Effect of Interaction between Mealworm Protein and Myofibrillar Protein on the Rheological Properties and Thermal Stability of the Prepared Emulsion Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Protein Extraction from Edible Insect
2.2. Myofibrillar Protein Extraction from Pork Ham
2.3. Preparation of Emulsion Systems
2.4. pH
2.5. Color Evaluation
2.6. Water Holding Capacity
2.7. Rheological Properties
2.8. Emulsion Stability
2.9. Differential Scanning Calorimetry
2.10. Texture Profile Analysis
2.11. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
2.12. Statistical Analysis
3. Results and Discussions
3.1. pH, Color, and Water Holding Capacity
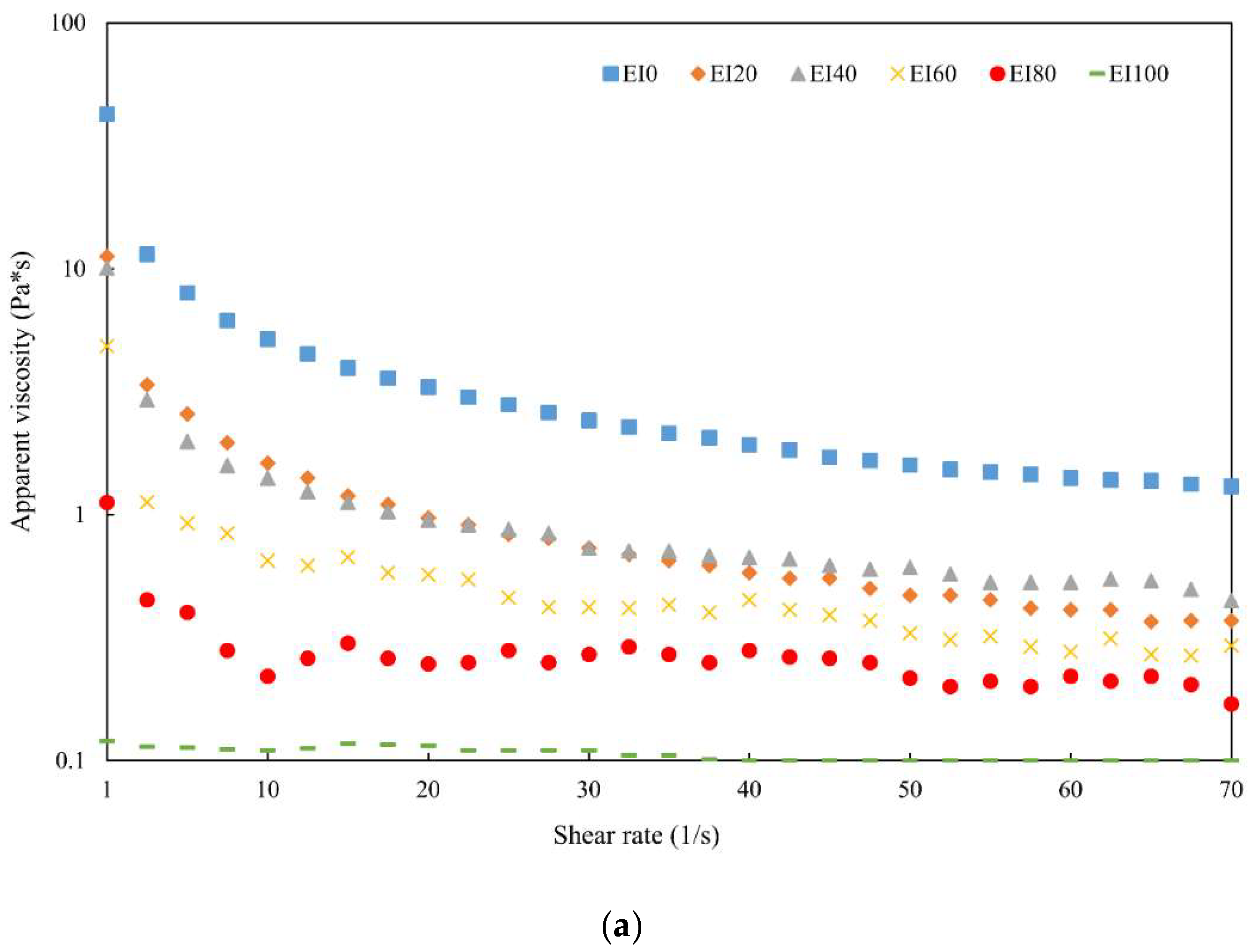
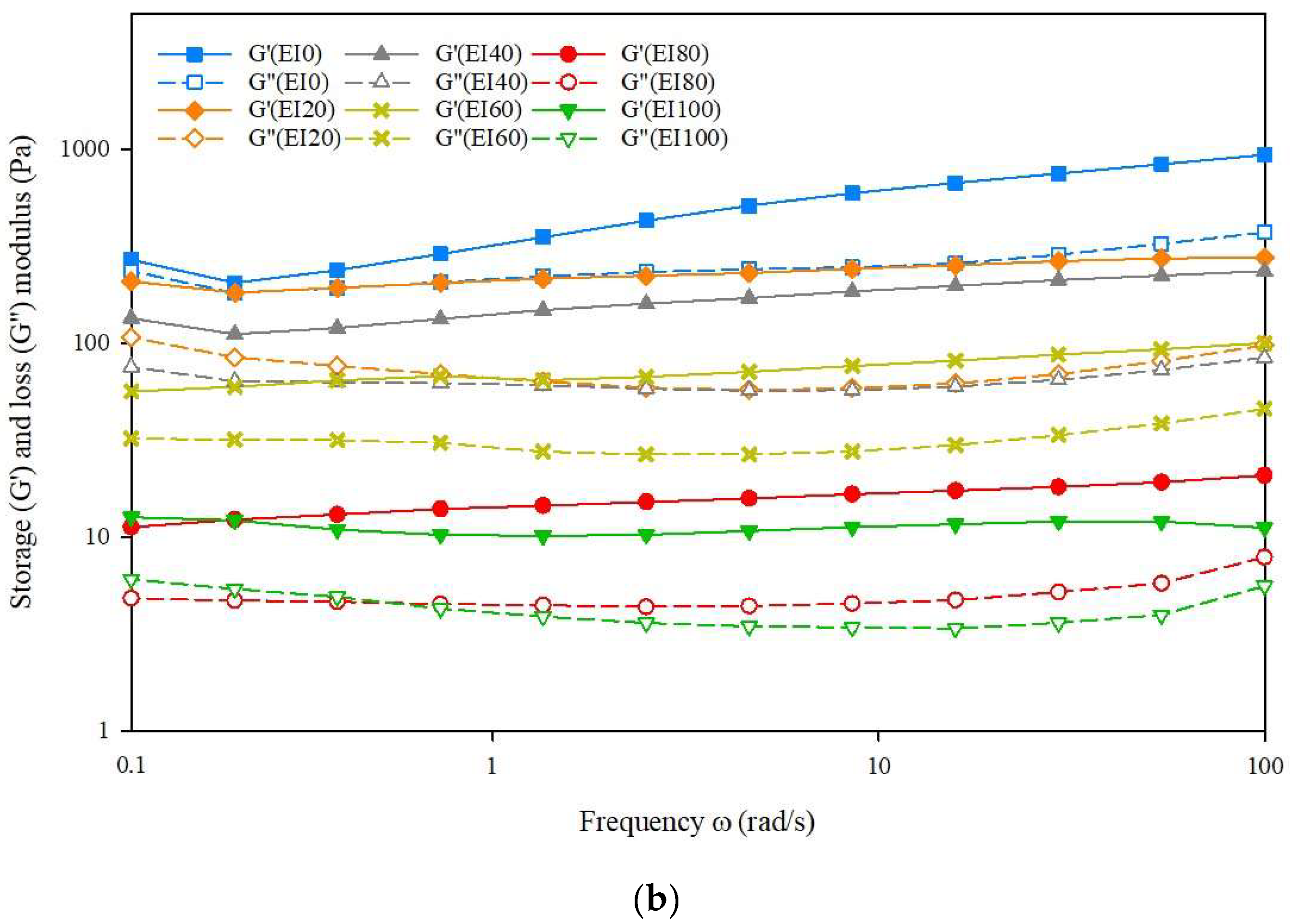
3.2. Rheological Properties
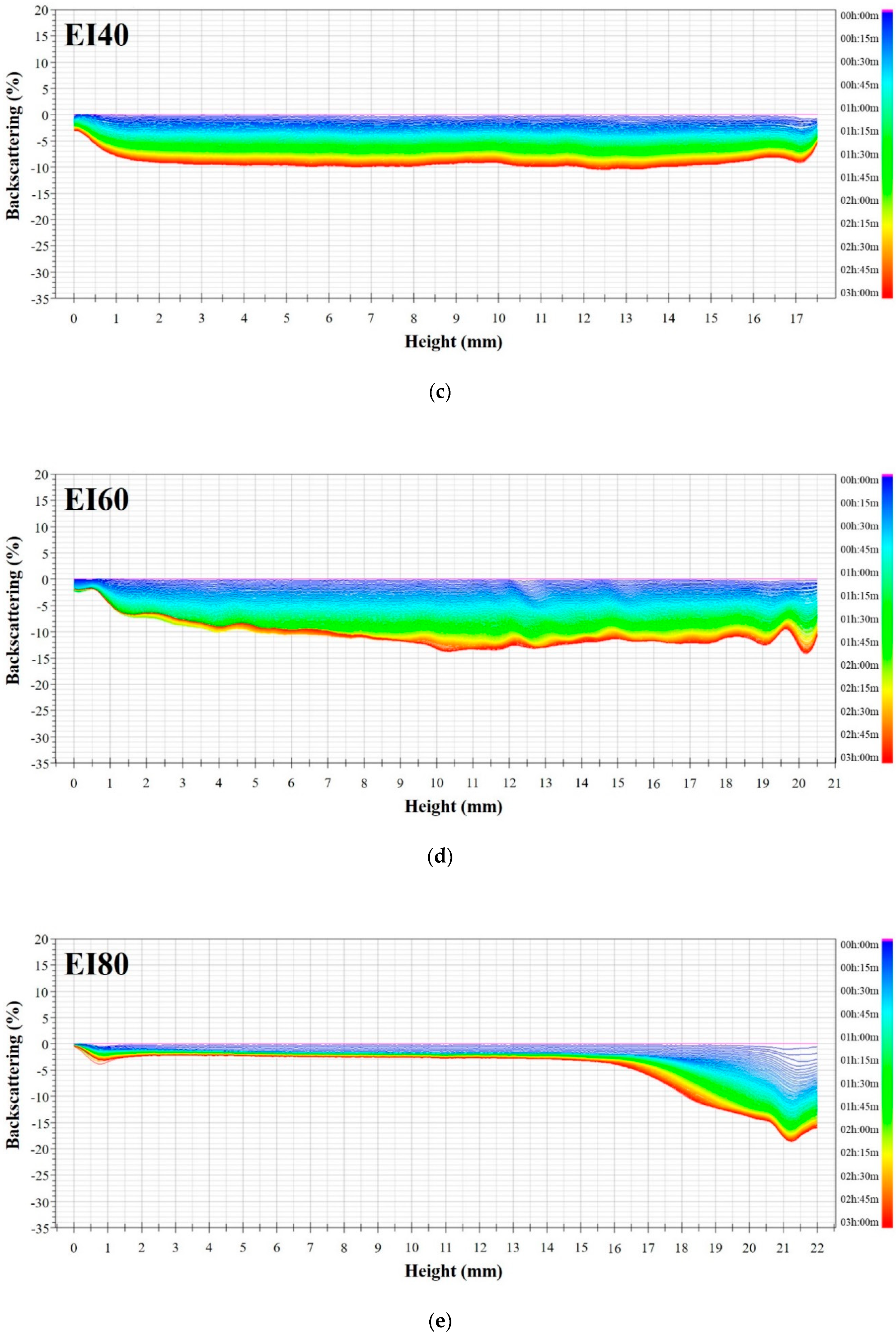
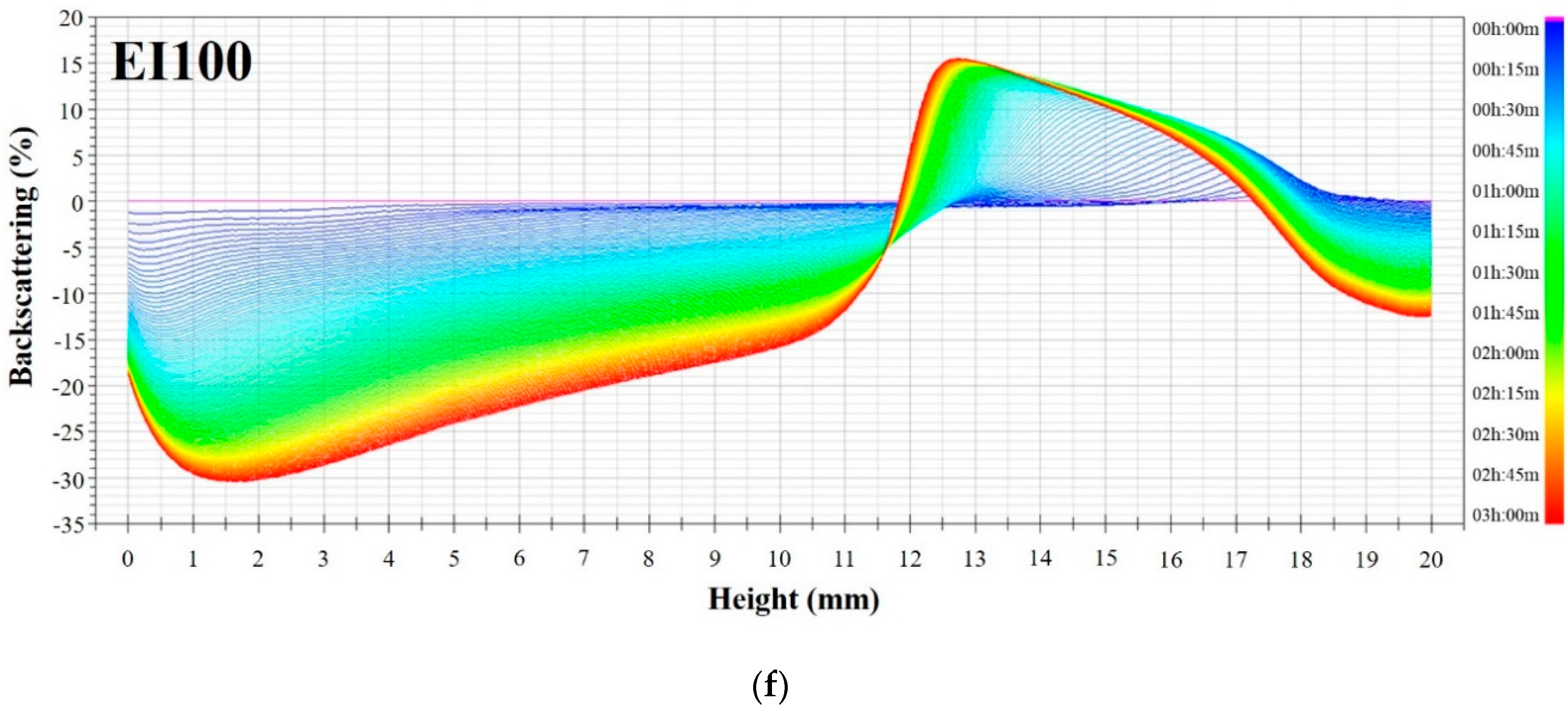
3.3. Emulsion Stability
3.4. Differential Scanning Calorimetry
3.5. Texture Profile Analysis
3.6. SDS-Polyacrylamide Gel Electrophoresis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, T.K.; Yong, H.I.; Jeong, C.H.; Han, S.G.; Kim, Y.B.; Paik, H.D.; Choi, Y.S. Technical functional properties of water- and salt-soluble proteins extracted from edible insects. Food Sci. Anim. Res. 2019, 39, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Kim, H.R.; Cho, H. Aroma characteristics of raw and cooked Tenebrio molitor larvae (mealworms). Food Sci. Anim. Resour. 2020, 40, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov. Food Sci. Emerg. