Comprehensive Characterization and Relative Quantification of α-Amylase/Trypsin Inhibitors from Wheat Cultivars by Targeted HPLC-MS/MS
Abstract
1. Introduction
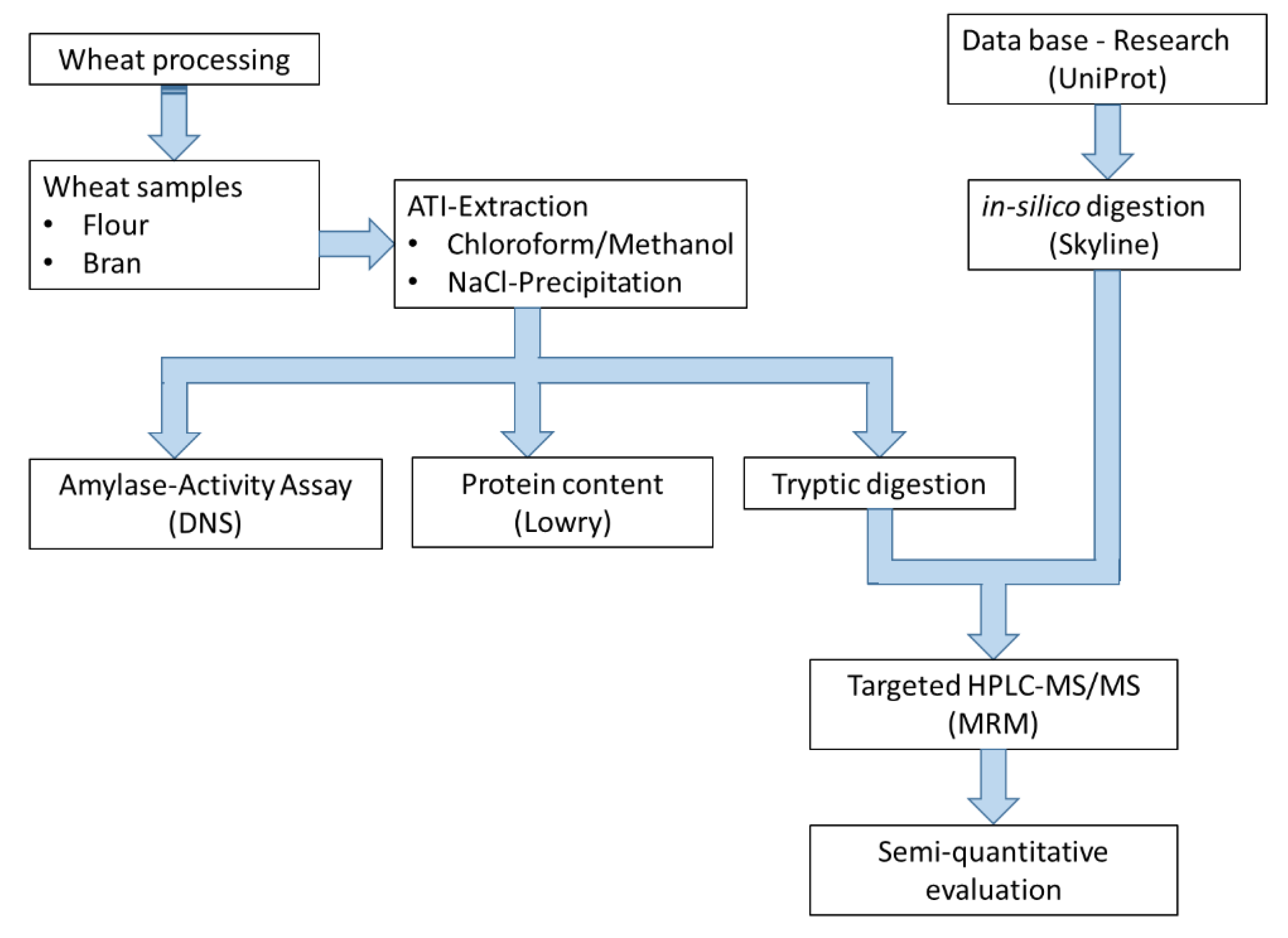
2. Materials and Methods
2.1. Materials
2.1.1. Samples Collected from Germany
2.1.2. Samples Collected from Turkey
2.1.3. Other Chemicals
2.2. Methods
2.2.1. Extraction of the ATIs Using the Chloroform/Methanol Method
2.2.2. Protein Determination
2.2.3. Inhibition of Amylase Activity
2.2.4. Inhibition of Trypsin Activity
2.2.5. SDS-Page
2.2.6. In-Solution Tryptic Digestion
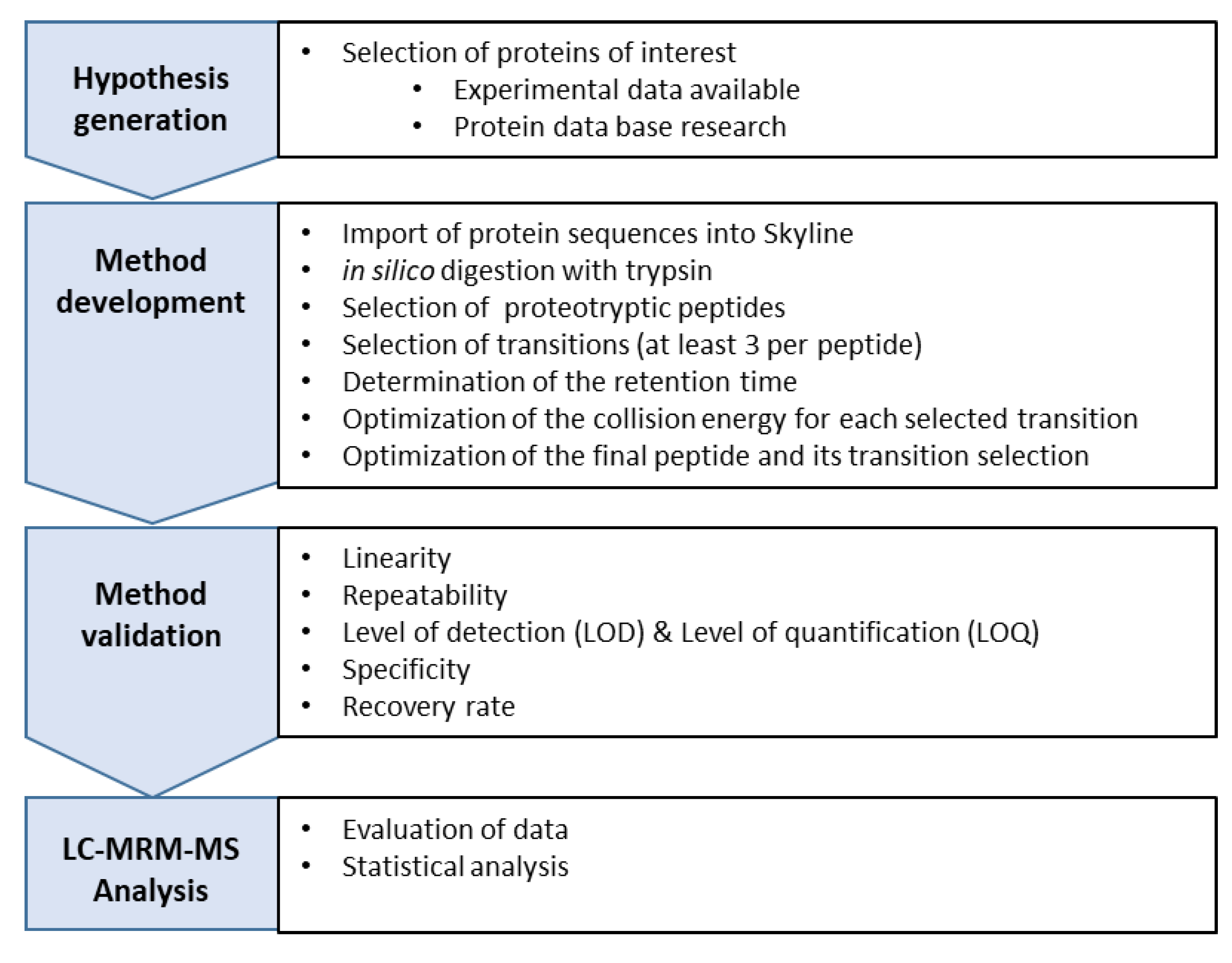
2.2.7. Development of a Multiple Reaction Monitoring (MRM) Assay Using HPLC-MS/MS
Hypothesis
Method Development
Validation of the HPLC-MS/MS
Normalization
2.2.8. Extraction of the ATIs Using Ammonium Bicarbonate Buffer
Ambi/Urea Extraction
Solid Phase Extraction for Sample Purification
Mass Spectrometric Analysis
2.2.9. Statistical Interpretation
3. Results and Discussion
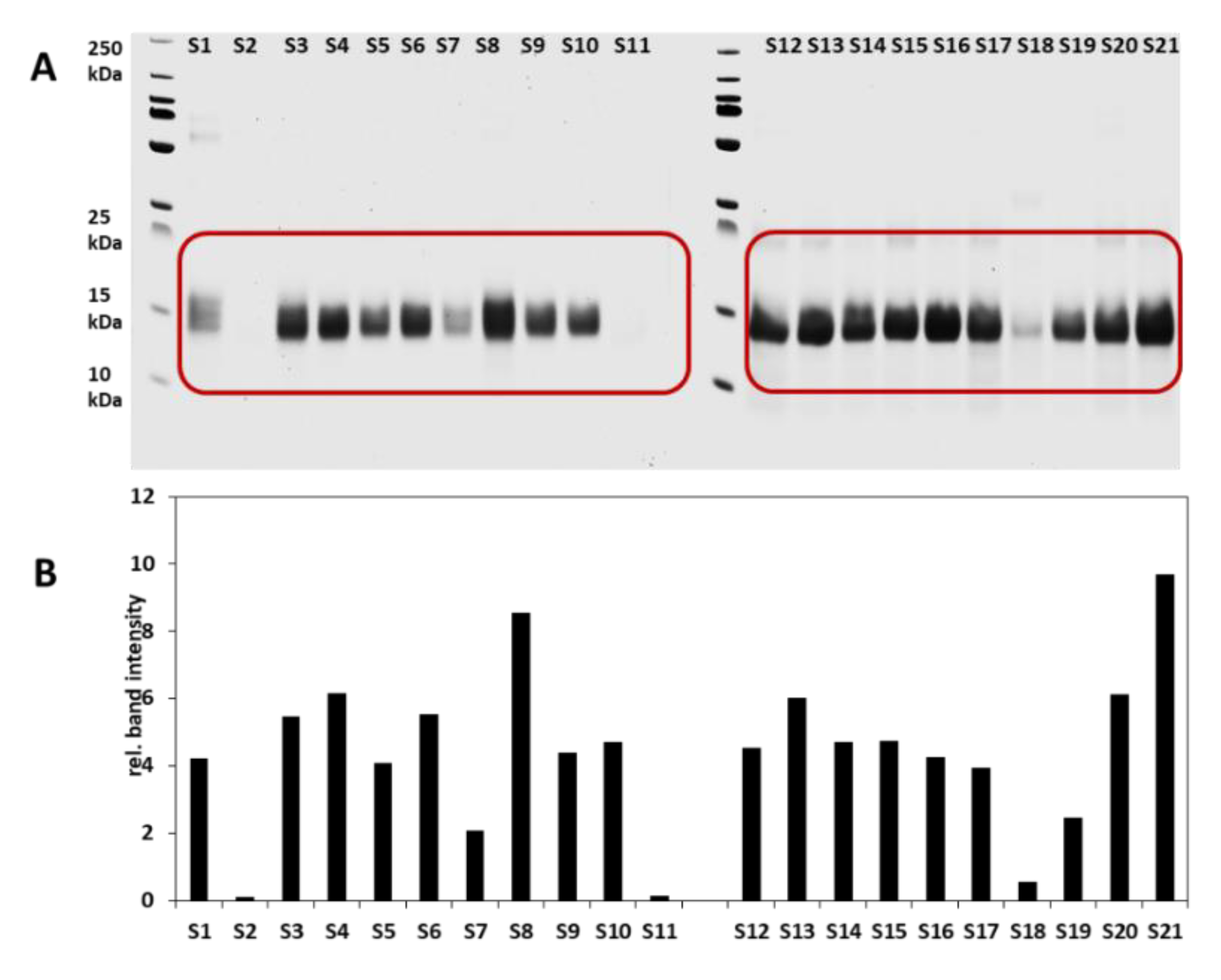
3.1. Intact Protein Analysis
3.1.1. SDS PAGE and Analysis of the Relative Band Intensities
3.1.2. Protein Contents According to Lowry
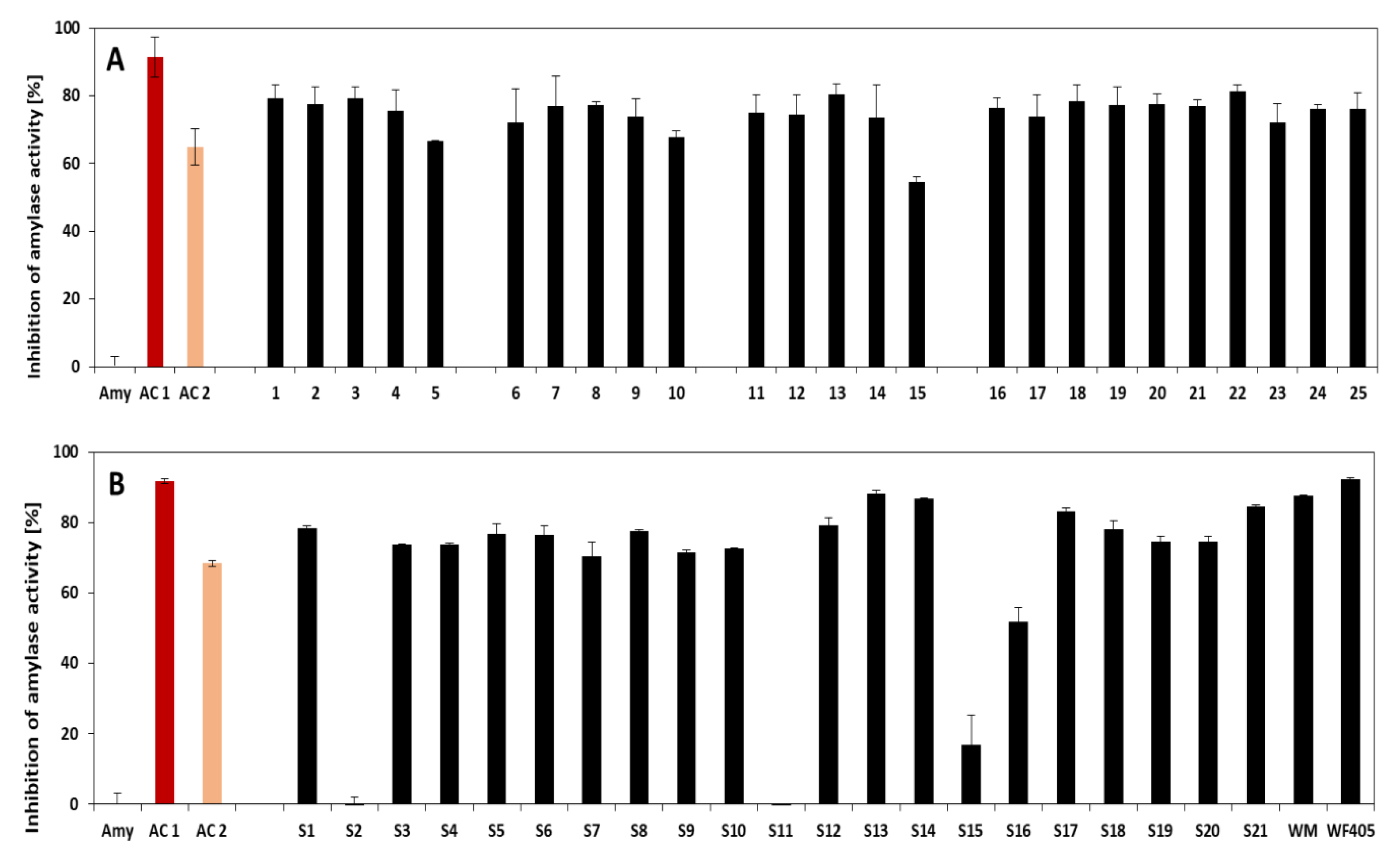
3.2. Amylase and Trypsin Inhibition
3.3. LC-MS/MS Analysis
3.3.1. Peptide Selection
3.3.2. Method Validation
Linearity of Responses
Specificity
Repeatability
Matrix Effects
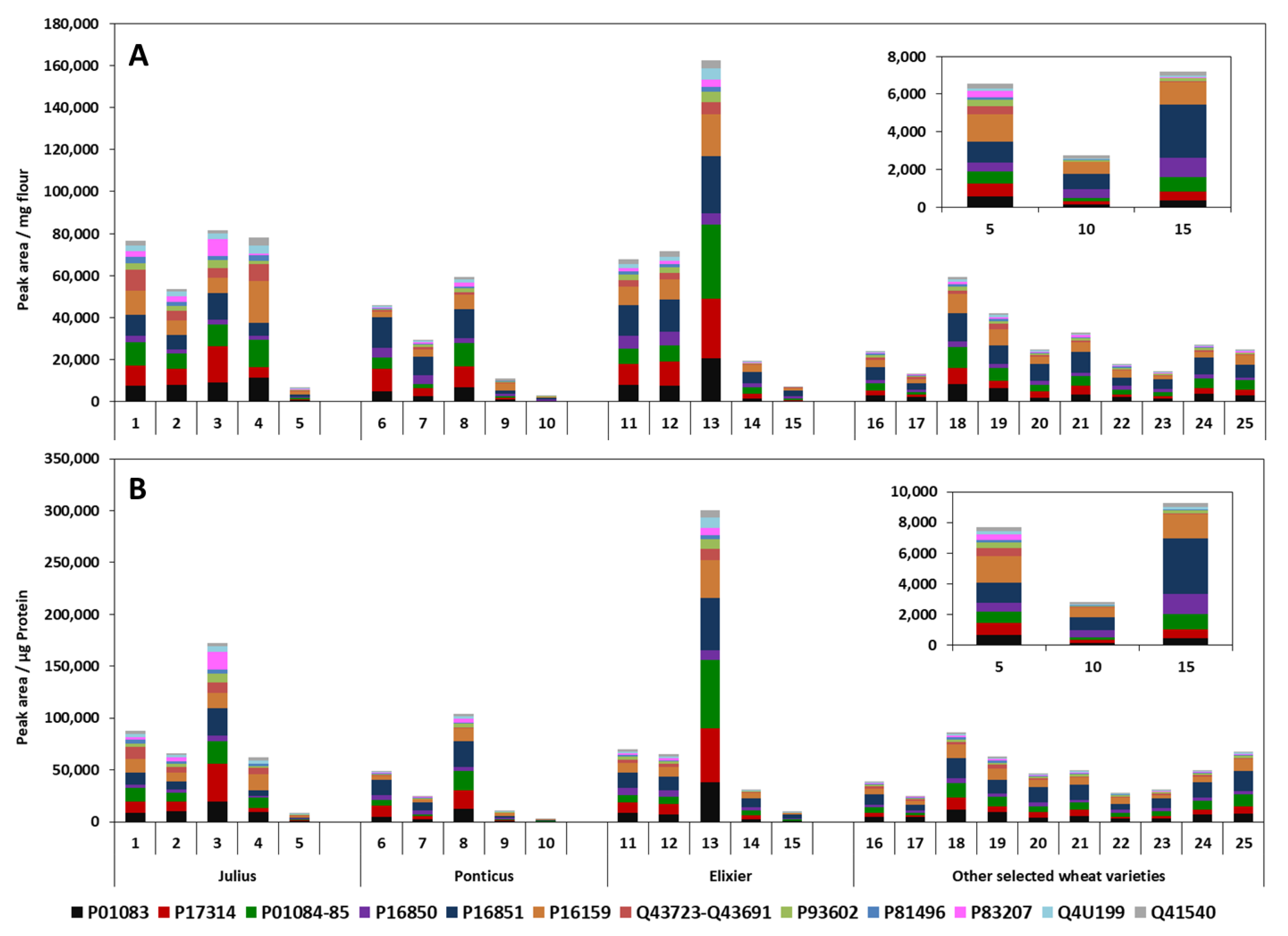
3.3.3. Analysis and Relative Quantification of ATIs in German Wheat Cultivars
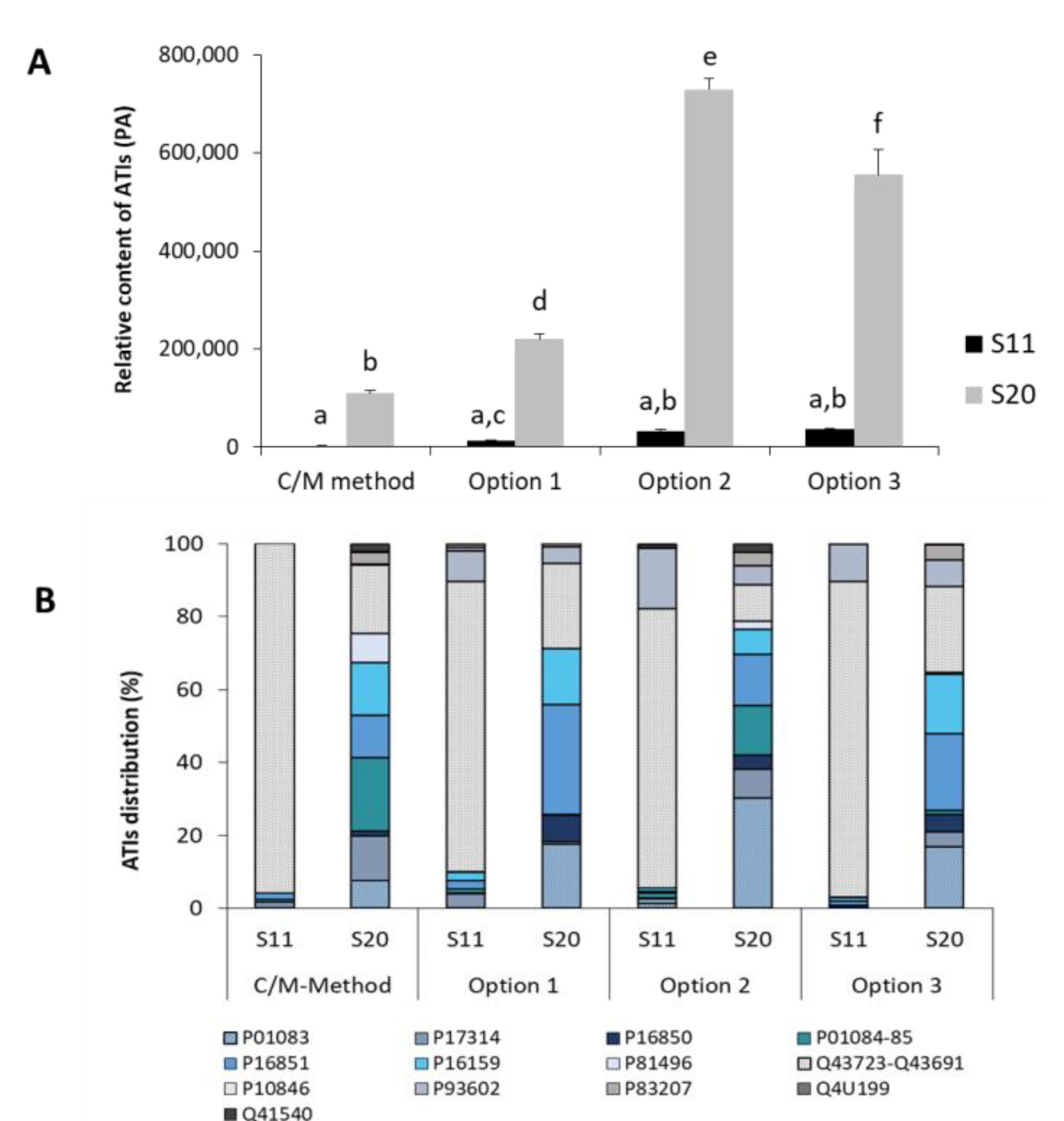
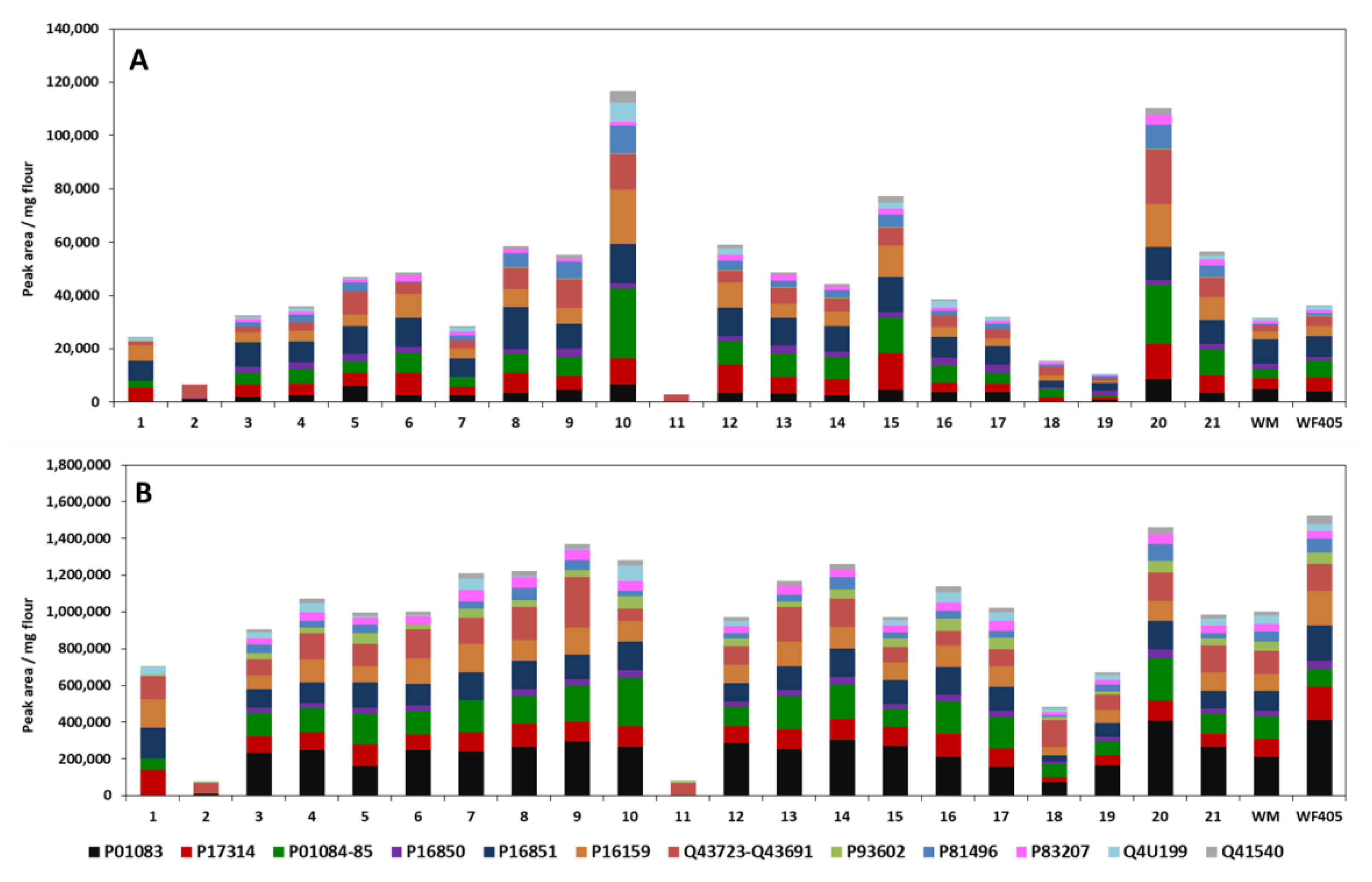
