The Effect of Apple Juice Concentration on Cider Fermentation and Properties of the Final Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Apple Juice Clarification and Concentration
2.2. Free Amino Acid Content
2.3. Cider Fermentation
2.4. Fructose and Malic acid Content
2.5. Analysis of Volatile Esters
2.6. Sensory Assessment of Hydrogen Sulfide Related Off-Flavor
2.7. Data Processing
3. Results and Discussion
3.1. Effect of Juice Concentration on Free Amino Acid Composition
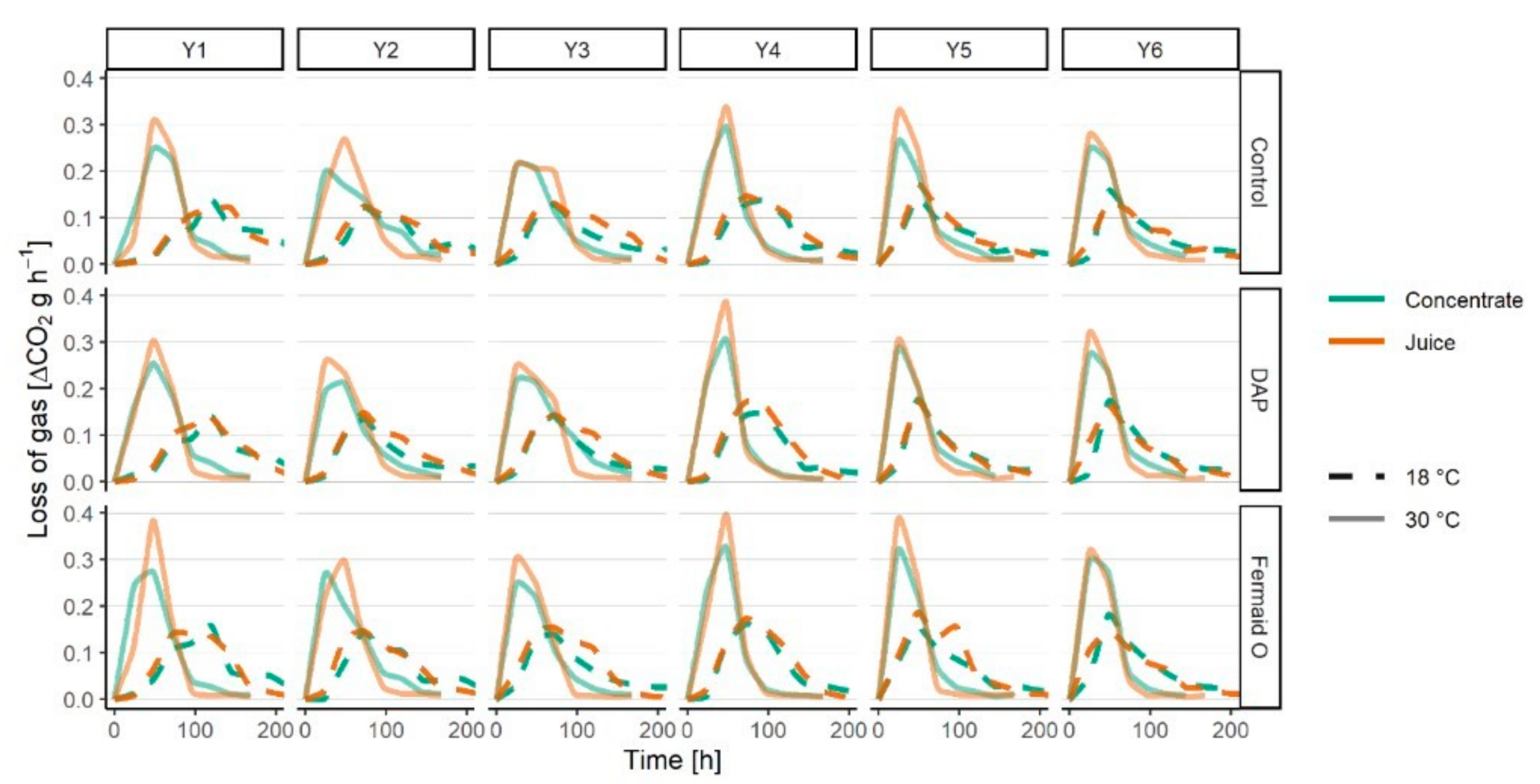
3.2. Fermentation Kinetics
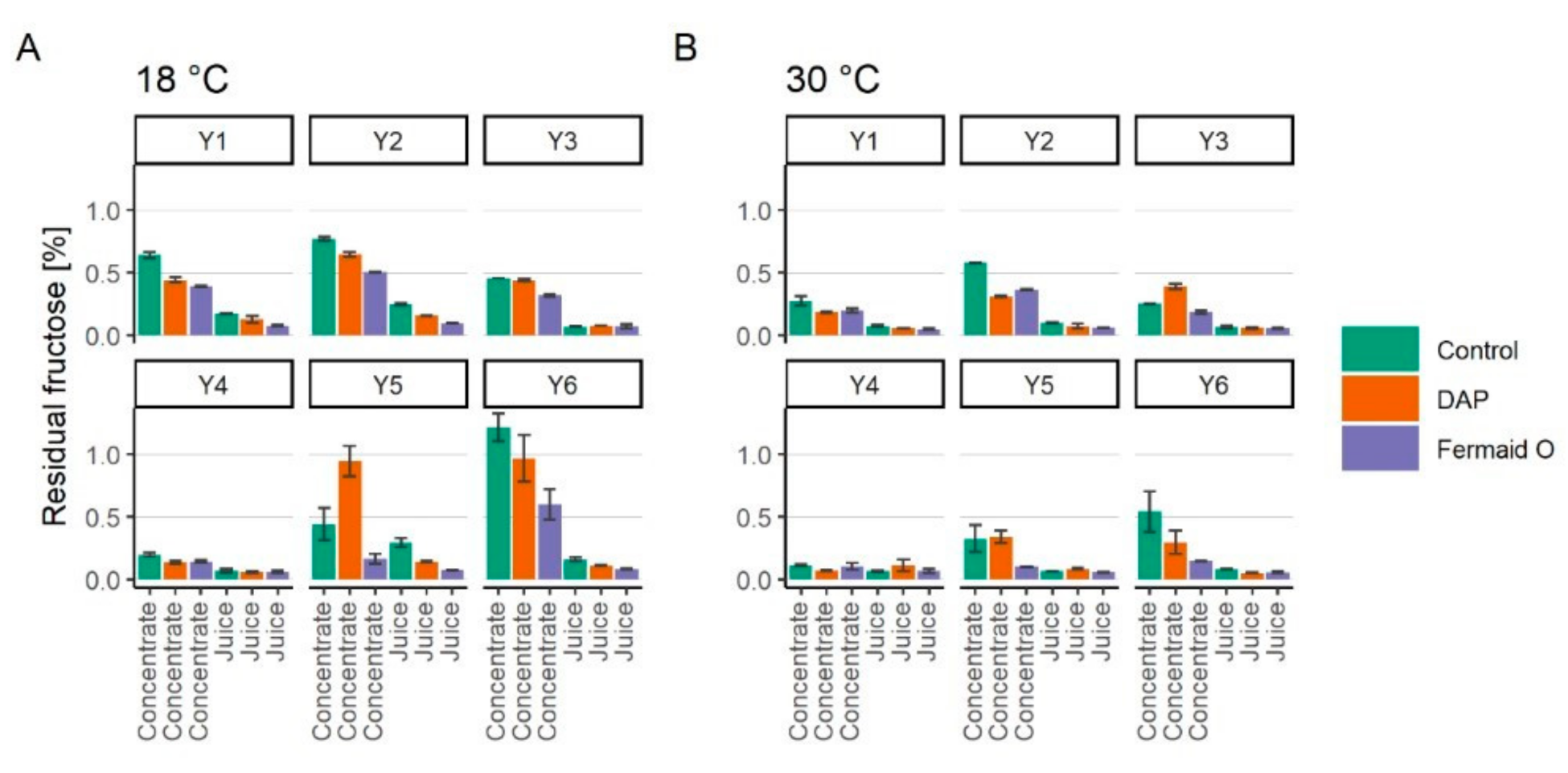
3.3. Assimilation of Fructose
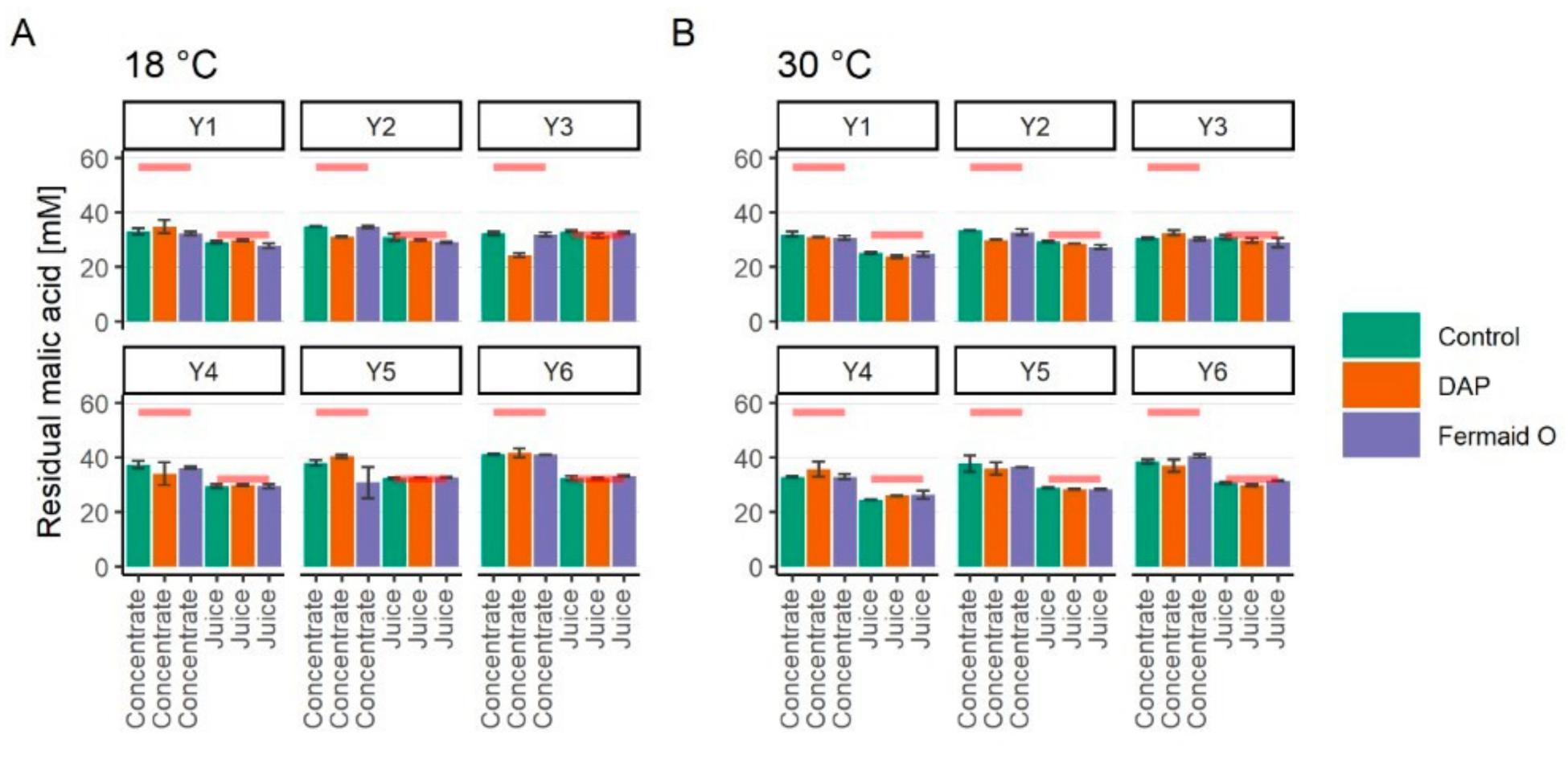
3.4. Assimilation of Malic Acid
3.5. Production of Volatile Esters
3.6. Hydrogen Sulfide Production and Off-Flavor Development
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riigiteataja. Alkoholi Määratlemise, Kirjeldamise, ja Müügiks Esitlemise Nõuded. Available online: https://www.riigiteataja.ee/akt/120092017010 (accessed on 31 August 2020).