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- Köhler, R.; Kariuki, L.; Lambert, C.; Biesalski, H.K. Protein, amino acid and mineral composition of some edible insects from Thailand. J. Asia Pac. Entomol. 2019, 22, 372–378. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Unlocking the biological potential of proteins from edible insects through enzymatic hydrolysis: A review. Innov. Food Sci. Emerg. 2017, 43, 239–252. [Google Scholar] [CrossRef]
- Halloran, A.; Roos, N.; Eilenberg, J.; Cerutti, A.; Bruun, S. Life cycle assessment of edible insects for food protein; a review. Agron Sustain. Dev. 2016, 36, 57. [Google Scholar] [CrossRef]
- Orsi, L.; Voege, L.L.; Stranieri, S. Eating edible insects as sustainable food? Exploring the determinants of consumer acceptance in Germany. Food Res. Int. 2019, 125, 108573. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, T.K.; Choi, H.D.; Park, J.D.; Sung, J.M.; Jeon, K.H.; Paik, H.D.; Kim, Y.B. Optimization of replacing pork meat with yellow worm (Tenebrio molitor L.) for frankfuters. Korean J. Food Sci. Anim. Resour. 2017, 37, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Chen, L.; Xu, X.; Zhou, G.; Li, Z.; Feng, X. Dose-dependent effects of rosmarinic acid on formation of oxidatively stressed myofibrillar protein emulsion gel at different NaCl concentrations. Food Chem. 2018, 243, 50–57. [Google Scholar] [CrossRef]
