Novel Technologies Based on Supercritical Fluids for the Encapsulation of Food Grade Bioactive Compounds
Abstract
1. Introduction
2. Techniques Used for the Encapsulation of Bioactive Compounds
3. Bioactive Compounds Worth to Encapsulate
3.1. Antioxidants and Vitamins
3.2. Vegetable and Essential Oils
4. Supercritical Carbon Dioxide as Encapsulation Solvent
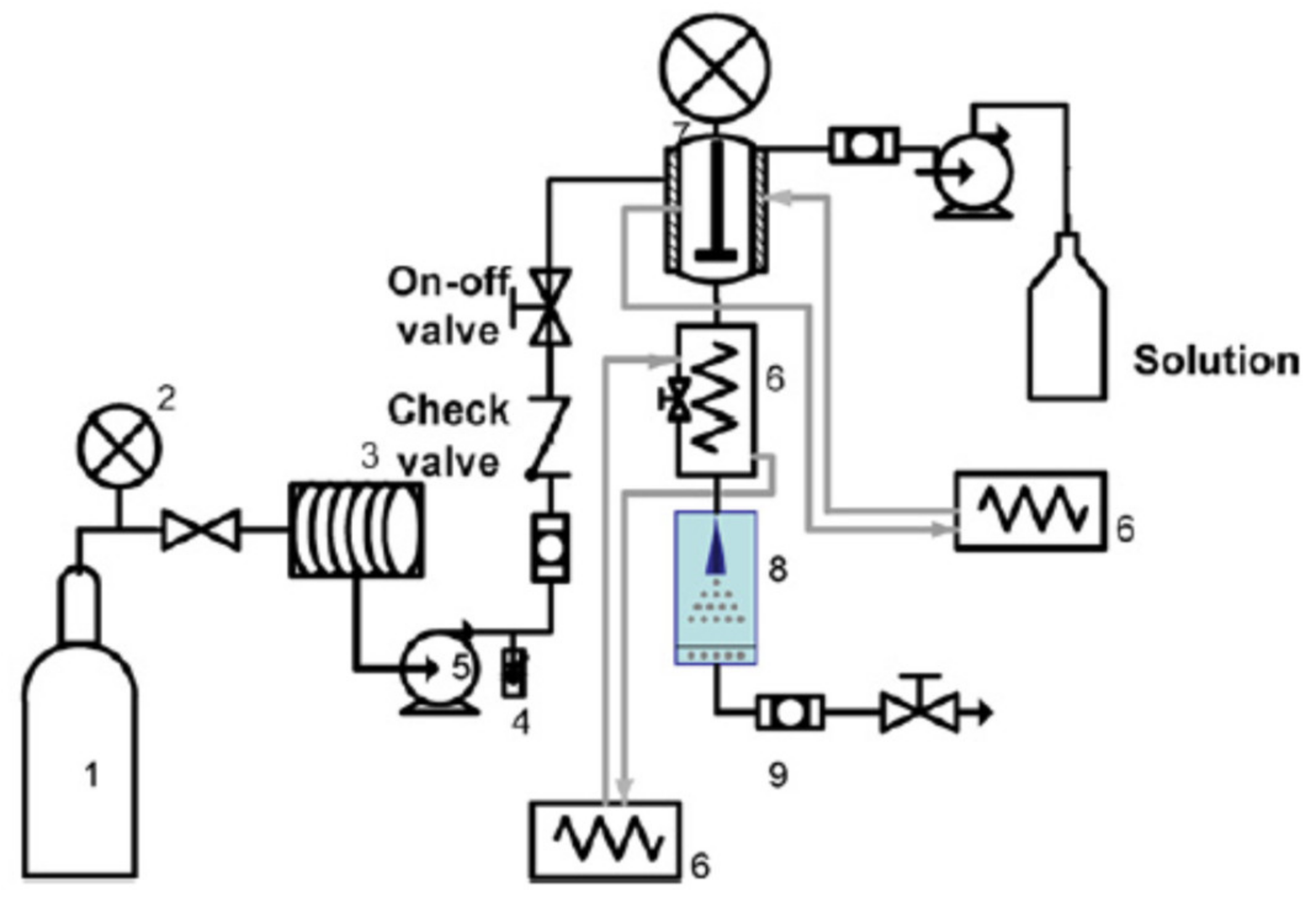
5. Supercritical Carbon Dioxide Technologies for the Encapsulation of Bioactives
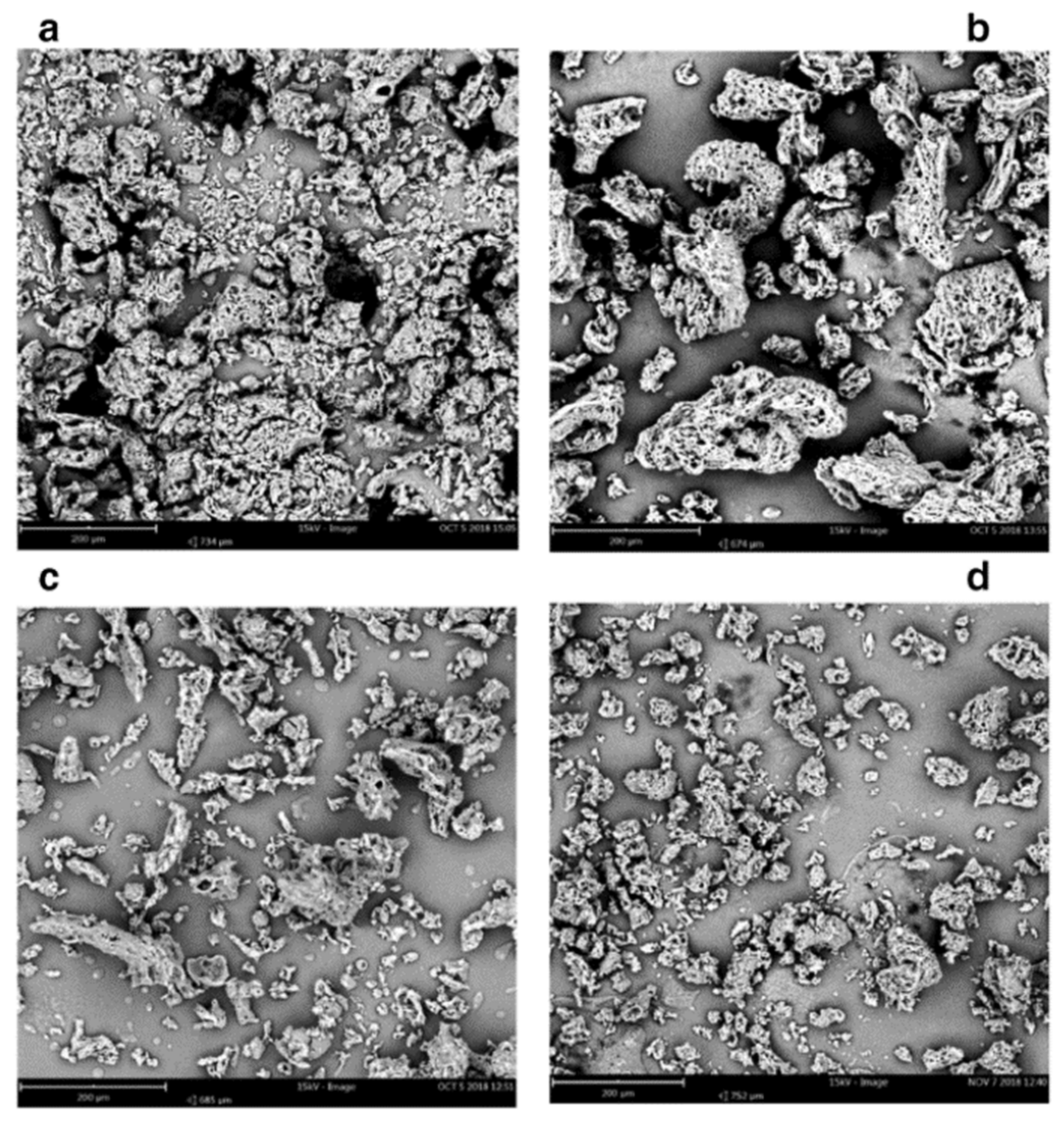
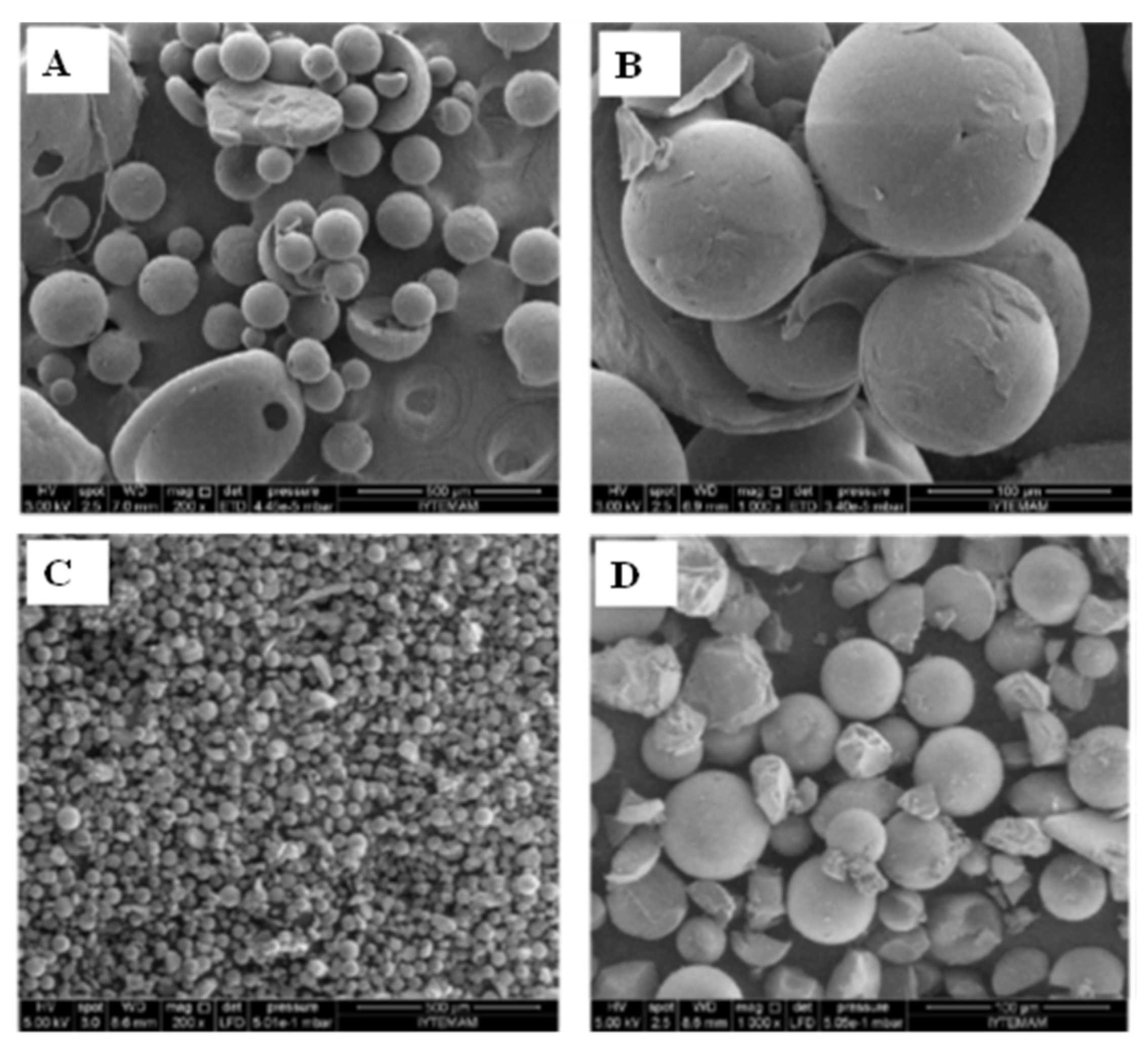
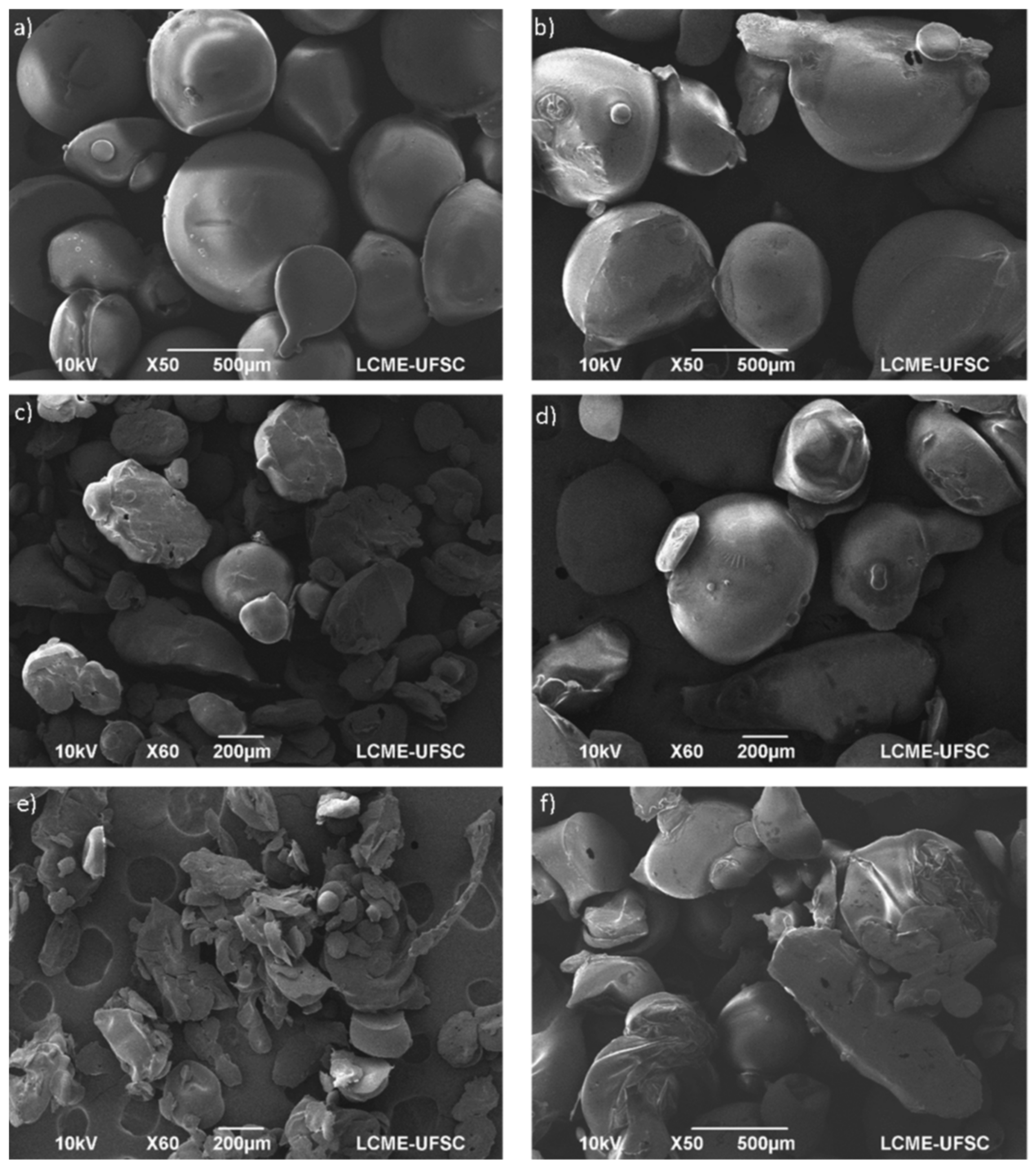
5.1. Particles from Gas Saturated Solutions
5.2. Particles from Gas Saturated Solutions Drying
5.3. Rapid Expansion of Supercritical Solutions
5.4. Gas Anti-Solvent Process
5.5. Supercritical Anti-Solvent Process
5.6. Solution Enhanced Dispersion by Supercritical Fluid
5.7. Supercritical Fluid Extraction of Emulsions
5.8. Liposomes Formation by Supercritical Fluids
6. Industrial Scale Applications
7. Future Perspectives and Final Remarks
Funding
Conflicts of Interest
References
- Temelli, F. Perspectives on the use of supercritical particle formation technologies for food ingredients. J. Supercrit. Fluids 2018, 134, 244–251. [Google Scholar] [CrossRef]
- Yada, R.Y.; Buck, N.; Canady, R.; Demerlis, C.; Duncan, T.; Janer, G.; Juneja, L.; Lin, M.; Mcclements, J.; Noonan, G.; et al. Engineered nanoscale food ingredients: Evaluation of current knowledge on material characteristics relevant to uptake from the gastrointestinal tract. Compr. Rev. Food Sci. Food Saf. 2014, 13, 730–744. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2014, 7, 452–490. [Google Scholar] [CrossRef]
- Keven Silva, E.; Angela, A.; Meireles, M. Encapsulation of Food Compounds Using Supercritical Technologies: Applications of Supercritical Carbon Dioxide as an Antisolvent. Food Public Health 2014, 4, 247–258. [Google Scholar] [CrossRef]
- Weidner, E. High pressure micronization for food applications. J. Supercrit. Fluids 2009, 47, 556–565. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Kermasha, S.; Alli, I.; Mulligan, C.N. Encapsulation in the food industry: A review. Int. J. Food Sci. Nutr. 1999, 50, 213–224. [Google Scholar] [CrossRef]
