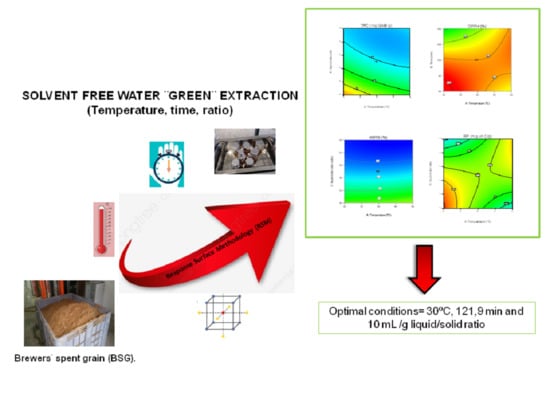
Optimization of Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant Activity in Craft Brewers’ Spent Grain Using Response Surface Methodology (RSM)
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Chemicals
2.2. Selection of Variables and the Extraction Process
2.3. Experimental Design
2.4. Quantification of Total Phenolics
2.5. Antioxidant In Vitro Assays
2.5.1. Determination of DPPH Radical Scavenging Activity
2.5.2. Reducing Power
2.5.3. ABTS Radical Cation Inhibition Antioxidant Assay
2.5.4. Validation of the Model
2.5.5. HPLC Analysis
3. Results and Discussion
3.1. Fitting the Model
3.2. Effect of the Extraction Variables on Total Phenolic Content (TPC)
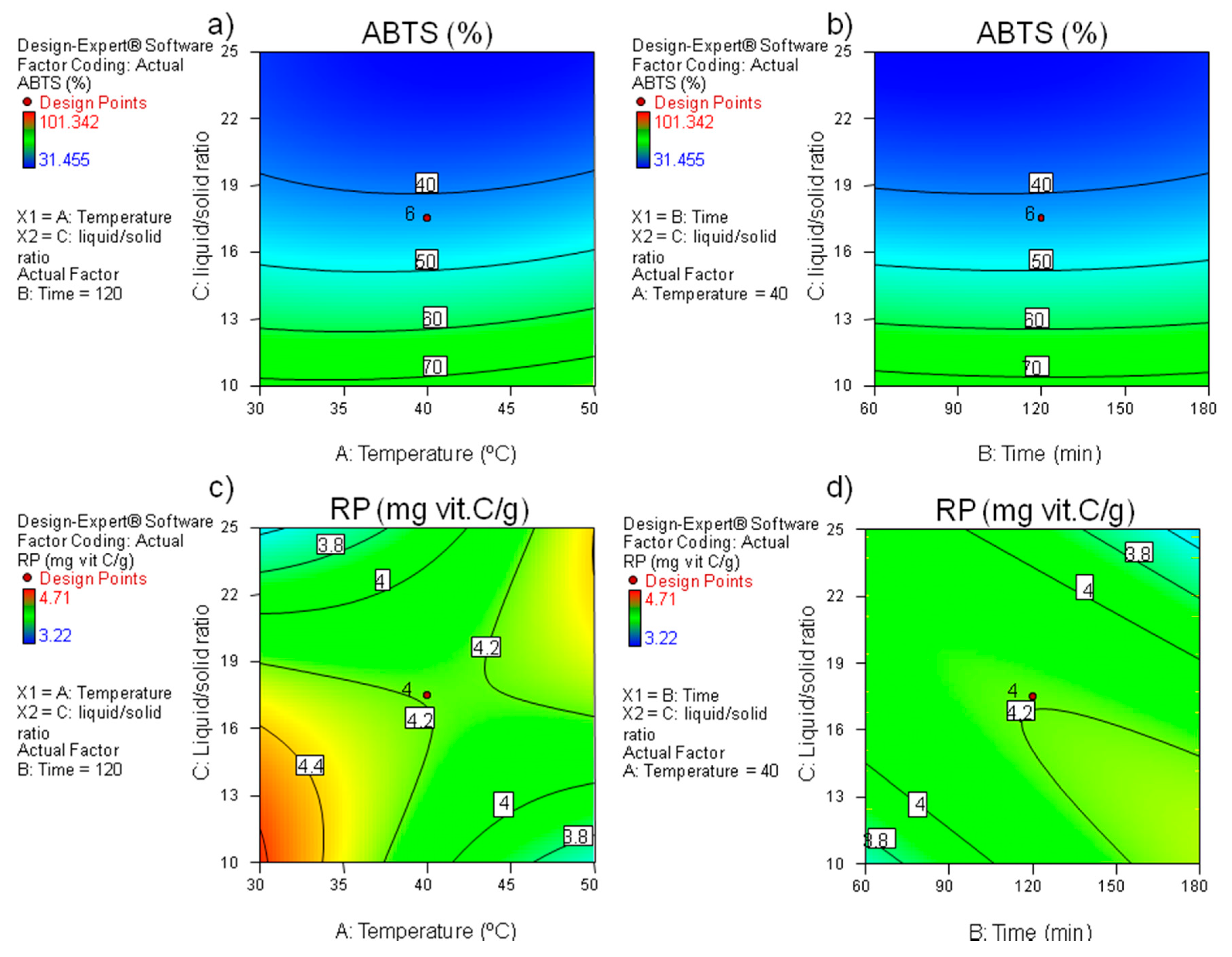
3.3. Effect of the Extraction Variables on the Antioxidant Potential of BSG Extracts
3.4. Optimization of the Extraction Parameters and Model Validation
3.5. HPLC Analysis of Phenolic Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The Brewers of Europe. Beer Statistics, 2019th ed.; The Brewers of Europe: Brussels, Belgium, 2019; Available online: https://brewersofeurope.org/site/media-centre/index.php?doc_id=840&class_id=31&detail=true (accessed on 3 June 2020).
- Roth, M.; Jekle, M.; Becker, T. Opportunities for upcycling cereal byproducts with special focus on Distiller’s grains. Trends Food Sci. Technol. 2019, 91, 282–293. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Verardo, V.; Berardinelli, A.; Marconi, E.; Caboni, M.F. A chemometric approach to determine the phenolic compounds in different barley samples by two different stationary phases: A comparison between C18 and pentafluorophenyl core shell columns. J. Chromatogr. A 2014, 1355, 134–142. [Google Scholar] [CrossRef]
- Guido, L.F.; Moreira, M.M. Techniques for Extraction of Brewer’s Spent Grain Polyphenols: A Review. Food Bioprocess. Technol. 2017, 10, 1192–1209. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Mass transfer process during extraction of phenolic compounds from milled berries. J. Food Eng. 2003, 59, 379–389. [Google Scholar] [CrossRef]
- Meneses, N.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Socaci, S.A.; Fărcaş, A.C.; Diaconeasa, Z.M.; Vodnar, D.C.; Rusu, B.; Tofană, M. Influence of the extraction solvent on phenolic content, antioxidant, antimicrobial and antimutagenic activities of brewers’ spent grain. J. Cereal Sci. 2018, 80, 180–187. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidović, S.; Redovniković, I.R.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Proces. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Reis, S.F.; Rai, D.K.; Ghannam, N.A. Water at room temperature as a solvent for the extraction of apple pomace phenolic compounds. Food Chem. 2012, 135, 1991–1998. [Google Scholar] [CrossRef]