3.3.4. Analysis of Turkish Wheat Cultivars: Comparison CM and Ambi/urea Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osborne, T.B.; Harris, I.F. Die Chemie der Proteine Körper des Weizenkorns. Teil II. Darstellung der Proteine in gen gender Menge fur die Hydrolyse. Z. Anal. Chem. 1907, 46, 749–756. [Google Scholar] [CrossRef][Green Version]
- Zilic, S.; Barac, M.; Pesic, M.; Dodig, D.; Ignjatovic-Micic, D. Characterization of proteins from grain of different bread and durum wheat genotypes. Int. J. Mol. Sci. 2011, 12, 5878–5894. [Google Scholar] [CrossRef] [PubMed]
- Sagu, S.T.; Huschek, G.; Bonick, J.; Homann, T.; Rawel, H.M. A New Approach of Extraction of alpha-Amylase/trypsin Inhibitors from Wheat (Triticum aestivum L.), Based on Optimization Using Plackett-Burman and Box-Behnken Designs. Molecules 2019, 24, 3589. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R. What is gluten? J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 78–81. [Google Scholar] [CrossRef] [PubMed]
- Iulek, J.; Franco, O.L.; Silva, M.; Slivinski, C.T.; Bloch, C., Jr.; Rigden, D.J.; Grossi de Sa, M.F. Purification, biochemical characterisation and partial primary structure of a new alpha-amylase inhibitor from Secale cereale (rye). Int. J. Biochem. Cell Biol. 2000, 32, 1195–1204. [Google Scholar] [CrossRef]
- Laudencia-Chingcuanco, D.L.; Stamova, B.S.; Lazo, G.R.; Cui, X.; Anderson, O.D. Analysis of the wheat endosperm transcriptome. J. Appl. Genet. 2006, 47, 287–302. [Google Scholar] [CrossRef]
- Altenbach, S.B.; Vensel, W.H.; Dupont, F.M. The spectrum of low molecular weight alpha-amylase/protease inhibitor genes expressed in the US bread wheat cultivar Butte 86. BMC Res. Notes 2011, 4, 242. [Google Scholar] [CrossRef]
- Maeda, K.; Wakabayashi, S.; Matsubara, H. Disulfide bridges in an alpha-amylase inhibitor from wheat kernel. J. Biochem. 1983, 94, 865–870. [Google Scholar] [CrossRef]
