, 323–331. [Google Scholar]
- Ferrara, L. Use of Chrysantellum americanum (L.) vatke as supplement. Eur. Sci. J. 2013, 9, 1–7. [Google Scholar]
- Mevy, J.P.; Bessiere, J.M.; Dherbomez, M. Composition, antimicrobial and antioxidant activities of the volatile oil of chrysanthellum americanum (linn.) vatke. J. Essent. Oil-Bearing Plants 2012, 15, 489–496. [Google Scholar] [CrossRef]
- Balmus, I.M.; Cojocariu, R.O.; Ciobica, A.; Strungaru, S.; Strungaru-Jijie, R.; Cantemir, A.; Galatanu, C.; Gorgan, L. Preliminary Study on the Tears Oxidative Stress Status and Sleep Disturbances in Irritable Bowel Syndrome Patients. Oxid. Med. Cell. Longev. 2020, 4690713. [Google Scholar] [CrossRef]
- Guenne, S.; Hilou, A.; Ouattara, N.; Nacoulma, O.G. Anti-bacterial activity and phytochemical composition of extracts of three medicinal Asteraceae species from Burkina Faso. Asian J. Pharm. Clin. Res. 2012, 5, 37–44. [Google Scholar]
- Ofodile, L.N.; Kanife, U.C.; Arojojoye, B.J. Antifungal activity of a Nigerian herbal plant Chrysanthellum americanum. J. Life Phys. Sci. 2010, 3, 60–63. [Google Scholar]
- Lievre, H.; Gullot, B.; Reymond, E. Chrysanthellum: Un hépatotrope, normolipémiant et vasculotrope: Confirmations et acquisitions. J. Jeune Prat. 1984, 7, 1–8. [Google Scholar]
- Séverine, J. Apport des Drogues Végétales Dans la Prévention des Maladies Cardiovasculaires Liées à L’hypercholestérolémie. Ph.D. Thesis, Université Henri Poincaré, Nancy, France, 2005. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics With Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lamaison, J.L.; Carnart, A. Teneurs en principaux flavonoides des fleurs et des feuilles de Crataegus Monogyna Jacq. et de Crataegus Laevigata (Poiret) Dc. en fonction de la période de végétation. Plantes Médicinales et Phytothérapie 1991, 25, 12–16. [Google Scholar]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1985, 25, 223–230. [Google Scholar] [CrossRef]
- Kanawati, B.; Bader, T.M.; Wanczek, K.P.; Li, Y.; Schmitt-Kopplin, P. Fourier transform (FT)-artifacts and power-function resolution filter in Fourier transform mass spectrometry. Rapid Commun. Mass Spectrom. 2017, 31, 1607–1615. [Google Scholar] [CrossRef]
- Hemmler, D.; Roullier-Gall, C.; Marshall, J.W.; Rychlik, M.; Taylor, A.J.; Schmitt-Kopplin, P. Insights into the Chemistry of Non-Enzymatic Browning Reactions in Different Ribose-Amino Acid Model Systems. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Roullier-Gall, C.; Boutegrabet, L.; Gougeon, R.D.; Schmitt-Kopplin, P. A grape and wine chemodiversity comparison of different appellations in Burgundy: Vintage vs terroir effects. Food Chem. 2014, 152, 100–107. [Google Scholar] [CrossRef]