- Gouin, S. Microencapsulation: Industrial appraisal of existing technologies and trends. In Proceedings of the Trends in Food Science and Technology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 15, pp. 330–347. [Google Scholar]
- Aherne, S.A.; Daly, T.; Jiwan, M.A.; O’Sullivan, L.; O’Brien, N.M. Bioavailability of β-carotene isomers from raw and cooked carrots using an in vitro digestion model coupled with a human intestinal Caco-2 cell model. Food Res. Int. 2010, 43, 1449–1454. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Gayoso, L.; Claerbout, A.S.; Calvo, M.I.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D. Bioaccessibility of rutin, caffeic acid and rosmarinic acid: Influence of the in vitro gastrointestinal digestion models. J. Funct. Foods 2016, 26, 428–438. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Xu, G.; Ye, X.; Liu, D.; Ma, Y.; Chen, J. Composition and distribution of phenolic acids in Ponkan (Citrus poonensis Hort. ex Tanaka) and Huyou (Citrus paradisi Macf. Changshanhuyou) during maturity. J. Food Compos. Anal. 2008, 21, 382–389. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Leshina, T.V. Certain aspects of the reactivity of carotenoids. Redox processes and complexation. Russ. Chem. Rev. 2006, 75, 1049–1064. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Palmero, P.; Lemmens, L.; Ribas-Agustí, A.; Sosa, C.; Met, K.; De Dieu Umutoni, J.; Hendrickx, M.; Van Loey, A. Novel targeted approach to better understand how natural structural barriers govern carotenoid in vitro bioaccessibility in vegetable-based systems. Food Chem. 2013, 141, 2036–2043. [Google Scholar] [CrossRef]
- Valerio, P.P.; Frias, J.M.; Cren, E.C. Thermal degradation kinetics of carotenoids: Acrocomia aculeata oil in the context of nutraceutical food and bioprocess technology. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Gangopadhyay, N.; O’Shea, N.; Brunton, N.P.; Gallagher, E.; Harrison, S.M.; Rai, D.K. Fate of beta-glucan, polyphenols and lipophilic compounds in baked crackers fortified with different barley-milled fractions. LWT 2019, 114, 108413. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; Barrow, C.J.; Adhikari, B. Microencapsulation of omega-3 fatty acids: A review of microencapsulation and characterization methods. J. Funct. Foods 2015, 19, 868–881. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Masui, E.; Tamogami, S.; Chen, J.; Zhang, H. Analysis of the Dynamic Decomposition of Unsaturated Fatty Acids and Tocopherols in Commercial Oils During Deep Frying. Anal. Lett. 2019, 52, 1991–2005. [Google Scholar] [CrossRef]