- Leite, P.; Silva, C.; Salgado, J.M.; Belo, I. Simultaneous production of lignocellulolytic enzymes and extraction of antioxidant compounds by solid-state fermentation of agro-industrial wastes. Ind. Crop. Prod. 2019, 137, 315–322. [Google Scholar] [CrossRef]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology RSM. Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef]
- Viacava, G.E.; Roura, S.I.; Agüero, M.V. Optimization of critical parameters during antioxidants extraction from butterhead lettuce to simultaneously enhance polyphenols and antioxidant activity. Chemometr. Intell. Lab. 2015, 146, 47–54. [Google Scholar] [CrossRef]
- García Paz, M. Los Residuos de Cerveza Como Fuente de Antioxidantes Naturales. Master’s Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2017. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Broncano, J.M.; Timón, M.L.; Parra, V.; Andrés, A.I.; Petrón, M.J. Use of proteases to improve oxidative stability of fermented sausages by increasing low molecular weight compounds with antioxidant activity 2011. Food Res. Int. 2011, 44, 2655–2659. [Google Scholar] [CrossRef]
- Świeca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Czyż, J. The influence of protein–flavonoid interactions on protein digestibility in vitro and the antioxidant quality of breads enriched with onion skin. Food Chem. 2013, 141, 451–458. [Google Scholar] [CrossRef]
- Karvela, E.; Makris, D.P.; Kalogeropoulos, N.; Karathanos, V.P.; Kefalas, P. Factorial design optimisation of grape Vitis vinifera seed polyphenol extraction. Eur. Food Res. Technol. 2009, 5, 731–742. [Google Scholar] [CrossRef]
- Pedro, A.C.; Granato, D.; Rosso, N.B. Extraction of anthocyanins and polyphenols from black rice Oryza sativa L. by modeling and assessing their reversibility and stability. Food Chem. 2016, 191, 12–20. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F. Antioxidant and free radical scavenging activities of whole wheat and milling fractions. Food Chem. 2007, 101, 1151–1157. [Google Scholar] [CrossRef]
- Liu, R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Jerez, M.; Pinelo, M.; Sineiro, J.; Núñez, M.J. Influence of extraction conditions on phenolic yields from pine bark: Assessment of procyanidins polymerization degree by thiolysis. Food Chem. 2006, 94, 406–414. [Google Scholar] [CrossRef]
- Balli, D.; Bellumori, M.; Orlandini, S.; Cecchi, L.; Mani, E.; Pieraccini, G.; Mulinacci, N.; Innocenti, M. Optimized hydrolytic methods by response surface methodology to accurately estimate the phenols in cereal by HPLC-DAD: The case of millet. Food Chem. 2020, 303, 125393. [Google Scholar] [CrossRef]