- Choi, Y.S.; Park, K.S.; Kim, H.Y.; Kim, H.W.; Song, D.H.; Chung, H.J.; Lee, J.W.; Kim, C.J. Interactions between chicken salt-soluble meat proteins and makgeolli lees fiber in heat-induced gels. Korean J. Food Sci. Anim. Resour. 2011, 31, 817–826. [Google Scholar] [CrossRef]
- Wu, M.; Xiong, Y.L.; Chen, J.; Tang, X.; Zhou, G. Rheological and microstructural properties of porcine myofibrillar protein–lipid emulsion composite gels. J. Food Sci. 2009, 74, E207–E217. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Jiang, X.; Zhou, H.; Han, M.; Liu, Y.; Bai, Y.; Xu, X.L.; Zhou, G.H. The effect of insoluble dietary fiber on myofibrillar protein emulsion gels: Oil particle size and protein network microstructure. LWT 2019, 534–542. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Choi, M.J.; Cho, Y. Improved physicochemical properties of pork patty supplemented with oil-in-water nanoemulsion. Food Sci. Anim. Resour. 2020, 40, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Uzlaşır, T.; Aktas, N.; Gercekaslan, K.E. Pumpkin seed oil as a partial animal fat replacer in bologna-type sausages. Food Sci. Anim. Resour. 2020, 40, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xiong, Y.; Chen, J. Rheology and microstructure of myofibrillar protein–plant lipid composite gels: Effect of emulsion droplet size and membrane type. J. Food Eng. 2011, 106, 318–324. [Google Scholar] [CrossRef]