- Légifrance. Décret n°53-978 du 30 Septembre 1953 Relatif à L’orientation de la Production Cidricole et à la Commercialisation des Cidres, Poirés et de Certaines Boissons Similaires. Available online: https://beta.legifrance.gouv.fr/loda/texte_lc/LEGITEXT000006060679/ (accessed on 31 August 2020).
- HM Revenue and Customs. Alcohol Duties. Excide Notice 163: Cider Production. Available online: https://www.gov.uk/government/publications/excise-notice-162-cider-production/excise-notice-162-cider-production (accessed on 31 August 2020).
- Alberti, A.; Vieira, R.G.; Drilleau, J.F.; Wosiacki, G.; Nogueira, A. Apple wine processing with different nitrogen contents. Braz. Arch. Biol. Technol. 2011, 54, 551–558. [Google Scholar] [CrossRef]
- Kelkar, S.; Dolan, K. Modeling the effects of initial nitrogen content and temperature on fermentation kinetics of hard cider. J. Food Eng. 2012, 109, 588–596. [Google Scholar] [CrossRef]
- Alberti, A.; Santos, T.P.M.; Ferreira Zielinski, A.A.; Santos, C.M.E.; Braga, C.M.; Demiante, I.M.; Nogueira, A. Impact on chemical profile in apple juice and cider made from unripe, ripe and senescent dessert varieties. LWT Food Sci. Technol. 2016, 65, 436–443. [Google Scholar] [CrossRef]
- Santos, C.M.E.; Alberti, A.; Pietrowski, G.A.M.; Zielinski, A.A.F.; Wosiacki, G.; Nogueira, A.; Jorge, R.M.M. Supplementation of amino acids in apple must for the standardization of volatile compounds in ciders. J. Inst. Brew. 2016, 122, 334–341. [Google Scholar] [CrossRef]
- Boudreau, T.F.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. The interactive effect of fungicide residues and yeast assimilable nitrogen on fermentation kinetics and hydrogen sulfide production during cider fermentation. J. Sci. Food Agric. 2017, 97, 693–704. [Google Scholar] [CrossRef]
- Laaksonen, O.; Kuldjärv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of apple cultivar, ripening stage, fermentation type and yeast strain on phenolic composition of apple ciders. Food Chem. 2017, 233, 29–37. [Google Scholar] [CrossRef]
- Boudreau, T.F.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Free amino nitrogen concentration correlates to total yeast assimilable nitrogen concentration in apple juice. Food Sci. Nutr. 2018, 6, 119–123. [Google Scholar] [CrossRef]
- Flores, M.G.; Rodriguez, M.E.; Origone, A.C.; Oteiza, J.M.; Querol, A.; Lopes, C.A. Saccharomyces uvarum isolated from patagonian ciders shows excellent fermentative performance for low temperature cidermaking. Food Res. Int. 2019, 126, 108656. [Google Scholar] [CrossRef]
- Guichard, H.; Poupard, P.; Legoahec, L.; Millet, M.; Bauduin, R.; Le Quere, J.M. Brettanomyces anomalus, a double drawback for cider aroma. LWT Food Sci. Technol. 2019, 102, 214–222. [Google Scholar] [CrossRef]
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. LWT Food Sci. Technol. 2019, 99, 224–230. [Google Scholar] [CrossRef]
- Rosend, J.; Kuldjärv, R.; Rosenvald, S.; Paalme, T. The effects of apple variety, ripening stage, and yeast strain on the volatile composition of apple cider. Heliyon 2019, 5, e01953. [Google Scholar] [CrossRef] [PubMed]