- Moreira, M.M.; Morais, S.; Carvalho, D.O.; Barros, A.A.; Delerue-Matos, C.; Guido, L.F. Brewer’s spent grain from different types of malt: Evaluation of the antioxidant activity and identification of the major phenolic compounds. Food Res. Int. 2013, 54, 382–388. [Google Scholar] [CrossRef]
- Pompeu, D.R.; Silva, E.M.; Rogez, H. Optimisation of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using Response Surface Methodology. Bioresour. Technol. 2009, 100, 6076–6082. [Google Scholar] [CrossRef]
- Gupta, S.; Jaiswal, A.K.; Abu-Ghannam, N. Optimization of fermentation conditions for the utilization of brewing waste to develop a nutraceutical rich liquid product. Ind. Crop. Prod. 2013, 44, 272–282. [Google Scholar] [CrossRef]
- Todaro, A.; Cimino, F.; Rapisarda, P.; Catalano, A.E.; Barbagallo, R.N.; Spagna, G. Recovery of anthocyanins from eggplant peel. Food Chem. 2009, 114, 434–439. [Google Scholar] [CrossRef]
- Ryu, D.; Koh, E. Application of response surface methodology to acidified water extraction of black soybeans for improving anthocyanin content, total phenols content and antioxidant activity. Food Chem. 2018, 261, 260–266. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y.; Zhu, Y.; Xu, Z. Assesment of the correlations between reducing power, scavenging DPPH activity and anti-lipid-oxidation capability of phenolic antioxidants. LWT-Food Sci. Technol. 2015, 63, 569–574. [Google Scholar] [CrossRef]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin-Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Prior, R.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods anddietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar]
- Ikawa, M.; Schafer, T.; Dollard, C.; Sasner, J. Utilization of Folin-Ciocalteu reagent for the detection of certain nitrogen compounds. J. Agric. Food Chem. 2003, 51, 1811–1815. [Google Scholar]
- Masisi, K.; Beta, T.; Moghadasian, M.H. Antioxidant properties of diverse cereal grains: A review on in vitro and in vivo studies. Food Chem. 2016, 196, 90–97. [Google Scholar] [CrossRef]
- Stefanello, F.S.; Obem dos Santos, C.; Cateano Bochi, V.; Burin Fruet, A.P.; Bromenberg Soquetta, M.; Dörr, A.C.; Laerte Nörnberg, J. Analysis of polyphenols in brewer’s spent grain and its comparison with corn silage and cereal brans commonly used for animal nutrition. Food Chem. 2018, 239, 385–401. [Google Scholar] [CrossRef]
- Da Costa Maia, I.; Thomaz dos Santos D’Almeida, D.; Guimarães Freire, D.M.; Larraz Ferreira, M.S. Effect of solid-state fermentation over the release of phenolic compounds from brewer’s spent grain revealed by UPLC-MSE. Food Sci. Technol. 2020, 133, 110–136. [Google Scholar] [CrossRef]