- Shin, H.; Kim, H.T.; Choi, M.J.; Ko, E.Y. Effects of bromelain and double emulsion on the physicochemical properties of pork loin. Food Sci. Anim. Resour. 2019, 39, 888–902. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, Y.; Wang, J.; Wei, S.; Li, S. Effects of low fat addition on chicken myofibrillar protein gelation properties. Food Hydrocoll. 2019, 90, 126–131. [Google Scholar] [CrossRef]
- Kocher, P.N.; Foegeding, E.A. Microcentrifuge-based method for measuring water-holding of protein gels. J. Food Sci. 1993, 58, 1040–1046. [Google Scholar] [CrossRef]
- Hwang, S.I.; Lee, E.J.; Hong, G.P. Effects of temperature and time on the cookery properties of sous-vide processed pork loin. Food Sci. Anim. Resour. 2019, 39, 65–72. [Google Scholar] [CrossRef]
- Bourne, M.C.; Kenny, J.F.; Barnard, J. Computer-assisted readout of data from texture profile analysis curves. J. Food Texture Stud. 1978, 9, 481–494. [Google Scholar] [CrossRef]
- Kim, T.K.; Yong, H.I.; Chun, H.H.; Lee, M.A.; Kim, Y.B.; Choi, Y.S. Changes of amino acid composition and protein technical functionality of edible insects by extracting steps. J. Asia Pac. Entomol. 2020, 23, 298–305. [Google Scholar] [CrossRef]
- Smarzyński, K.; Sarbak, P.; Musia, S.; Jezowski, P.; Piatek, M.; Kowalczewski, P.L. Nutritional analysis and evaluation of the consumer acceptance of pork pâté enriched with cricket powder—Preliminary study. Open Agric. 2019, 4, 159–163. [Google Scholar] [CrossRef]