- Jung, M.Y.; Yoon, S.H.; Min, D.B. Effects of processing steps on the contents of minor compounds and oxidation of soybean oil. J. Am. Oil Chem. Soc. 1989, 66, 118–120. [Google Scholar] [CrossRef]
- Krygier, K.; Płatek, T. Comparison of Pressed and Extracted Rapeseed Oils Characteristics. Available online: http://www.regional.org.au/au/gcirc/6/641.htm (accessed on 4 September 2020).
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Impact of different storage conditions on the quality of selected essential oils. Food Res. Int. 2012, 46, 341–353. [Google Scholar] [CrossRef]
- Misharina, T.A.; Polshkov, A.N. Antioxidant properties of essential oils: Autoxidation of essential oils from laurel and fennel and effects of mixing with essential oil from coriander. Prikl. Biokhimiia i Mikrobiol. 2005, 41, 693–702. [Google Scholar]
- Ariyarathna, I.R.; Karunaratne, D.N. Microencapsulation stabilizes curcumin for efficient delivery in food applications. Food Packag. Shelf Life 2016, 10, 79–86. [Google Scholar] [CrossRef]
- Ayan, A.K.; Yenilmez, A.; Eroglu, H. Evaluation of radiolabeled curcumin-loaded solid lipid nanoparticles usage as an imaging agent in liver-spleen scintigraphy. Mater. Sci. Eng. C 2017, 75, 663–670. [Google Scholar] [CrossRef]
- de Paz, E.; Martín, Á.; Duarte, C.M.M.; Cocero, M.J. Formulation of β-carotene with poly-(ε-caprolactones) by PGSS process. Powder Technol. 2012, 217, 77–83. [Google Scholar] [CrossRef]
- Haq, M.; Chun, B.S. Microencapsulation of omega-3 polyunsaturated fatty acids and astaxanthin-rich salmon oil using particles from gas saturated solutions (PGSS) process. LWT 2018, 92, 523–530. [Google Scholar] [CrossRef]
- Vo, D.T.; Saravana, P.S.; Woo, H.C.; Chun, B.S. Fucoxanthin-rich oil encapsulation using biodegradable polyethylene glycol and particles from gas-saturated solutions technique. J. CO2 Util. 2018, 26, 359–369. [Google Scholar] [CrossRef]
- Rodrigues, M.; Peiriço, N.; Matos, H.; Gomes De Azevedo, E.; Lobato, M.R.; Almeida, A.J. Microcomposites theophylline/hydrogenated palm oil from a PGSS process for controlled drug delivery systems. J. Supercrit. Fluids 2004, 29, 175–184. [Google Scholar] [CrossRef]