- Rosend, J.; Kuldjärv, R.; Arju, G.; Nisamedtinov, I. Yeast performance characterisation in different cider fermentation matrices. Agron Res. 2019, 17, 2040–2053. [Google Scholar] [CrossRef]
- Wei, J.; Wang, S.; Zhang, Y.; Yuan, Y.; Yue, T. Characterization and screening of non-Saccharomyces yeasts used to produce fragrant cider. LWT Food Sci. Technol. 2019, 107, 191–198. [Google Scholar] [CrossRef]
- Li, C.X.; Zhao, X.H.; Zuo, W.F.; Zhang, T.L.; Zhang, Z.Y.; Chen, X.S. The effects of simultaneous and sequential inoculation of yeast and autochthonous Oenococcus oeni on the chemical composition of red-fleshed apple cider. LWT Food Sci. Technol. 2020, 124, 109184. [Google Scholar] [CrossRef]
- Song, Y.; Gibney, P.; Cheng, L.; Liu, S.; Peck, G. Yeast assimilable nitrogen concentrations influence yeast gene expression and hydrogen sulfide production during cider fermentation. Front. Microbiol. 2020, 11, 1264. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Wang, Y.; Ju, H.; Niu, C.; Song, Z.; Yuan, Y.; Yue, T. Assessment of chemical composition and sensorial properties of ciders fermented with different non-Saccharomyces yeasts in pure and mixed fermentations. Int. J. Food Microbiol. 2020, 318, 108471. [Google Scholar] [CrossRef]
- Gampp, E.; Schmitt, R.; Urlaub, R. Method for Clarification of Fruit Juice. US patent US4109017A, 22 August 1978. Available online: https://patents.google.com/patent/US4109017A/en (accessed on 1 February 2017).
- Lahtvee, P.J.; Seiman, A.; Arike, L.; Adamberg, K.; Vilu, R. Protein turnover forms one of the highest maintenance costs in Lactococcus lactis. Microbiol 2014, 160, 1501–1512. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 2, 165–177. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Nogueira, A.; Wosiacki, G. Apple cider fermentation. In Handbook of Plant-Based Fermented Food and Beverage Technology, 2nd ed.; Hui, Y.H., Evranuz, E.Ö., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 209–236. [Google Scholar]
- Maccarone, E.; Campisi, S.; Cataldi Lupo, M.C.; Fallico, B.; Nicolosi Asmundo, C. Thermal treatments effect on the red orange juice constituents. Ind. Bevande 1996, 25, 335–341. [Google Scholar]
- Gunko, S.; Vebycha, S.; Bryka, M.; Hilal, N. Concentration of apple juice using direct contact membrane distillation. Desalination 2006, 190, 117–124. [Google Scholar] [CrossRef]
- Kozák, Á.; Békássy-Molnár, E.; Vatai, G. Production of black-currant juice concentrate by using membrane distillation. Desalination 2009, 241, 309–314. [Google Scholar] [CrossRef]
- Juliana, H.; Yaohanny, F.; Devina, A.; Purwadi, R.; Wenten, G. Apple juice concentration using submerged direct contact membrane distillation (SDCMD). J. Food Eng. 2020, 272, 109807. [Google Scholar] [CrossRef]
- Sharf, R.; Margalith, P. The effect of temperature on spontaneous wine fermentation. Eur. J. Appl. Microbiol. Biotechnol. 1983, 17, 311–313. [Google Scholar] [CrossRef]
- Gao, C.; Fleet, G.H. The effects of temperature and pH on the ethanol tolerance of the wine yeasts: Saccharomyces cerevisiae, Candida stellata and Kloeckera apiculata. J. Appl. Bacteriol. 1988, 65, 405–410. [Google Scholar] [CrossRef]