- Kan, Y.; Wan, Y.; Beaudoin, F.; Leader, D.J.; Edwards, K.; Poole, R.; Wang, D.; Mitchell, R.A.C.; Shewry, P.R. Transcriptome analysis reveals differentially expressed storage protein transcripts in seeds of Aegilops and wheat. J. Cereal. Sci. 2006, 44, 75–85. [Google Scholar] [CrossRef]
- Breiteneder, H.; Radauer, C. A classification of plant food allergens. J. Allergy Clin. Immunol. 2004, 113, 821–830. [Google Scholar] [CrossRef]
- Oda, Y.; Matsunaga, T.; Fukuyama, K.; Miyazaki, T.; Morimoto, T. Tertiary and quaternary structures of 0.19 alpha-amylase inhibitor from wheat kernel determined by X-ray analysis at 2.06 A resolution. Biochemistry 1997, 36, 13503–13511. [Google Scholar] [CrossRef] [PubMed]
- Islamov, R.A.; Furusov, O.V. Bifunctional inhibitor of alpha-amylase/trypsin from wheat grain. Prikladnaia Biokhimiia i Mikrobiologiia 2007, 43, 419–423. [Google Scholar] [PubMed]
- Rogniaux, H.; Pavlovic, M.; Lupi, R.; Lollier, V.; Joint, M.; Mameri, H.; Denery, S.; Larre, C. Allergen relative abundance in several wheat varieties as revealed via a targeted quantitative approach using MS. Proteomics 2015, 15, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R.; Wei, Y.M.; Deng, M.; Nevo, E.; Yan, Z.H.; Zheng, Y.L. The impact of single nucleotide polymorphism in monomeric alpha-amylase inhibitor genes from wild emmer wheat, primarily from Israel and Golan. BMC Evol. Biol. 2010, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Dupont, F.M.; Vensel, W.H.; Tanaka, C.K.; Hurkman, W.J.; Altenbach, S.B. Deciphering the complexities of the wheat flour proteome using quantitative two-dimensional electrophoresis, three proteases and