- Suhre, K.; Schmitt-Kopplin, P. MassTRIX: Mass translator into pathways. Nucleic Acids Res. 2008, 36, 481–484. [Google Scholar] [CrossRef]
- Nasreddine, R.; Orlic, L.; Banni, G.A.H.D.; Fayad, S.; Marchal, A.; Piazza, F.; Lopin-Bon, C.; Hamacek, J.; Nehmé, R. Polyethylene glycol crowding effect on hyaluronidase activity monitored by capillary electrophoresis. Anal. Bioanal. Chem. 2020, 412, 4195–4207. [Google Scholar] [CrossRef]
- Fayad, S.; Nehmé, R.; Langmajerová, M.; Ayela, B.; Colas, C.; Maunit, B.; Jacquinet, J.C.; Vibert, A.; Lopin-Bon, C.; Zdeněk, G.; et al. Hyaluronidase reaction kinetics evaluated by capillary electrophoresis with UV and high-resolution mass spectrometry (HRMS) detection. Anal. Chim. Acta 2017, 951, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Ratnasooriya, W.D.; Abeysekera, W.P.K.M.; Ratnasooriya, C.T.D. In vitro anti-hyaluronidase activity of Sri Lankan low grown orthodox orange pekoe grade black tea (Camellia sinensis L.). Asian Pac. J. Trop. Biomed. 2014, 4, 959–963. [Google Scholar] [CrossRef]
- Messaili, S.; Colas, C.; Fougère, L.; Destandau, E. Combination of molecular network and centrifugal partition chromatography fractionation for targeting and identifying Artemisia annua L. antioxidant compounds. J. Chromatogr. A 2020, 1615, 460785. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Collina, S.; Rossi, D.; Bazzoni, D.; Gaggeri, R.; Bracco, F.; Azzolina, O. Influence of the extraction mode on the yield of hyperoside, vitexin and vitexin-2-O-rhamnoside from Crataegus monogyna Jacq. (Hawthorn). Phytochem. Anal. 2008, 19, 534–540. [Google Scholar] [CrossRef]
- Pan, X.; Niu, G.; Liu, H. Comparison of microwave-assisted extraction and conventional extraction techniques for the extraction of tanshinones from Salvia miltiorrhiza bunge. Biochem. Eng. J. 2002, 12, 71–77. [Google Scholar] [CrossRef]
- Boucherit, A.; Khalaf, H.; Bonete, P.; Todolí Torró, J.L. Comparison of Microwave-assisted, ultrasound-assisted and conventional solvent extraction techniques for the extraction of molybdenum with tributyl phosphate. J. Mater. Environ. Sci. 2019, 10, 274–289. [Google Scholar]
- Liazid, A.; Schwarz, M.; Varela, R.M.; Palma, M.; Guillén, D.A.; Brigui, J.; Macías, F.A.; Barroso, C.G. Evaluation of various extraction techniques for obtaining bioactive extracts from pine seeds. Food Bioprod. Process. 2010, 88, 247–252. [Google Scholar] [CrossRef]
- Islami, F.; Poustchi, H.; Pourshams, A.; Khoshnia, M.; Gharavi, A.; Kamangar, F.; Dawsey, S.M.; Abnet, C.C.; Brennan, P.; Sheikh, M.; et al. A prospective study of tea drinking temperature and risk of esophageal squamous cell carcinoma. Int. J. Cancer 2020, 146, 18–25. [Google Scholar] [CrossRef]