- Kim, T.K.; Yong, H.I.; Jang, H.W.; Kim, Y.B.; Choi, Y.S. Functional properties of extracted protein from edible insect larvae and their interaction with transglutaminase. Foods 2020, 9, 591. [Google Scholar] [CrossRef] [PubMed]
- Wittkopp, P.J.; Beldade, P. Development and evolution of insect pigmentation: Genetic mechanisms and the potential consequences of pleiotropy. Semin. Cell Dev. Biol. 2009, 20, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Khampakool, A.; Soisungwan, S.; You, S.G.; Park, S.H. Infrared assisted freeze-drying (IRAFD) to produce shelf-stable insect food from Protaetia brevitarsis (white-spotted flower chafer) larva. Food Sci. Anim. Resour. 2020, 40, 813. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Guan, H.; Zhao, X.; Chen, Q.; Kong, B. Properties and oxidative stability of emulsions prepared with myofibrillar protein and lard diacylglycerols. Meat Sci. 2016, 115, 16–23. [Google Scholar] [CrossRef]
- Li, K.; Fu, L.; Zhao, Y.Y.; Xue, S.W.; Wang, P.; Xu, X.L.; Bai, Y.H. Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocoll. 2020, 98, 105275. [Google Scholar] [CrossRef]
- Gould, J.; Wolf, B. Interfacial and emulsifying properties of mealworm protein at the oil/water interface. Food Hydrocoll. 2018, 77, 57–63. [Google Scholar] [CrossRef]
- Gravel, A.; Doyen, A. The use of edible insect proteins in food: Challenges and issues related to their functional properties. Innov. Food Sci. Emerg. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Zhou, L.; Feng, X.; Yang, Y.; Chen, Y.; Tang, X.; Wei, S.; Li, S. Effects of high-speed shear homogenization on the emulsifying and structural properties of myofibrillar protein under low-fat conditions. J. Sci. Food Agric. 2019, 99, 6500–6508. [Google Scholar] [CrossRef]
- Heussen, P.; Ye, P.; Menard, K.; Courtney, P. Practical food applications of differential scanning calorimetry (DSC). In Differential Scanning Calorimetry; PerkinElmer: Waltham, MA, USA, 2011; pp. 2–3. [Google Scholar]
- Lee, H.J.; Kim, J.H.; Ji, D.S.; Lee, C.H. Effects of heating time and temperature on functional properties of proteins of yellow mealworm larvae (Tenebrio molitor L.). Food Sci. Anim. Resour. 2019, 39, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Choi, Y.S.; Hwang, K.E.; Kim, T.K.; Lee, C.W.; Shin, D.M.; Han, S.G. Physicochemical properties of meat batter added with edible silkworm pupae (Bombyx mori) and transglutaminase. Korean J. Food Sci. Anim. Resour. 2017, 37, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U. SDS-page Laemmli method. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Van Boekel, M.A.J.S.; Boeren, S.; Lakemond, C.M.M. Protein identification and in vitro digestion of fractions from Tenebrio molitor. Eur. Food Res. Technol. 2016, 242, 1285–1297. [Google Scholar] [CrossRef]