tandem mass spectrometry. Proteome Sci. 2011, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Tatham, A.S.; Shewry, P.R. Allergens to wheat and related cereals. Clin. Exp. Allergy 2008, 38, 1712–1726. [Google Scholar] [CrossRef]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Russel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells. Gastroenterology 2017, 152, 1100–1113. [Google Scholar] [CrossRef]
- Zevallos, V.F.; Raker, V.K.; Maxeiner, J.; Scholtes, P.; Steinbrink, K.; Schuppan, D. Dietary wheat amylase trypsin inhibitors exacerbate murine allergic airway inflammation. Eur. J. Nutr. 2019, 58, 1507–1514. [Google Scholar] [CrossRef]
- Sander, I.; Rozynek, P.; Rihs, H.P.; van Kampen, V.; Chew, F.T.; Lee, W.S.; Kotschy-Lang, N.; Merget, R.; Bruning, T.; Raulf-Heimsoth, M. Multiple wheat flour allergens and cross-reactive carbohydrate determinants bind IgE in baker’s asthma. Allergy 2011, 66, 1208–1215. [Google Scholar] [CrossRef]
- Sanchez-Monge, R.; Gomez, L.; Barber, D.; Lopez-Otin, C.; Armentia, A.; Salcedo, G. Wheat and barley allergens associated with baker’s asthma. Glycosylated subunits of the alpha-amylase-inhibitor family have enhanced IgE-binding capacity. Biochem. J. 1992, 281 Pt 2, 401–405. [Google Scholar] [CrossRef]
- Franken, J.; Stephan, U.; Meyer, H.E.; Konig, W. Identification of alpha-amylase inhibitor as a major allergen of wheat flour. Int. Arch. Allergy Immunol. 1994, 104, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Junker, Y.; Zeissig, S.; Kim, S.J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Larre, C.; Lupi, R.; Gombaud, G.; Brossard, C.; Branlard, G.; Moneret-Vautrin, D.A.; Rogniaux, H.; Denery-Papini, S. Assessment of allergenicity of diploid and hexaploid wheat genotypes: Identification of allergens in the albumin/globulin fraction. J. Proteom. 