- Tai, W.P.; Nie, G.J.; Chen, M.J.; Yaz, T.Y.; Guli, A.; Wuxur, A.; Huang, Q.Q.; Lin, Z.G.; Wu, J. Hot food and beverage consumption and the risk of esophageal squamous cell carcinoma: A case-control study in a northwest area in China. Medicine 2017, 96, e9325. [Google Scholar] [CrossRef]
- Liu, P.; Yang, B.; Kallio, H. Characterization of phenolic compounds in Chinese hawthorn (Crataegus pinnatifida Bge. var. major) fruit by high performance liquid chromatography-electrospray ionization mass spectrometry. Food Chem. 2010, 121, 1188–1197. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Gorzelany, J.; Kapusta, I. Identification and characterization of low molecular weight polyphenols in berry leaf extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011, 59, 12830–12835. [Google Scholar] [CrossRef] [PubMed]
- Vagiri, M.; Ekholm, A.; Johansson, E.; Andersson, S.C.; Rumpunen, K. Major phenolic compounds in black currant (Ribes nigrum L.) buds: Variation due to genotype, ontogenetic stage and location. Food Chem. 2015, 63, 1274–1280. [Google Scholar] [CrossRef]
- Vagiri, M.; Ekholm, A.; Andersson, S.C.; Johansson, E.; Rumpunen, K. An optimized method for analysis of phenolic compounds in buds, leaves, and fruits of black currant (Ribes nigrum L.). J. Agric. Food Chem. 2012, 60, 10501–10510. [Google Scholar] [CrossRef]
- Shimokoriyama, M. Anthochlor Pigments of Coreopsis tinctoria. J. Am. Chem. Soc. 1957, 79, 214–220. [Google Scholar] [CrossRef]
- Uehara, A.; Nakata, M.; Kitajima, J.; Iwashina, T. Internal and external flavonoids from the leaves of Japanese Chrysanthemum species (Asteraceae). Biochem. Syst. Ecol. 2012, 41, 142–149. [Google Scholar] [CrossRef]
- Li, Y.; Yang, P.; Luo, Y.; Gao, B.; Sun, J.; Lu, W.; Liu, J.; Chen, P.; Zhang, Y.; Yu, L. (Lucy) Chemical compositions of chrysanthemum teas and their anti-inflammatory and antioxidant properties. Food Chem. 2019, 286, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Harnly, J.M. Identification of the phenolic components of chrysanthemum flower (Chrysanthemum morifolium Ramat). Food Chem. 2010, 120, 319–326. [Google Scholar] [CrossRef]
- Chen, L.X.; Hu, D.J.; Lam, S.C.; Ge, L.; Wu, D.; Zhao, J.; Long, Z.R.; Yang, W.J.; Fan, B.; Li, S.P. Comparison of antioxidant activities of different parts from snow chrysanthemum (Coreopsis tinctoria Nutt.) and identification of their natural antioxidants using high performance liquid chromatography coupled with diode array detection and mass spectrometry and 2,2′-azinobis (3-ethylbenzthiazoline-sulfonic acid) diammonium salt-based assay. J. Chromatogr. A 2016, 1428, 134–142. [Google Scholar]
- Roullier-Gall, C.; Witting, M.; Gougeon, R.D.; Schmitt-Kopplin, P. High precision mass measurements for wine metabolomics. Front. Chem. 2014, 2, 102. [Google Scholar] [CrossRef]