- Sampaio de Sousa, A.R.; Simplício, A.L.; de Sousa, H.C.; Duarte, C.M.M. Preparation of glyceryl monostearate-based particles by PGSS®-Application to caffeine. J. Supercrit. Fluids 2007, 43, 120–125. [Google Scholar] [CrossRef]
- García-González, C.A.; Argemí, A.; De Sousa, A.R.S.; Duarte, C.M.M.; Saurina, J.; Domingo, C. Encapsulation efficiency of solid lipid hybrid particles prepared using the PGSS® technique and loaded with different polarity active agents. J. Supercrit. Fluids 2010, 54, 342–347. [Google Scholar] [CrossRef]
- Couto, R.; Alvarez, V.; Temelli, F. Encapsulation of Vitamin B2 in solid lipid nanoparticles using supercritical CO2. J. Supercrit. Fluids 2017, 120, 432–442. [Google Scholar] [CrossRef]
- Salminen, H.; Gömmel, C.; Leuenberger, B.H.; Weiss, J. Influence of encapsulated functional lipids on crystal structure and chemical stability in solid lipid nanoparticles: Towards bioactive-based design of delivery systems. Food Chem. 2016, 190, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Varona, S.; Kareth, S.; Martín, Á.; Cocero, M.J. Formulation of lavandin essential oil with biopolymers by PGSS for application as biocide in ecological agriculture. J. Supercrit. Fluids 2010, 54, 369–377. [Google Scholar] [CrossRef]
- Varona, S.; Martín, Á.; Cocero, M.J.; Duarte, C.M.M. Encapsulation of Lavandin Essential Oil in Poly-(ε{lunate}-caprolactones) by PGSS Process. Chem. Eng. Technol. 2013, 36, 1187–1192. [Google Scholar] [CrossRef]
- Hu, X.; Guo, Y.; Wang, L.; Hua, D.; Hong, Y.; Li, J. Coenzyme Q10 nanoparticles prepared by a supercritical fluid-based method. J. Supercrit. Fluids 2011, 57, 66–72. [Google Scholar] [CrossRef]
- Bule, M.V.; Singhal, R.S.; Kennedy, J.F. Microencapsulation of ubiquinone-10 in carbohydrate matrices for improved stability. Carbohydr. Polym. 2010, 82, 1290–1296. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, H.; Hu, X.; Bao, S.; Huang, H. Synthesis and release studies of microalgal oil-containing microcapsules prepared by complex coacervation. Colloids Surf. B Biointerfaces 2012, 89, 61–66. [Google Scholar] [CrossRef]
- Bejrapha, P.; Min, S.-G.; Surassmo, S.; Choi, M.-J. Physicothermal Properties of Freeze-Dried Fish Oil Nanocapsules Frozen under Different Conditions. Dry. Technol. 2010, 28, 481–489. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Zhong, Q. Physical and antimicrobial properties of spray-dried zein-casein nanocapsules with co-encapsulated eugenol and thymol. J. Food Eng. 2014, 144, 93–102. [Google Scholar] [CrossRef]