2011, 74, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Prandi, B.; Faccini, A.; Tedeschi, T.; Galaverna, G.; Sforza, S. LC/MS analysis of proteolytic peptides in wheat extracts for determining the content of the allergen amylase/trypsin inhibitor CM3: Influence of growing area and variety. Food Chem. 2013, 140, 141–146. [Google Scholar] [CrossRef]
- Geisslitz, S.; Ludwig, C.; Scherf, K.A.; Koehler, P. Targeted LC-MS/MS Reveals Similar Contents of alpha-Amylase/Trypsin-Inhibitors as Putative Triggers of Nonceliac Gluten Sensitivity in All Wheat Species except Einkorn. J. Agric. Food Chem. 2018, 66, 12395–12403. [Google Scholar] [CrossRef]
- Bose, U.; Juhasz, A.; Broadbent, J.A.; Byrne, K.; Howitt, C.A.; Colgrave, M.L. Identification and Quantitation of Amylase Trypsin Inhibitors Across Cultivars Representing the Diversity of Bread Wheat. J. Proteome Res. 2020, 19, 2136–2148. [Google Scholar] [CrossRef]
- Meißner, M. Standard-Methoden für Getreide, Mehl und Brot. 8th Revised and Extended Edition with Updated ICC-Standards; Schäfer, M., Ed.; Arbeitsgemeinschaft Getreideforschung e. V. (AGF): Detmold, Germany, 2016. [Google Scholar]
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.L.; Liebler, D.C.; MacCoss, M.J. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef]
- Ali, M.; Homann, T.; Khalil, M.; Kruse, H.P.; Rawel, H. Milk whey protein modification by coffee-specific phenolics: Effect on structural and functional properties. J. Agric. Food Chem. 2013, 61, 6911–6920. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Sagu, S.T.; Nso, E.J.; Homann, T.; Kapseu, C.; Rawel, H.M. Extraction and purification of beta-amylase from stems of Abrus precatorius by three phase partitioning. Food Chem. 2015, 183, 144–153. [Google Scholar] [CrossRef]