- Fărcaş, A.C.; Socaci, S.A.; Dulf, F.V.; Tofană, M.; Mudura, E.; Diaconeasa, Z. Volatile profile, fatty acids composition and total phenolics content of brewers’ spent grain by-product with potential use in the development of new functional foods. J. Cereal Sci. 2015, 64, 34–42. [Google Scholar] [CrossRef]
- McCarthy, A.; O’Callaghan, Y.; Connolly, A.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. In vitro antioxidant and anti-inflammatory effects of brewers’ spent grain protein rich isolate and its associated hydrolysates. Food Res. Int. 2013, 50, 205–212. [Google Scholar]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar]

| Independent Variables | Responses | ||||||
|---|---|---|---|---|---|---|---|
| Run | Temperature (X1) | Time (X2) | Ratio v/w (X3) | TPC | DPPH | RP | ABTS |
| 1 | 40 | 120 | 17.5 | 2.20 ± 0.08 | 86.38 ± 1.41 | 12.50 ± 0.51 | 46.65 ± 1.12 |
| 2 | 50 | 60 | 10 | 3.01 ± 0.06 | 77.56 ± 2.35 | 12.09 ± 0.48 | 78.58 ± 2.43 |
| 3 | 30 | 60 | 25 | 1.51 ± 0.02 | 39.83 ± 1.21 | 13.85 ± 0.55 | 34.25 ± 1.43 |
| 4 | 40 | 220.91 | 17.5 | 2.01 ± 0.05 | 62.10 ± 1.88 | 13.85 ± 0.55 | 46.75 ± 2.13 |
| 5 | 30 | 180 | 25 | 0.91 ± 0.02 | 45.57 ± 1.38 | 13.22 ± 0.53 | 35.96 ± 1.43 |
| 6 | 50 | 180 | 10 | 3.61 ± 0.06 | 98.23 ± 2.98 | 13.96 ± 0.56 | 81.43 ± 1.90 |
| 7 | 23.18 | 120 | 17.5 | 3.81 ± 0.07 | 96.51 ± 2.92 | 14.50 ± 0.58 | 50.56 ± 1.55 |
| 8 | 40 | 19.09 | 17.5 | 1.11 ± 0.03 | 92.49 ± 2.80 | 13.90 ± 0.56 | 44.55 ± 2.23 |
| 9 | 40 | 120 | 17.5 | 1.21 ± 0.04 | 93.61 ± 2.84 | 14.46 ± 0.58 | 48.42 ± 2.27 |
| 10 | 50 | 180 | 25 | 1.51 ± 0.04 | 84.49 ± 2.56 | 14.17 ± 0.57 | 37.04 ± 1.65 |
| 11 | 40 | 120 | 30.11 | 2.91 ± 0.06 | 84.70 ± 2.57 | 13.26 ± 0.53 | 34.30 ± 1.56 |
| 12 | 40 | 120 | 17.5 | 2.51 ± 0.05 | 96.67 ± 2.93 | 14.31 ± 0.57 | 47.72 ± 1.88 |
| 13 | 56.82 | 120 | 17.5 | 1.71 ± 0.03 | 95.49 ± 2.89 | 14.24 ± 0.57 | 56.15 ± 2.53 |
| 14 | 50 | 60 | 25 | 1.71 ± 0.03 | 85.78 ± 2.60 | 14.71 ± 0.59 | 33.76 ± 1.03 |
| 15 | 30 | 60 | 10 | 5.11 ± 0.011 | 95.87 ± 2.91 | 14.29 ± 0.57 | 73.97 ± 2.36 |
| 16 | 40 | 120 | 17.5 | 2.41 ± 0.05 | 87.60 ± 2.65 | 13.92 ± 0.56 | 39.72 ± 1.81 |
| 17 | 40 | 120 | 4.89 | 5.51 ± 0.01 | 93.77 ± 2.84 | 13.80 ± 0.55 | 101.34 ± 5.511 |
| 18 | 30 | 180 | 10 | 4.21 ± 0.08 | 90.50 ± 2.74 | 14.71 ± 0.59 | 69.89 ± 3.18 |
| 19 | 40 | 120 | 17.5 | 2.51 ± 0.05 | 79.92 ± 2.42 | 14.11 ± 0.56 | 31.45 ± 1.23 |
| 20 | 40 | 120 | 17.5 | 1.61 ± 0.02 | 91.79 ± 2.78 | 13.12 ± 0.52 | 43.37 ± 2.97 |
| Regression Coefficients (β) | ||||
|---|---|---|---|---|