- Kim, T.K.; Yong, H.I.; Kim, Y.B.; Kim, H.W.; Choi, Y.S. Edible insects as a protein source: A review of public perception, processing technology, and research trends. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Lee, M.H.; Yu, M.H.; Yong, H.I.; Jang, H.W.; Jung, S.; Choi, Y.S. Thermal stability and rheological properties of heat-induced gels prepared using edible insect proteins in a model system. LWT 2020, 134, 110270. [Google Scholar] [CrossRef]

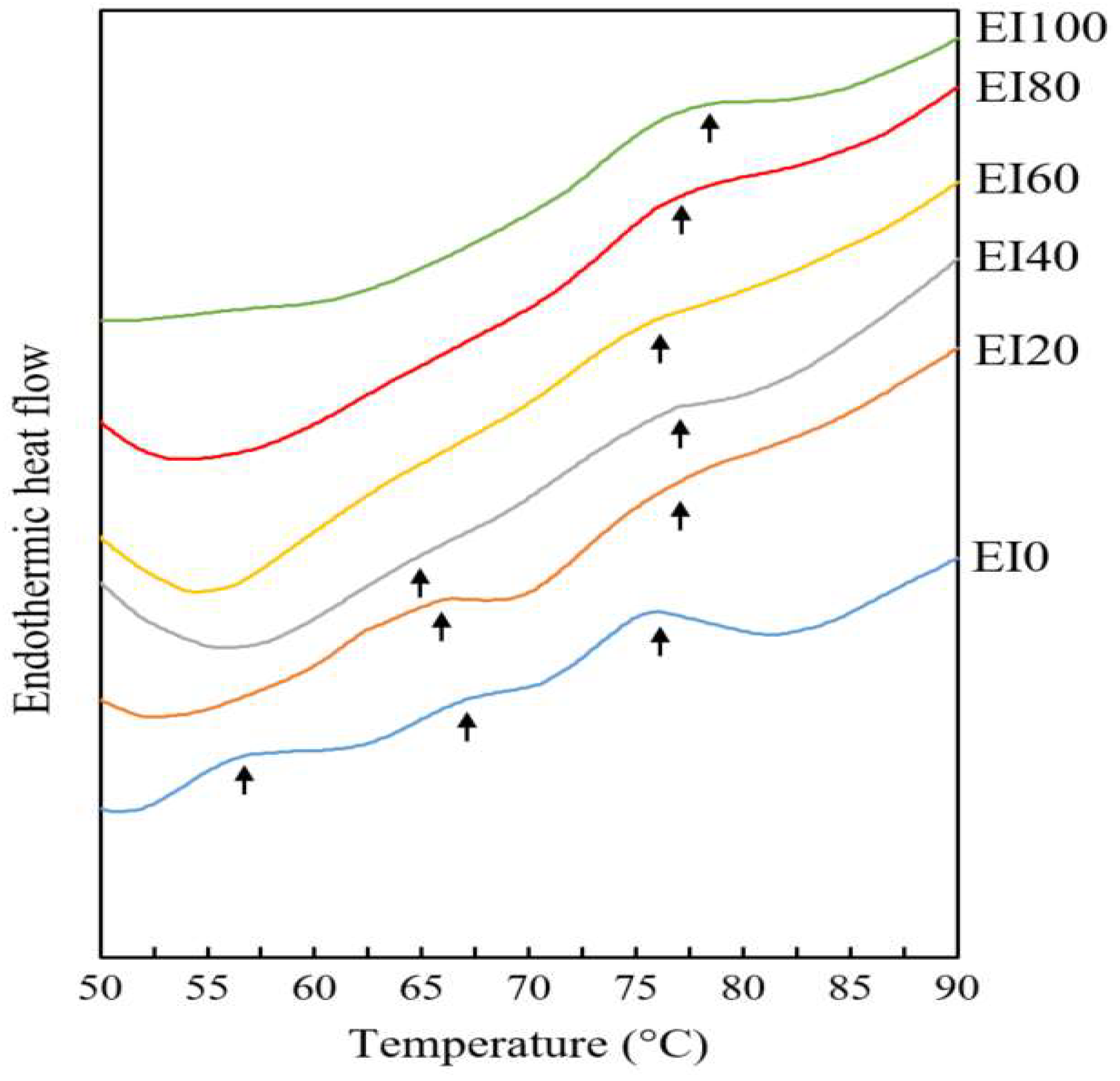
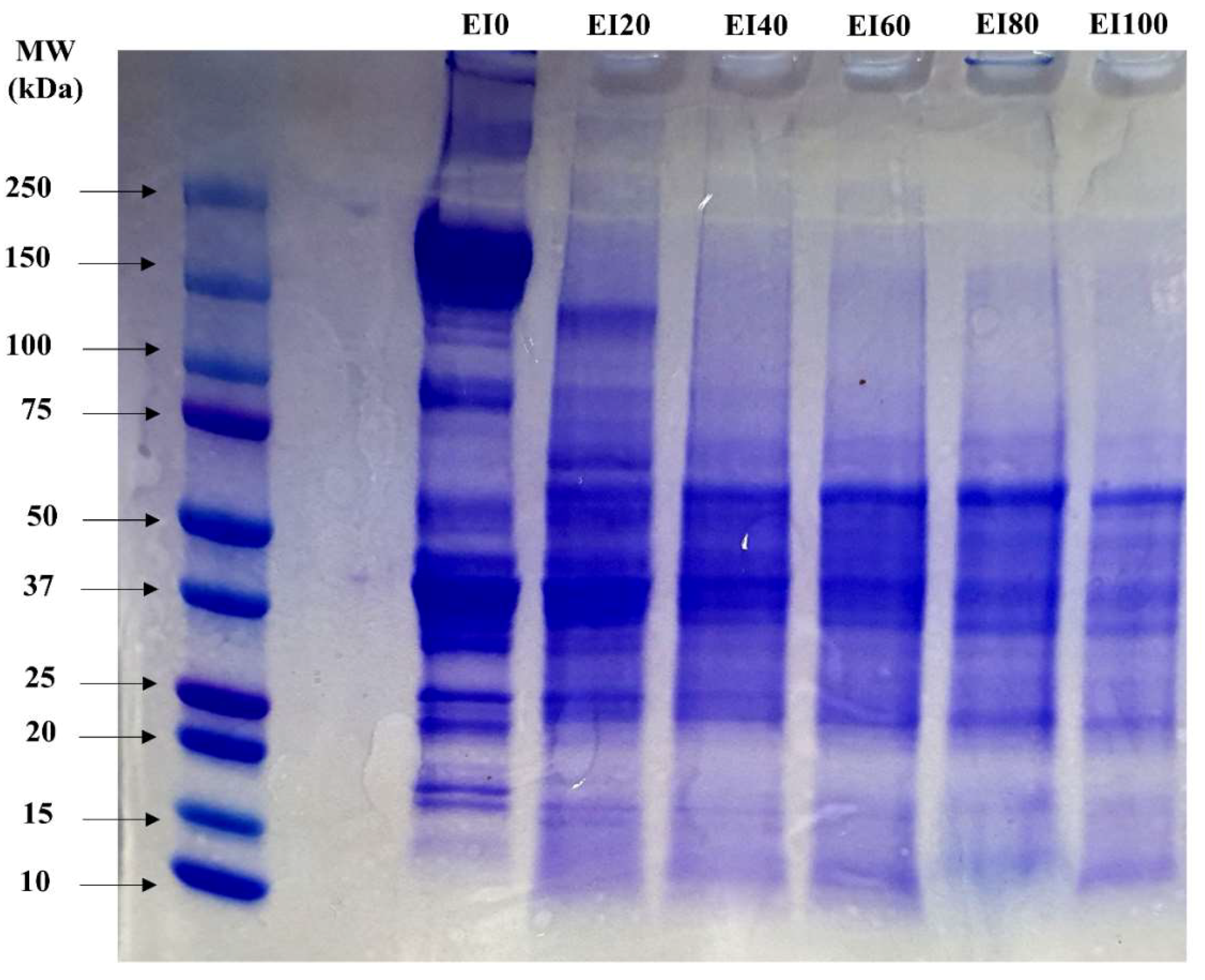
| EI0 | EI20 | EI40 | EI60 | EI80 | EI100 | |
|---|---|---|---|---|---|---|
| pH | 6.29 ± 0.02 e | 6.32 ± 0.01 d | 6.38 ± 0.02 c | 6.39 ± 0.01 bc | 6.41 ± 0.01 ab | 6.43 ± 0.02 a |
| CIE (1) L* | 73.76 ± 0.08 a | 65.08 ± 0.15 b | 59.75 ± 0.18 c | 55.25 ± 0.17 d | 51.12 ± 0.21 e | 48.84 ± 0.02 f |
| CIE a* | −0.13 ± 0.03 f | 0.73 ± 0.03 e | 1.82 ± 0.03 d | 2.37 ± 0.02 c | 2.52 ± 0.01 b | 2.85 ± 0.02 a |
| CIE b* | 9.15 ± 0.06 f | 9.92 ± 0.10 e | 10.11 ± 0.05 d | 10.63 ± 0.12 c | 10.78 ± 0.09 b | 11.19 ± 0.01 a |
| WHC (%) | 53.32 ± 3.42 a | 18.69 ± 5.28 bc | 22.53 ± 2.58 b | 16.01 ± 1.78 d | 16.45 ± 3.55 d | 9.82 ± 0.39 e |
| EI0 | EI20 | EI40 | EI60 | EI80 | EI100 | |
|---|---|---|---|---|---|---|
| Hardness (kg) | 0.60 ± 0.06 a | 0.22 ± 0.03 b | 0.15 ± 0.01 c | 0.08 ± 0.01 d | 0.06 ± 0.01 d | 0.05 ± 0.02 d |
| Springiness | 0.99 ± 0.01 a | 0.97 ± 0.01 b | 0.97 ± 0.01 b | 0.97 ± 0.01 b | 0.97 ± 0.01 b | 0.86 ± 0.11 b |
| Cohesiveness | 0.47 ± 0.05 a | 0.47 ± 0.03 a | 0.53 ± 0.03 a | 0.51 ± 0.03 a | 0.47 ± 0.07 a | 0.29 ± 0.22 b |
| Gumminess (kg) | 0.28 ± 0.04 a | 0.10 ± 0.01 b | 0.08 ± 0.01 c | 0.04 ± 0.01 d | 0.03 ± 0.01 de | 0.01 ± 0.01 e |