- Pearson, W.R.; Lipman, D.J. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 1988, 85, 2444–2448. [Google Scholar] [CrossRef] [PubMed]
- Bereman, M.S.; MacLean, B.; Tomazela, D.M.; Liebler, D.C.; MacCoss, M.J. The development of selected reaction monitoring methods for targeted proteomics via empirical refinement. Proteomics 2012, 12, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Bennion, B.J.; Daggett, V. The molecular basis for the chemical denaturation of proteins by urea. Proc. Natl. Acad. Sci. USA 2003, 100, 5142–5147. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.; Sanchez-Monge, R.; Garcia-Olmedo, F.; Salcedo, G. Wheat tetrameric inhibitors of insect alpha-amylases: Alloploid heterosis at the molecular level. Proc. Natl. Acad. Sci. USA 1989, 86, 3242–3246. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.H.; Richardson, M.; Chen, M.S.; Kramer, K.J.; Morgan, T.D.; Reeck, G.R. alpha-Amylase inhibitors from wheat: Amino acid sequences and patterns of inhibition of insect and human alpha-amylases. Insect Biochem. Mol. Biol. 1996, 26, 419–426. [Google Scholar] [CrossRef]
- Csapó, J.; Albert, C. Methods and procedures for reducing soy trypsin inhibitor activity by means of heat treatment combined with chemical methods. Acta Univ. Sapientiae Aliment. 2018, 11, 58–80. [Google Scholar] [CrossRef]
- Hwang, D.L.; Foard, D.E.; Wei, C.H. A soybean trypsin inhibitor. Crystallization and x-ray crystallographic study. J. Biol. Chem. 1977, 252, 1099–1101. [Google Scholar]