| TPC | DPPH | RP | ABTS | |
| Intercept | ||||
| X0 | 2.057 | 89.999 | 14.194 | 45.130 |
| Linear | ||||
| X1 | −0.397 * | 0.588 | −0.029 | 0.682 |
| X2 | 0.030 | −7.021 ** | −0.010 | 0.546 |
| X3 | −1.074 ** | −4.189 | −0.087 | −20.181 *** |
| Cross product | ||||
| X12 | 0.237 | 10.443 ** | 0.046 | 1.060 |
| X13 | 0.437 | 3.862 | 0.448 ** | −1.946 |
| X23 | −0.062 | −9.423 ** | −0.286 ** | 0.778 |
| Quadratic | ||||
| X12 | 0.209 | 1.709 | 0.084 | 1.366 |
| X22 | −0.214 | −4.905 * | −0.090 | 0.410 |
| X32 | 0.722 ** | −0.682 | −0.212 ** | 8.248 ** |
| R2 | 0.89 | 0.88 | 0.90 | 0.99 |
| F value (model) | 7.91 ** | 6.47 ** | 7.13 ** | 66.19 *** |
| F value (lack of fit) | 1.21 | 1.22 | 0.54 | 0.72 |
| TPC | DPPH | RP | ABTS | |
|---|---|---|---|---|
| TPC | 1 | 0.434 | 0.179 | 0.788 ** |
| DPPH | 1 | 0.513 * | 0.402 | |
| RP | 1 | 0.155 | ||
| ABTS | 1 |
| Dependent Variables | Predicted Value | Experimental Value | % CV |
|---|---|---|---|
| TPC | 4.89 | 5.42 | 8.3 |
| DPPH | 98.23 | 86.26 | 9.2 |
| RP | 14.64 | 13.93 | 11.6 |
| ABTS | 72.26 | 71.58 | 0.6 |
| Phenolic Compounds (µg/g) | |
|---|---|
| Epicatechin | 28.91 ± 2.90 |
| Gallic acid | 83.30 ± 9.58 |
| Protocatechuic acid | 15.72 ± 5.79 |
| 4-Hydroxy benzoic acid | 4.04 ± 2.44 |
| 4-Hydroxyphenylacetic acid | 32.40 ± 5.13 |
| Vanillic acid | 1.07 ± 0.73 |
| Caffeic acid | 1.69 ± 1.65 |
| Syringic acid | 2.51 ± 1.61 |
| Coumaric acid | 0.27 ± 0.06 |
| Ferulic acid | 0.88 ± 0.11 |
| Sinapic acid | 7.16 ± 2.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andres, A.I.; Petron, M.J.; Lopez, A.M.; Timon, M.L. Optimization of Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant Activity in Craft Brewers’ Spent Grain Using Response Surface Methodology (RSM). Foods 2020, 9, 1398. https://doi.org/10.3390/foods9101398
Andres AI, Petron MJ, Lopez AM, Timon ML. Optimization of Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant Activity in Craft Brewers’ Spent Grain Using Response Surface Methodology (RSM). Foods. 2020; 9(10):1398. https://doi.org/10.3390/foods9101398
Chicago/Turabian StyleAndres, Ana Isabel, Maria Jesus Petron, Ana Maria Lopez, and Maria Luisa Timon. 2020. "Optimization of Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant Activity in Craft Brewers’ Spent Grain Using Response Surface Methodology (RSM)" Foods 9, no. 10: 1398. https://doi.org/10.3390/foods9101398
APA StyleAndres, A. I., Petron, M. J., Lopez, A. M., & Timon, M. L. (2020). Optimization of Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant Activity in Craft Brewers’ Spent Grain Using Response Surface Methodology (RSM). Foods, 9(10), 1398. https://doi.org/10.3390/foods9101398