- Cocero, M.J.; Martín, Á.; Mattea, F.; Varona, S. Encapsulation and co-precipitation processes with supercritical fluids: Fundamentals and applications. J. Supercrit. Fluids 2009, 47, 546–555. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F.; Curtis, J.M.; Chen, L. Encapsulation of lutein in liposomes using supercritical carbon dioxide. Food Res. Int. 2017, 100, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Thiering, R.; Dehghani, F.; Foster, N.R. Current issues relating to anti-solvent micronisation techniques and their extension to industrial scales. J. Supercrit. Fluids 2001, 21, 159–177. [Google Scholar] [CrossRef]
- Knez, Z.; Weidner, E. Particles formation and particle design using supercritical fluids. Curr. Opin. Solid State Mater. Sci. 2003, 7, 353–361. [Google Scholar] [CrossRef]
- Fages, J.; Lochard, H.; Letourneau, J.J.; Sauceau, M.; Rodier, E. Particle generation for pharmaceutical applications using supercritical fluid technology. Powder Technol. 2004, 141, 219–226. [Google Scholar] [CrossRef]
- Monteagudo-Olivan, R.; Cocero, M.J.; Coronas, J.; Rodríguez-Rojo, S. Supercritical CO2 encapsulation of bioactive molecules in carboxylate based MOFs. J. CO2 Util. 2019, 30, 38–47. [Google Scholar] [CrossRef]
- Jung, J.; Perrut, M. Particle design using supercritical fluids: Literature and patent survey. J. Supercrit. Fluids 2001, 20, 179–219. [Google Scholar] [CrossRef]
- Nunes, A.V.M.; Duarte, C.M.M. Dense CO2 as a Solute, Co-Solute or Co-Solvent in Particle Formation Processes: A review. Materials 2011, 4, 2017–2041. [Google Scholar] [CrossRef]
- Visentin, A.; Rodríguez-Rojo, S.; Navarrete, A.; Maestri, D.; Cocero, M.J. Precipitation and encapsulation of rosemary antioxidants by supercritical antisolvent process. J. Food Eng. 2012, 109, 9–15. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Cetin-Uyanikgil, E.O. In vitro release kinetics of polycaprolactone encapsulated plant extract fabricated by supercritical antisolvent process and solvent evaporation method. J. Supercrit. Fluids 2012, 62, 219–225. [Google Scholar] [CrossRef]
- Hu, D.; Lin, C.; Liu, L.; Li, S.; Zhao, Y. Preparation, characterization, and in vitro release investigation of lutein/zein nanoparticles via solution enhanced dispersion by supercritical fluids. J. Food Eng. 2012, 109, 545–552. [Google Scholar] [CrossRef]