- Liu, P.; Kallio, H.; Yang, B. Phenolic compounds in hawthorn (Crataegus grayana) fruits and leaves and changes during fruit ripening. J. Agric. Food Chem. 2011, 59, 11141–11149. [Google Scholar] [CrossRef]
- Liu, P. Composition of Hawthorn (Crataegus spp.) Fruits and Leaves and Emblic Leaf Flower (Phyllanthus emblica) Fruits. Ph.D. Thesis, University of Turku, Turku, Finland, 2012. [Google Scholar]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Stern, R. Hyaluronidases in cancer biology. Semin. Cancer Biol. 2008, 18, 275–280. [Google Scholar] [CrossRef]
- Mohamed, E.M.; Hetta, M.H.; Rateb, M.E.; Selim, M.A.; AboulMagd, A.M.; Badria, F.A.; Abdelmohsen, U.R.; Alhadrami, H.A.; Hassan, H.M. Bioassay-guided isolation, metabolic profiling, and docking studies of hyaluronidase inhibitors from ravenala madagascariensis. Molecules 2020, 25, 1714. [Google Scholar] [CrossRef] [PubMed]
- González-Peña, D.; Colina-Coca, C.; Char, C.D.; Cano, M.P.; De Ancos, B.; Sánchez-Moreno, C. Hyaluronidase inhibiting activity and radical scavenging potential of flavonols in processed onion. J. Agric. Food Chem. 2013, 61, 4862–4872. [Google Scholar] [CrossRef] [PubMed]
- Tomohara, K.; Ito, T.; Onikata, S.; Kato, A.; Adachi, I. Discovery of hyaluronidase inhibitors from natural products and their mechanistic characterization under DMSO-perturbed assay conditions. Bioorganic Med. Chem. Lett. 2017, 27, 1620–1623. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, K.E.; Khan, Z.; Giani, J.F.; Cao, D.Y.; Bernstein, E.A.; Shen, X.Z. Angiotensin-converting enzyme in innate and adaptive immunity. Nat. Rev. Nephrol. 2018, 14, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Fuku, N.; Kumagai, H.; Ahmetov, I.I. Genetics of muscle fiber composition. Sport. Exerc. Nutr. Genomics Curr. Status Futur. Dir. 2019, 295–314. [Google Scholar]
- Miller, A.J.; Arnold, A.C. The renin–angiotensin system in cardiovascular autonomic control: Recent developments and clinical implications. Clin. Auton. Res. 2019, 29, 231–243. [Google Scholar] [CrossRef]
- Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Natural Angiotensin Converting Enzyme (ACE) Inhibitors with Antihypertensive Properties. In Natural Products Targeting Clinically Relevant Enzymes; Wiley-VCH: Weinheim, Germany, 2017; pp. 45–67. [Google Scholar]
- Cloud, A.; Vilcins, D.; McEwen, B. The effect of hawthorn (Crataegus spp.) on blood pressure: A systematic review. Adv. Integr. Med. 2020, 7, 167–175. [Google Scholar] [CrossRef]
- Chang, Q.; Zuo, Z.; Harrison, F.; Sing, M.; Chow, S. Hawthorn. J. Clin. Pharmacol. 2002, 42, 605–612. [Google Scholar] [CrossRef]
- Han, J.; Tan, D.; Liu, G. Hawthorn—A health food. Appl. Mech. Mater. 2012, 140, 350–354. [Google Scholar] [CrossRef]