- Heard, G.M.; Fleet, G.H. The effects of temperature and pH on the growth of yeast species during the fermentation of grape juice. J. Appl. Bacteriol. 1988, 65, 23–28. [Google Scholar] [CrossRef]
- Boles, E.; Jong-Gubbels, P.; Pronk, J.T. Identification and characterization of MAE1, the Saccharomyces cerevisiae structural gene encoding mitochondrial malic enzyme. J. Bacteriol. 1998, 180, 2875–2882. [Google Scholar] [CrossRef]
- Volschenk, H.; van Vuuren, H.J.J.; Viljoen-Bloom, M. Malo-ethanolic fermentation in Saccharomyces and Schizosaccharomyces. Curr. Genet. 2003, 43, 379–391. [Google Scholar] [CrossRef]
- Eden, A.; Simchen, G.; Benvenisty, N. Two yeast homologs of ECA39, a target for c-Myc regulation, code for cytosolic and mitochondrial branched chain amino acid aminotransferases. J. Biol Chem. 1996, 271, 20242–20425. [Google Scholar] [CrossRef]
- Plata, C.; Millan, C.; Mauricio, J.C.; Ortega, J.M. Formation of ethyl acetate and isoamyl acetate by various species of wine yeasts. Food Microbiol. 2003, 20, 217–224. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.C.; de Revel, G. Development, validation and application of a specific method for the quantitative determination of wine esters by headspace-solid-phase microextraction-gas chromatography–mass spectrometry. Food Chem. 2010, 121, 1236–1245. [Google Scholar] [CrossRef]
- Viejo, C.G.; Fuentes, S.; Torrico, D.D.; Godbole, A.; Dunshea, F.R. Chemical characterization of aromas in beer and their effect on consumers liking. Food Chem. 2019, 293, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Jin, G.J.; Mei, W.C.; Li, T.; Tao, Y.S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, H.; Benatier, M.G. Yeast autolysis in sparkling wine—A review. Aust. J. Grape Wine Res. 2006, 12, 119–127. [Google Scholar] [CrossRef]
- Saerens, S.; Thevelein, J.; Delvaux, F.; De Cooman, L. Ethyl ester production during brewery fermentation, a review. Cerevisia 2008, 33, 82–90. [Google Scholar]
- Wang, J.; Li, M.; Zheng, F.; Niu, C.; Liu, C.; Li, Q.; Sun, J. Cell wall polysaccharides: Before and after autolysis of brewer’s yeast. World J. Microbiol. Biotechnol. 2018, 34, 137. [Google Scholar] [CrossRef]
- Torrea, D.; Varela, C.; Ugliano, M.; Ancin-Azpilicueta, C.; Leigh Francis, I.; Henschke, P.A. Comparison of inorganic and organic nitrogen supplementation of grape juice—Effect on volatile composition and aroma profile of a Chardonnay wine fermented with Saccharomyces cerevisiae yeast. Food Chem. 2011, 127, 1072–1083. [Google Scholar] [CrossRef]
- Mendes-Ferreira, A.; Mendes-Faia, A.; Leao, C. Survey of hydrogen sulphide production by wine yeasts. J. Food Protect. 2002, 65, 1033–1037. [Google Scholar] [CrossRef]
- Nowak, A.; Kusewicz, D.; Kalinowska, H.; Turkiewicz, M.; Patelski, P. Production of H2S and properties of sulfite reductase from selected strains of wine-producing yeasts. Eur. Food Res. Technol. 2004, 219, 84–89. [Google Scholar] [CrossRef]
- Torija, M.J.; Beltran, G.; Novo, M.; Poblet, M.; Guillamon, J.M.; Mas, A.; Rozes, N. Effects of fermentation temperature and Saccharomyces species on the cell fatty acid composition and presence of volatile compounds in wine. Int. J. Food Microbiol. 2003, 85, 127–136. [Google Scholar] [CrossRef]