- Münüklü, P.; Jansens, P.J. Particle formation of an edible fat (rapeseed 70) using the supercritical melt micronization (ScMM) process. J. Supercrit. Fluids 2007, 40, 433–442. [Google Scholar] [CrossRef]
- Vatai, T.; Škerget, M.; Knez, Ž.; Kareth, S.; Wehowski, M.; Weidner, E. Extraction and formulation of anthocyanin-concentrates from grape residues. J. Supercrit. Fluids 2008, 45, 32–36. [Google Scholar] [CrossRef]
- Pemsel, M.; Schwab, S.; Scheurer, A.; Freitag, D.; Schatz, R.; Schlücker, E. Advanced PGSS process for the encapsulation of the biopesticide Cydia pomonella granulovirus. J. Supercrit. Fluids 2010, 53, 174–178. [Google Scholar] [CrossRef]
- Gîtin, L.; Varona, S.; José, M.; Alonso, C. Encapsulation of Garlic Essential Oil by Batch PGSS Process. Innov. Rom. Food Biotechnol. “Dunărea de Jos” University – Galaţi 2011, 9, 60–67. [Google Scholar]
- Yun, J.-H.; Lee, S.-M.; Park, J.-N.; Chun, B.-S. Particle Formation of Lecithin Process with Particles from Gas-Saturated Solutions using Supercritical Carbon Dioxide. APCBEE Procedia 2012. [Google Scholar] [CrossRef][Green Version]
- Yun, J.H.; Lee, H.Y.; Asaduzzaman, A.K.M.; Chun, B.S. Micronization and characterization of squid lecithin/polyethylene glycol composite using particles from gas saturated solutions (PGSS) process. J. Ind. Eng. Chem. 2013, 19, 686–691. [Google Scholar] [CrossRef]
- Gonçalvesab, V.S.S.; Poejoab, J.; Matiasab, A.A.; Nogueirac, I.D.; Duarteab, C.M.M. Encapsulation and precipitation of aqueous natural hydroxytyrosol-rich concentrate into a solid lipid matrix through PGSS®. In Proceedings of the 14th European meeting of supercritical fluids, Marseille, France, 18–21 May 2014. [Google Scholar]
- Perko, T.; Ravber, M.; Knez, Ž.; Škerget, M. Isolation, characterization and formulation of curcuminoids and in vitro release study of the encapsulated particles. J. Supercrit. Fluids 2015, 103, 48–54. [Google Scholar] [CrossRef]
- Yun, J.H.; Haque, A.S.M.T.; Chun, B.S. Characterization of Wheat Germ Oil Particles Formed by Gas-Saturated Solutions Process with Polyethylene Glycol. J. Food Process. Preserv. 2015, 39, 1720–1728. [Google Scholar] [CrossRef]