- Kumar, D.; Arya, V.; Bhat, Z.A.; Khan, N.A.; Prasad, D.N. The genus Crataegus: Chemical and pharmacological perspectives. Braz. J. Pharmacogn. 2012, 22, 1187–1200. [Google Scholar] [CrossRef]
- Holubarsch, C.J.F.; Colucci, W.S.; Eha, J. Benefit-Risk Assessment of Crataegus Extract WS 1442: An Evidence-Based Review. Am. J. Cardiovasc. Drugs 2018, 18, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.; Malek, F.A. Standardized extracts from hawthorn leaves and flowers in the treatment of cardiovascular disorders preclinical and clinical studies. Planta Med. 2011, 77, 1123–1128. [Google Scholar] [CrossRef]
- Tassell, M.; Kingston, R.; Gilroy, D.; Lehane, M.; Furey, A. Hawthorn (Crataegus spp.) in the treatment of cardiovascular disease. Pharmacogn. Rev. 2010, 4, 32–41. [Google Scholar]
- Zick, S.M.; Gillespie, B.; Aaronson, K.D. The effect of Crataegus oxycantha special extract WS 1442 on clinical progression in patients with mild to moderate symptoms of heart failure. Eur. J. Heart Fail. 2008, 10, 587–593. [Google Scholar] [CrossRef]
- Alirezalu, A.; Salehi, P.; Ahmadi, N.; Sonboli, A.; Aceto, S.; Maleki, H.H.; Ayyari, M. Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp.) from different regions of iran. Int. J. Food Prop. 2018, 21, 452–470. [Google Scholar] [CrossRef]
- Tabart, J.; Schini-Kerth, V.; Pincemail, J.; Kevers, C.; Pirotte, B.; Defraigne, J.O.; Dommes, J. The leaf extract of Ribes nigrum L. is a potent stimulator of the endothelial formation of NO in cultured endothelial cells and porcine coronary artery rings. J. Berry Res. 2016, 6, 277–289. [Google Scholar] [CrossRef]
- Lievre, H.; Gullot, B. Chrysanthellum americanum: Une plante tropicale au service des vaisseaux, du foie et de l’orthodoxie des métabolismes lipo-protéiques. La Revue du Jeune Médecin, 1984. [Google Scholar]
- Kouchmeshky, A.; Jameie, S.B.; Amin, G.; Ziai, S.A. Investigation of angiotensin-convertings enzyme inhibitory effects of medicinal plants used in traditional Persian medicine for treatment of hypertension: Screening study. Thrita Stud. J. Med. Sci. 2012, 1, 13–23. [Google Scholar] [CrossRef]
- Chiou, S.Y.; Sung, J.M.; Huang, P.W.; Lin, S.D. Antioxidant, Antidiabetic, and Antihypertensive Properties of Echinacea purpurea Flower Extract and Caffeic Acid Derivatives Using in Vitro Models. J. Med. Food 2017, 20, 171–179. [Google Scholar] [CrossRef]
- Liu, J.C.; Hsu, F.L.; Tsai, J.C.; Chan, P.; Liu, J.Y.H.; Thomas, G.N.; Tomlinson, B.; Lo, M.Y.; Lin, J.Y. Antihypertensive effects of tannins isolated from traditional Chinese herbs as non-specific inhibitors of angiontensin converting enzyme. Life Sci. 2003, 73, 1543–1555. [Google Scholar] [CrossRef]