- Das, A.; Mukhopadhyay, C. Urea-mediated protein denaturation: A consensus view. J. Phys. Chem. B 2009, 113, 12816–12824. [Google Scholar] [CrossRef]
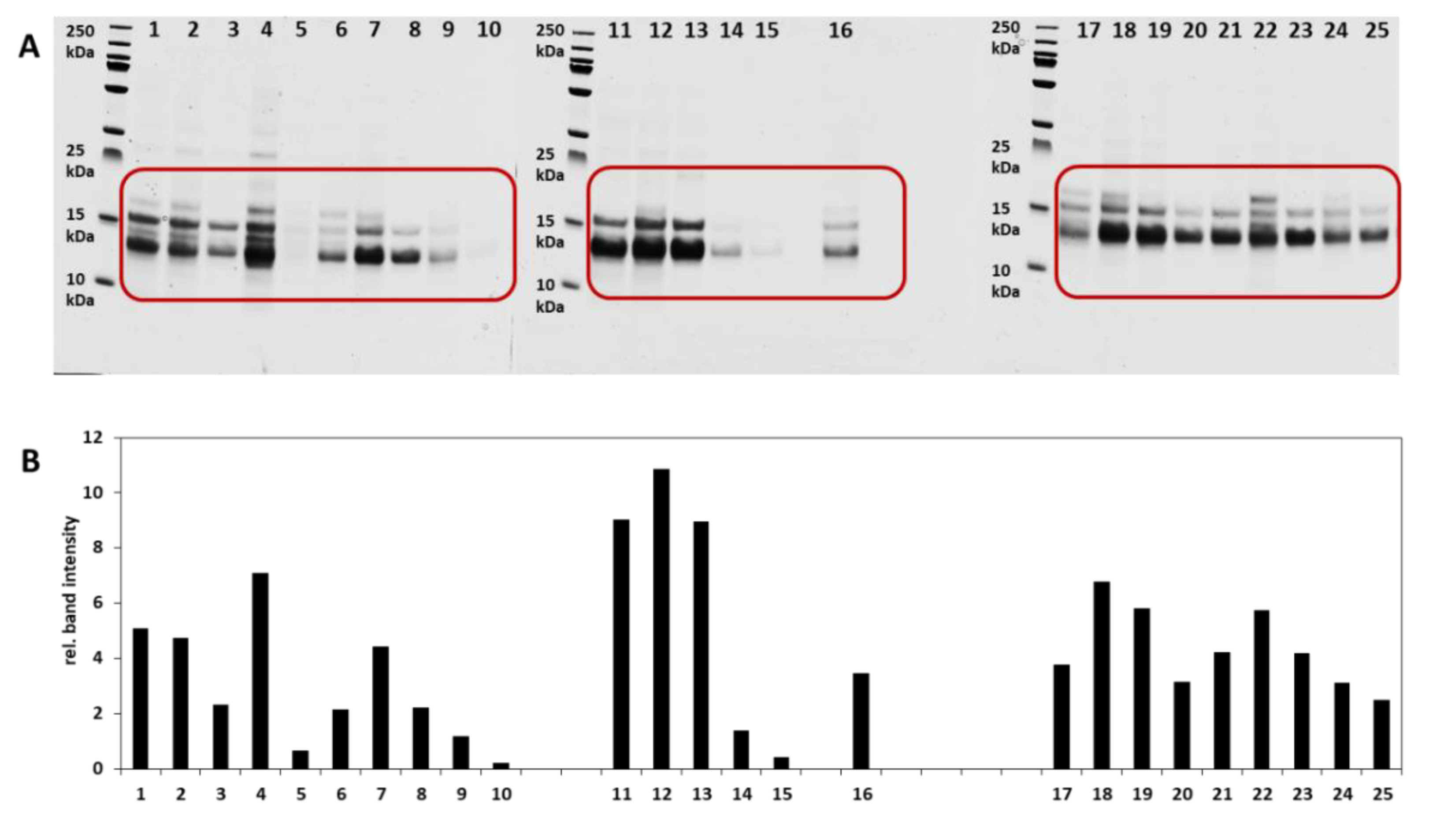
| Cultivar | Cultivation Location | Harvest Year | Quality * | Protein ** (g/100 g) |
|---|---|---|---|---|
| Julius | Münchenberg | 2018 | A | 14.6 |
| RGT Reform | Grünow | 2018 | A | 14.7 |
| Findus | Sonnewalde | 2017 | A | 13.6 |
| Nordkap | Sonnewalde | 2017 | A | 12.4 |
| Patras | Sonnewalde | 2018 | A | 12.4 |
| Ponticus | Sonnewalde | 2018 | E | 15.7 |
| Kerubino | Münchenberg | 2018 | E | 12.5 |
| Tobias | Angermünde | 2018 | E | 14.0 |
| Capo | Schmogrow | 2018 | E | 11.3 |
| Elixer | n.a | 2018 | C | 12.8 |
| Ackermanns Bayernkönig | Münchenberg | 2018 | X | 19.4 |
| Sample No | Cultivar | Cultivation Location | Harvest Year | Type | Protein * (g/100 g) |
|---|---|---|---|---|---|
| S1 | Kunduru | Central Anatolia | 2004 | Hard | 11.8 |
| S2 | Siyazan | Easthern Anatolia | 2019 | Soft | 12.1 |
| S3 | Tosunbey | Central Anatolia | 2019 | Hard | 13.0 |
| S4 | Tosunbey | Central Anatolia | 2016 | Hard | 13.0 |
| S5 | Esperya | Central Anatolia | 2019 | Hard | 14.3 |
| S6 | Kayra | Aegean Region | 2018 | Half hard | 13.5 |
| S7 | Sivas 111/33 | Central Anatolia | 2018 | Soft | 12.7 |
| S8 | Ceyhan-99 | Southeasthern Anatolia | 2019 | Hard | 14.0 |
| S9 | Ceyhan-99 | Blacksea Region | 2019 | Hard | 14.0 |
| S10 | Pehlivan | Central Anatolia | 2016 | Hard | 12.4 |
| S11 | Siyazan | Central Anatolia | 2019 | Soft | 15.6 |
| S12 | Russian | Blacksea Region | 2019 | Hard | n.a |
| S13 | Esperya | Marmara | 2019 | Hard | 14.3 |
| S14 | Ceyhan-99 | Southeasthern Anatolia | 2016 | Hard | 14.0 |
| S15 | Russian | Mediterranean Region | 2019 | Hard | 13.1 |