- Yang, J.; Ciftci, O.N. Development of free-flowing peppermint essential oil-loaded hollow solid lipid micro- and nanoparticles via atomization with carbon dioxide. Food Res. Int. 2016, 87, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Tanbirul Haque, A.S.M.; Chun, B.S. Particle formation and characterization of mackerel reaction oil by gas saturated solution process. J. Food Sci. Technol. 2016, 53, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Getachew, A.T.; Chun, B.S. Optimization of coffee oil flavor encapsulation using response surface methodology. LWT-Food Sci. Technol. 2016, 70, 126–134. [Google Scholar] [CrossRef]
- Bánvölgyi, S.Z.; Vatai, T.; Molnár, Z.S.; Kiss, I.; Knez, Z.; Vatai, G.Y.; Škerget, M. Integrated process to obtain anthocyanin enriched palm-fat particles from elderberry juice. Acta Aliment. 2016, 45, 206–214. [Google Scholar] [CrossRef]
- Ciftci, O.N.; Temelli, F. Formation of solid lipid microparticles from fully hydrogenated canola oil using supercritical carbon dioxide. J. Food Eng. 2016, 178, 137–144. [Google Scholar] [CrossRef]
- Lan, H.; Zhu, L.; He, B.; Hong, W.; Li, J. Encapsulation of menthol in beeswax by a supercritical fluid technique. Int. J. Chem. Eng. 2010, 2010, 608680. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Chun, B.S. Formation, characterization and release behavior of citrus oil-polymer microparticles using particles from gas saturated solutions (PGSS) process. J. Ind. Eng. Chem. 2018, 63, 201–207. [Google Scholar] [CrossRef]
- Akolade, J.O.; Balogun, M.; Swanepoel, A.; Ibrahim, R.B.; Yusuf, A.A.; Labuschagne, P. Microencapsulation of eucalyptol in polyethylene glycol and polycaprolactone using particles from gas-saturated solutions. RSC Adv. 2019, 9, 34039–34049. [Google Scholar] [CrossRef]
- Machado, L.C.; Pelegati, V.B.; Oliveira, A.L. Study of simple microparticles formation of limonene in modified starch using PGSS—Particles from gas-saturated suspensions. J. Supercrit. Fluids 2016, 107, 260–269. [Google Scholar] [CrossRef]
- Kravanja, G.; Knez, Ž.; Kotnik, P.; Ljubec, B.; Hrnčič, M.K. Formulation of nimodipine, fenofibrate, and o-vanillin with Brij S100 and PEG 4000 using the PGSSTM process. J. Supercrit. Fluids 2018, 135, 245–253. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Ferrentino, G.; Nabil, H.; Scampicchio, M. Encapsulation of Oils Recovered from brewer’s Spent Grain by Particles from Gas Saturated Solutions Technique. Food Bioprocess Technol. 2020, 13, 256–264. [Google Scholar] [CrossRef]