- Keser, S.; Celik, S.; Turkoglu, S.; Yilmaz, O.; Turkoglu, I. The Investigation of Some Bioactive Compounds and Antioxidant Properties of Hawthorn (Crataegus monogyna subsp. monogyna jacq.). J. Intercult. Ethnopharmacol. 2014, 3, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Birasuren, B.; Oh, H.L.; Kim, C.R.; Kim, N.Y.; Jeon, H.L.; Kim, M.R. Antioxidant activities of Ribes diacanthum pall extracts in the northern region of mongolia. Prev. Nutr. Food Sci. 2012, 17, 261–268. [Google Scholar] [CrossRef] [PubMed]

| Kinetics of Extraction a | TPC b, TFC b, OPC b | UHPLC-MS c | ESI(-) FT-ICR-MS c | Enzymatic Activity c | |
|---|---|---|---|---|---|
| Chrysanthellum americanum (CA) | 559980 (infusion and maceration) | 559980 d | 559980 e, CP44120 e, NH558088 e | 559980 e, CP44120 e, NH558088 e | 559980 e |
| Blackcurrant (Ribes nigrum) leaves (BC) | 55870 (infusion and maceration) | 55870 d | 55870 e, NH558024 e | 55870 e | 55870 e |
| Hawthorn (Crataegus) flowering tops (HAW) | 20335 (infusion, maceration and ultrasonic) [4] | 20335 d [4] | 20335 d/R78927 e, 1221478 e, H18001534 e, CB58120 e [4] | CB58120 e, APC27031904 e | 20335 (infusion) f CB58120 e |
| Plant Nature and Granulometry | Extraction Mode | Extraction Time to Get 70% of the Abs at 30 min (min) | Absorbance at 30 min | 10 min Extraction Time | 30 min Extraction Time | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Extraction Yield (%) b | TPC c | TFC d | OPC e | Extraction Yield (%) b | TPC c | TFC d | OPC e | ||||
| CA (raw) | Infusion | 2 | 1.177 | 22.70 ± 0.94 | 11.29 ± 0.30 | 2.64 ± 0.06 | 0.36 ± 0.03 | 25.12 ± 1.96 | 14.79 ± 0.60 | 3.12 ± 0.07 | 0.43 ± 0.03 |
| Maceration | 7 | 1.161 | 22.41 ± 0.45 | 8.37 ± 0.60 | 2.23 ± 0.18 | 0.34 ± 0.03 | 24.86 ± 1.48 | 11.48 ± 0.93 | 2.64 ± 0.22 | 0.37 ± 0.02 | |
| Ultrasonic | - | - | 24.12 ± 1.03 | 8.91 ± 0.44 | 3.21 ± 0.15 | 0.39 ± 0.03 | - | - | - | - | |
| Microwave | - | - | 27.61 ± 1.62 | 13.86 ± 0.60 | 3.29 ± 0.12 | 0.48 ± 0.02 | - | - | - | - | |
| Percolation | - | - | 16.60 ± 0.01 | 8.29 ± 0.05 | 2.08 ± 0.10 | 0.26 ± 0.02 | - | - | - | - | |
| CA (ground 1 mm) | Infusion | <1.5 | 1.647 | 31.81 ± 0.18 | 17.08 ± 0.31 | 4.42 ± 0.28 | 0.66 ± 0.04 | 32.34 ± 0.80 | 18.37 ± 0.51 | 3.62 ± 0.16 | 0.67 ± 0.06 |
| Maceration | <1.5 | 1.478 | 30.85 ± 0.41 | 12.87 ± 0.35 | 3.76 ± 0.05 | 0.50 ± 0.02 | 31.50 ± 0.47 | 14.59 ± 0.80 | 3.76 ± 0.16 | 0.54 ± 0.02 | |
| Ultrasonic | - | - | 31.65 ± 1.42 | 12.74 ± 0.94 | 4.21 ± 0.07 | 0.63 ± 0.05 | - | - | - | - | |
| Microwave | - | - | 31.29 ± 1.05 | 17.38 ± 0.83 | 4.21 ± 0.08 | 0.63 ± 0.02 | - | - | - | - | |
| Percolation | - | - | 31.09 ± 0.89 | 13.04 ± 0.48 | 4.32 ± 0.05 | 0.67 ± 0.04 | - | - | - | - | |
| BC (raw) | Infusion | 8.5 | 1.824 | 15.16 ± 0.39 | 15.84 ± 0.45 | 1.72 ± 0.08 | 0.55 ± 0.04 | 20.69 ± 0.72 | 23.92 ± 1.38 | 2.48 ± 0.04 | 0.83 ± 0.07 |