| Chewiness (kg) | 0.28 ± 0.04 a | 0.10 ± 0.01 b | 0.08 ± 0.01 c | 0.04 ± 0.01 d | 0.03 ± 0.01 de | 0.01 ± 0.01 e |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-K.; Lee, M.H.; Yong, H.I.; Jung, S.; Paik, H.-D.; Jang, H.W.; Choi, Y.-S. Effect of Interaction between Mealworm Protein and Myofibrillar Protein on the Rheological Properties and Thermal Stability of the Prepared Emulsion Systems. Foods 2020, 9, 1443. https://doi.org/10.3390/foods9101443
Kim T-K, Lee MH, Yong HI, Jung S, Paik H-D, Jang HW, Choi Y-S. Effect of Interaction between Mealworm Protein and Myofibrillar Protein on the Rheological Properties and Thermal Stability of the Prepared Emulsion Systems. Foods. 2020; 9(10):1443. https://doi.org/10.3390/foods9101443
Chicago/Turabian StyleKim, Tae-Kyung, Min Hyeock Lee, Hae In Yong, Samooel Jung, Hyun-Dong Paik, Hae Won Jang, and Yun-Sang Choi. 2020. "Effect of Interaction between Mealworm Protein and Myofibrillar Protein on the Rheological Properties and Thermal Stability of the Prepared Emulsion Systems" Foods 9, no. 10: 1443. https://doi.org/10.3390/foods9101443
APA StyleKim, T.-K., Lee, M. H., Yong, H. I., Jung, S., Paik, H.-D., Jang, H. W., & Choi, Y.-S. (2020). Effect of Interaction between Mealworm Protein and Myofibrillar Protein on the Rheological Properties and Thermal Stability of the Prepared Emulsion Systems. Foods, 9(10), 1443. https://doi.org/10.3390/foods9101443