| FAA, mg L−1 | Juice, 9.8% Brix | Concentrate, 10% Brix |
|---|---|---|
| His | 1.71 ± 0.32 | 2.27 ± 0.44 |
| Asn | 269.60 ± 24.48 | 315.03 ± 32.70 |
| Ser | 6.28 ± 0.93 | 9.21 ± 0.82 |
| Gln | 3.26 ± 0.60 | 2.87 ± 0.26 |
| Arg | 21.68 ± 1.10 | 19.36 ± 3.63 |
| Gly | 0.32 ± 0.04 | 1.19 ± 0.21 |
| Asp | 38.10 ± 5.26 | 44.42 ± 4.46 |
| Glu | 32.43 ± 3.54 | 38.15 ± 3.67 |
| Thr | 2.75 ± 0.45 | 3.62 ± 0.33 |
| Ala | 8.85 ± 1.56 | 10.91 ± 1.01 |
| Pro | 2.32 ± 0.37 | 3.22 ± 0.33 |
| Orn | 0.27 ± 0.02 | 0.51 ± 0.10 |
| Cys-cys | 0.30 ± 0.03 | 0.34 ± 0.03 |
| Lys | 0.82 ± 0.09 | 1.93 ± 0.08 |
| Tyr | 0.88 ± 0.06 | 1.04 ± 0.22 |
| Met | 1.06 ± 0.16 | 1.27 ± 0.12 |
| Val | 3.93 ± 0.73 | 4.41 ± 0.46 |
| Ile | 2.34 ± 0.45 | 2.90 ± 0.33 |
| Leu | 1.18 ± 0.23 | 3.21 ± 0.39 |
| Phe | 1.97 ± 0.16 | 1.98 ± 0.21 |
| Trp | 0.57 ± 0.10 | 0.41 ± 0.03 |
| FAN, mg L−1 | 76.13 ± 7.29 | 87.91 ± 9.62 |
| + DAP YAN, mg L−1 | 95.03 ± 7.29 1 | 106.81 ± 9.62 1 |
| + Fermaid O YAN, mg L−1 | 93.33 ± 7.29 2 | 105.11 ± 9.62 2 |
| Nutrient | Control | Fermaid O | DAP * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Matrix | Juice | Concentrate | Juice | Concentrate | Juice | Concentrate | ||||||
| °C | 18 | 30 | 18 | 30 | 18 | 30 | 18 | 30 | 18 | 30 | 18 | 30 |
| Y1 | ++ | − | ++ | ++ | +++ | ++ | ++ | ++ | +++ | ++ | ++ | ++ |
| Y2 | ++ | − | ++ | − | ++ | − | ++ | ++ | ++ | − | ++ | + |
| Y3 | ++ | ++ | ++ | ++ | ++ | + | ++ | ++ | ++ | + | ++ | + |
| Y4 | +++ | − | +++ | + | +++ | − | +++ | ++ | +++ | − | +++ | +++ |
| Y5 | + | + | + | + | ++ | ++ | ++ | ++ | +++ | +++ | +++ | +++ |
| Y6 | − | − | − | − | + | − | + | − | − | − | − | − |
| Nutrient | Control | Fermaid O | DAP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Matrix | Juice | Concentrate | Juice | Concentrate | Juice | Concentrate | ||||||
| °C | 18 | 30 | 18 | 30 | 18 | 30 | 18 | 30 | 18 | 30 | 18 | 30 |
| Y1 | + | − | − | − | ++ | − | − | − | ++ | − | − | − |
| Y2 | − | − | − | − | − | − | − | − | − | − | − | − |
| Y3 | − | − | − | − | − | − | − | − | − | − | − | − |
| Y4 | + | − | ++ | − | + | − | ++ | − | ++ | − | ++ | ++ |
| Y5 | − | − | − | − | − | − | − | − | ++ | ++ | ++ | ++ |
| Y6 | − | − | − | − | − | − | − | − | − | − | − | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosend, J.; Kaleda, A.; Kuldjärv, R.; Arju, G.; Nisamedtinov, I. The Effect of Apple Juice Concentration on Cider Fermentation and Properties of the Final Product. Foods 2020, 9, 1401. https://doi.org/10.3390/foods9101401
Rosend J, Kaleda A, Kuldjärv R, Arju G, Nisamedtinov I. The Effect of Apple Juice Concentration on Cider Fermentation and Properties of the Final Product. Foods. 2020; 9(10):1401. https://doi.org/10.3390/foods9101401
Chicago/Turabian StyleRosend, Julia, Aleksei Kaleda, Rain Kuldjärv, Georg Arju, and Ildar Nisamedtinov. 2020. "The Effect of Apple Juice Concentration on Cider Fermentation and Properties of the Final Product" Foods 9, no. 10: 1401. https://doi.org/10.3390/foods9101401
APA StyleRosend, J., Kaleda, A., Kuldjärv, R., Arju, G., & Nisamedtinov, I. (2020). The Effect of Apple Juice Concentration on Cider Fermentation and Properties of the Final Product. Foods, 9(10), 1401. https://doi.org/10.3390/foods9101401