| Maceration | 15.5 | 1.137 | 8.44 ± 0.42 | 6.99 ± 0.35 | 0.81 ± 0.06 | 0.24 ± 0.02 | 18.49 ± 1.19 | 17.09 ± 0.28 | 1.98 ± 0.11 | 0.64 ± 0.01 | |
| Ultrasonic | - | - | 17.61 ± 1.48 | 18.82 ± 0.30 | 2.28 ± 0.17 | 1.02 ± 0.02 | - | - | - | - | |
| Microwave | - | - | 20.45 ± 0.21 | 23.08 ± 1.09 | 2.74 ± 0.21 | 1.02 ± 0.10 | - | - | - | - | |
| Percolation | - | - | 7.40 ± 0.38 | 6.50 ± 0.26 | 0.88 ± 0.01 | 0.30 ± 0.05 | - | - | - | - | |
| BC (ground 1 mm) | Infusion | <1.5 | 3.615 a | 30.49 ± 1.22 | 47.28 ± 0.57 | 4.75 ± 0.09 | 2.35 ± 0.17 | 32.41 ± 0.52 | 45.39 ± 1.25 | 4.82 ± 0.13 | 2.66 ± 0.16 |
| Maceration | <1.5 | 3.399 a | 30.39 ± 0.36 | 45.75 ± 2.34 | 4.73 ± 0.10 | 2.07 ± 0.05 | 30.51 ± 0.44 | 44.94 ± 1.35 | 4.70 ± 0.14 | 2.74 ± 0.16 | |
| Ultrasonic | - | - | 29.54 ± 0.35 | 42.42 ± 1.67 | 4.45 ± 0.32 | 2.55 ± 0.01 | - | - | - | - | |
| Microwave | - | - | 33.13 ± 0.27 | 50.62 ± 1.30 | 5.25 ± 0.09 | 3.60 ± 0.32 | - | - | - | - | |
| Percolation | - | - | 31.80 ± 0.96 | 46.05 ± 0.18 | 4.77 ± 0.01 | 2.42 ± 0.07 | - | - | - | - | |
| Nature of the Plant | Lot Number | Extraction Yield (%) | TPC a | TFC b | OPC c |
|---|---|---|---|---|---|
| Chrysanthellum americanum (CA) | 559980 | 25.9 ± 0.9 | 15.51 ± 0.05 | 3.31 ± 0.04 | 0.42 ± 0.01 |
| Blackcurrant leaves (Ribes nigrum) (BC) | 55870 | 28.6 ± 0.5 | 39.60 ± 0.49 | 4.44 ± 0.17 | 1.67 ± 0.10 |
| Hawthorn (Crataegus) (HAW) [4] | CB58120 | 21.7 ± 0.1 | 20.1 ± 0.4 | 2.86 ± 0.02 | 1.81 ± 0.05 |
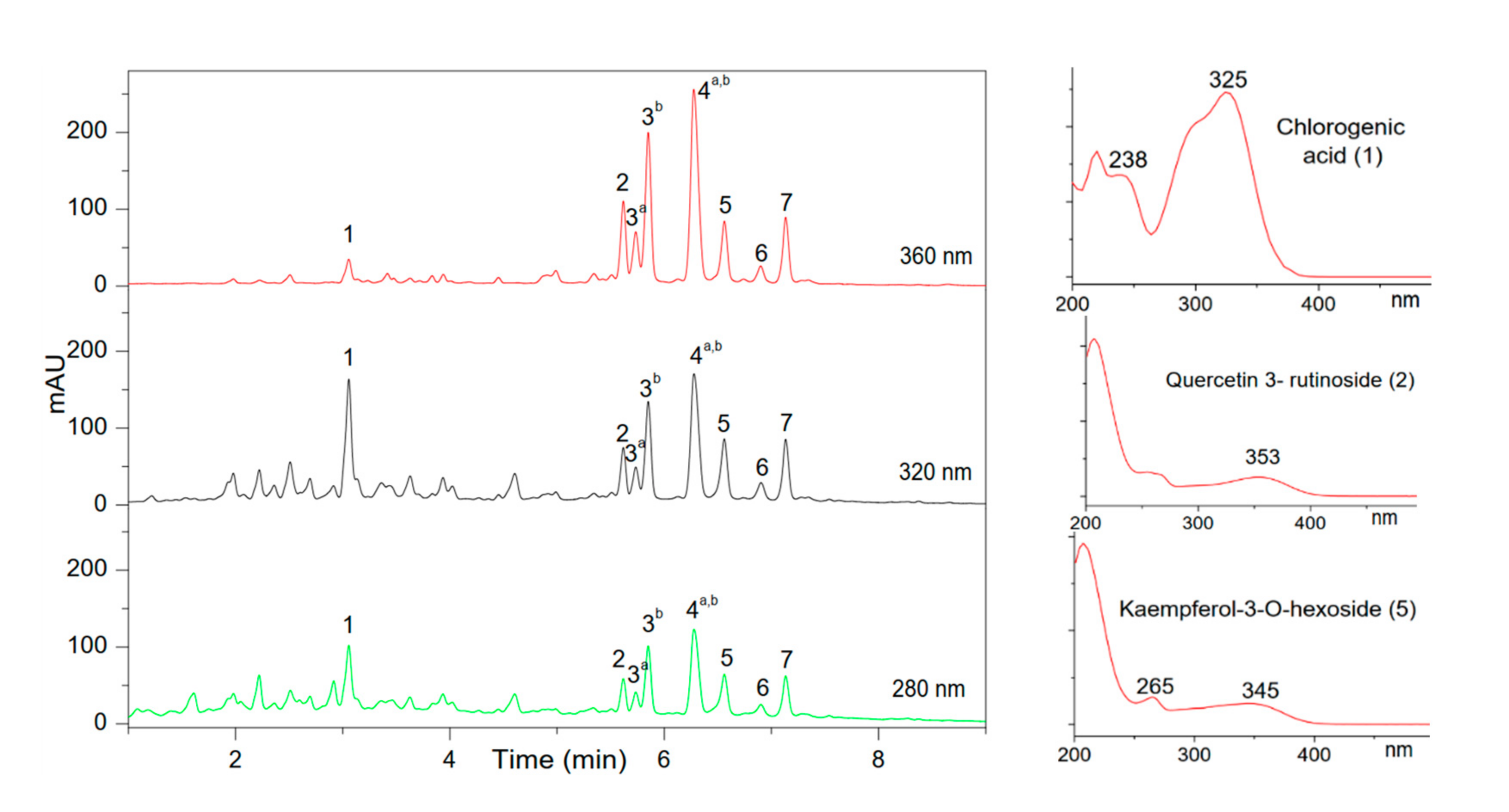
| Peak | Retention Time (min) | ƛmax (nm) | [M+H]+/[M−H]− | Other Ions in the Spectrum (Positive/Negative Mode) | Family (Subclass) | Identified Compound |
|---|---|---|---|---|---|---|
| 1 | 3.25 | 219, 238, 325 | 355/353 | 377, 163/191, 179 | Phenolic acid (hydroxycinnamic acid) | Chlorogenic acid |
| 2 | 5.64 | 254, 353 | 611/609.2 | 303/301, 179 | Flavonoid (Flavonol) | Quercetin 3-rutinoside |
| 3a | 5.75 | 254, 353 | 465/463.1 | 303/301 | Flavonoid (Flavonol) | Quercetin 3-O-galactoside (hyperoside) |
| 3b | 5.85 | 254, 353 | 465/463.1 | 303/301 | Flavonoid (Flavonol) | Quercetin 3-O-glucoside (isoquercetin) |
| 4a | 6.24 | 255, 353 | 551/549 | 303/505.1, 301 | Flavonoid (Flavonol) | Quercetin-3-6-malonyl-glucoside |
| 4b | 6.24 | 263, 347 | 287/593 | 287/285 | Flavonoid (Flavonol) | Kaempferol-3-O-rutinoside |
| 5 | 6.51 | 265, 345 | 287/447.1 | 287/285 | Flavonoid (Flavonol) | Kaempferol-3-O-hexoside |
| 6 | 6.84 | 265, 344 | 535/533 | 287/489, 285 | Flavonoid (Flavonol) | Kaempferol-malonylglucoside |
| 7 | 7.05 | 265, 345 | 535/533 | 535, 287/489, 285 | Flavonoid (Flavonol) | Kaempferol-malonyl-glucoside isomer |