| S16 | Pehlivan | Marmara | 2018 | Hard | 12.4 |
| S17 | Bezostaja | Central Anatolia | 2019 | Hard | 12.0 |
| S18 | Siyazan | Blacksea Region | 2019 | Soft | 11.8 |
| S19 | AK-702 | Central Anatolia | 2018 | Soft | 10.9 |
| S20 | Esperya | Central Anatolia | 2016 | Hard | 13.9 |
| S21 | Altay | Central Anatolia | 2016 | Half Hard | 11.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagu, S.T.; Zimmermann, L.; Landgräber, E.; Homann, T.; Huschek, G.; Özpinar, H.; Schweigert, F.J.; Rawel, H.M. Comprehensive Characterization and Relative Quantification of α-Amylase/Trypsin Inhibitors from Wheat Cultivars by Targeted HPLC-MS/MS. Foods 2020, 9, 1448. https://doi.org/10.3390/foods9101448
Sagu ST, Zimmermann L, Landgräber E, Homann T, Huschek G, Özpinar H, Schweigert FJ, Rawel HM. Comprehensive Characterization and Relative Quantification of α-Amylase/Trypsin Inhibitors from Wheat Cultivars by Targeted HPLC-MS/MS. Foods. 2020; 9(10):1448. https://doi.org/10.3390/foods9101448
Chicago/Turabian StyleSagu, Sorel Tchewonpi, Lynn Zimmermann, Eva Landgräber, Thomas Homann, Gerd Huschek, Haydar Özpinar, Florian J. Schweigert, and Harshadrai M. Rawel. 2020. "Comprehensive Characterization and Relative Quantification of α-Amylase/Trypsin Inhibitors from Wheat Cultivars by Targeted HPLC-MS/MS" Foods 9, no. 10: 1448. https://doi.org/10.3390/foods9101448
APA StyleSagu, S. T., Zimmermann, L., Landgräber, E., Homann, T., Huschek, G., Özpinar, H., Schweigert, F. J., & Rawel, H. M. (2020). Comprehensive Characterization and Relative Quantification of α-Amylase/Trypsin Inhibitors from Wheat Cultivars by Targeted HPLC-MS/MS. Foods, 9(10), 1448. https://doi.org/10.3390/foods9101448