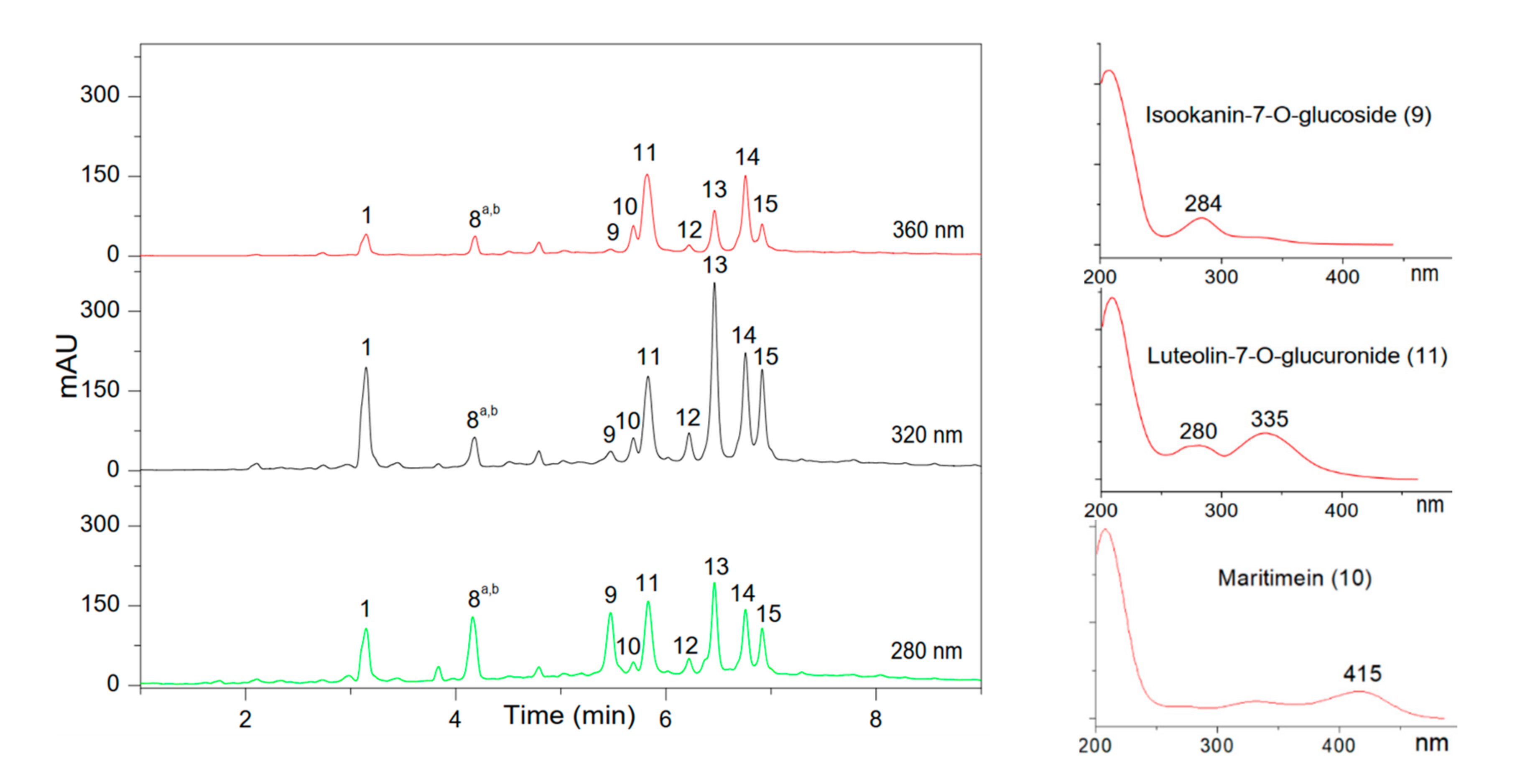
| Peak | Retention Time (min) | ƛmax (nm) | [M−H]− | Other Ions in the Spectrum | Family (Subclass) | Identified Compound |
|---|---|---|---|---|---|---|
| 1 | 3.24 | 219, 238, 325 | 353 | 191, 179 | Phenolic acid (Hydroxycinnamic acid) | Chlorogenic acid |
| 8a | 4.25 | 282 | 449 | 287, 151, 135 | Flavonoid (Flavanone) | Eriodicyol-7-O-glucoside |
| 8b | 4.25 | 267 | 593 | 473, 353, 191 | Flavonoid (Flavone) | 6,8-C,C-diglucosylapigenin |
| 9 | 5.51 | 284 | 449 | 287, 269, 151, 135 | Flavonoid (Flavanone) | Isookanin-7-O-glucoside (Flavanomarein) |
| 10 | 5.71 | 415 | 447 | 285, 151, 135, 133 | Flavonoid (Aurone) | Maritimetin-6-O-glucoside (Maritimein) |
| 11 | 5.83 | 280, 335 | 461 | 285 | Flavonoid (Flavone) | Luteolin-7-O-glucuronide |
| 12 | 6.24 | 208, 323 | 515 | 353, 191, 179, 173, 135 | Phenolic acid (Hydroxycinnamic acid) | di-caffeoylquinic acid |
| 13 | 6.45 | 211, 327 | 515 | 353, 191, 179, 173, 135 | Phenolic acid (Hydroxycinnamic acid) | di-caffeoylquinic acid isomer |
| 14 | 6.74 | 207, 266, 334 | 445 | 269 | Flavonoid (Flavone) | Apigenin-7-glucuronide |
| 15 | 6.90 | 209, 326 | 515 | 353, 191, 179, 173, 135 | Phenolic acid (Hydroxycinnamic acid) | di-caffeoylquinic acid isomer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao-Ngoc, P.; Leclercq, L.; Rossi, J.-C.; Hertzog, J.; Tixier, A.-S.; Chemat, F.; Nasreddine, R.; Al Hamoui Dit Banni, G.; Nehmé, R.; Schmitt-Kopplin, P.; et al. Water-Based Extraction of Bioactive Principles from Blackcurrant Leaves and Chrysanthellum americanum: A Comparative Study. Foods 2020, 9, 1478. https://doi.org/10.3390/foods9101478
Cao-Ngoc P, Leclercq L, Rossi J-C, Hertzog J, Tixier A-S, Chemat F, Nasreddine R, Al Hamoui Dit Banni G, Nehmé R, Schmitt-Kopplin P, et al. Water-Based Extraction of Bioactive Principles from Blackcurrant Leaves and Chrysanthellum americanum: A Comparative Study. Foods. 2020; 9(10):1478. https://doi.org/10.3390/foods9101478
Chicago/Turabian StyleCao-Ngoc, Phu, Laurent Leclercq, Jean-Christophe Rossi, Jasmine Hertzog, Anne-Sylvie Tixier, Farid Chemat, Rouba Nasreddine, Ghassan Al Hamoui Dit Banni, Reine Nehmé, Philippe Schmitt-Kopplin, and et al. 2020. "Water-Based Extraction of Bioactive Principles from Blackcurrant Leaves and Chrysanthellum americanum: A Comparative Study" Foods 9, no. 10: 1478. https://doi.org/10.3390/foods9101478
APA StyleCao-Ngoc, P., Leclercq, L., Rossi, J.-C., Hertzog, J., Tixier, A.-S., Chemat, F., Nasreddine, R., Al Hamoui Dit Banni, G., Nehmé, R., Schmitt-Kopplin, P., & Cottet, H. (2020). Water-Based Extraction of Bioactive Principles from Blackcurrant Leaves and Chrysanthellum americanum: A Comparative Study. Foods, 9(10), 1478. https://doi.org/10.3390/foods9101478