- Meterc, D.; Petermann, M.; Weidner, E. Drying of aqueous green tea extracts using a supercritical fluid spray process. J. Supercrit. Fluids 2008, 45, 253–259. [Google Scholar] [CrossRef]
- Varona, S.; Martín, Á.; Cocero, M.J. Liposomal incorporation of lavandin essential oil by a thin-film hydration method and by particles from gas-saturated solutions. Ind. Eng. Chem. Res. 2011, 50, 2088–2097. [Google Scholar] [CrossRef]
- De Paz, E.; Martín, Á.; Cocero, M.J. Formulation of β-carotene with soybean lecithin by PGSS (Particles from Gas Saturated Solutions)-drying. J. Supercrit. Fluids 2012, 72, 125–133. [Google Scholar] [CrossRef]
- Dübbert, K.; Rubio-Rodriguez, N.; Kareth, S.; Beltran, S.; Petermann, M. ENCAPSULATION OF FISH OIL IN BIODEGRADABLE POLYMERS BY THE PGSS PROCESS. Available online: https://www.semanticscholar.org/paper/ENCAPSULATION-OF-FISH-OIL-IN-BIODEGRADABLE-POLYMERS-D%C3%BCbbert-Kareth/fe55de2c25d7174b1f93011fec2bf98ed7e9f788 (accessed on 22 September 2020).
- Salgado, M.; Rodríguez-Rojo, S.; Alves-Santos, F.M.; Cocero, M.J. Encapsulation of resveratrol on lecithin and β-glucans to enhance its action against Botrytis cinerea. J. Food Eng. 2015, 165, 13–21. [Google Scholar] [CrossRef]
- Gonçalves, V.S.S.; Poejo, J.; Matias, A.A.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.M.M. Using different natural origin carriers for development of epigallocatechin gallate (EGCG) solid formulations with improved antioxidant activity by PGSS-drying. RSC Adv. 2016, 6, 67599–67609. [Google Scholar] [CrossRef]
- Lévai, G.; Martín, Á.; Moro, A.; Matias, A.A.; Gonçalves, V.S.S.; Bronze, M.R.; Duarte, C.M.M.; Rodríguez-Rojo, S.; Cocero, M.J. Production of encapsulated quercetin particles using supercritical fluid technologies. Powder Technol. 2017, 317, 142–153. [Google Scholar] [CrossRef]
- Melgosa, R.; Benito-Román, Ó.; Sanz, M.T.; de Paz, E.; Beltrán, S. Omega–3 encapsulation by PGSS-drying and conventional drying methods. Particle characterization and oxidative stability. Food Chem. 2019, 270, 138–148. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Sanz, T.; Beltrán, S. Microencapsulation of rice bran oil using pea protein and maltodextrin mixtures as wall material. Heliyon 2020, 6, e03615. [Google Scholar] [CrossRef]
- Liu, N.; Couto, R.; Seifried, B.; Moquin, P.; Delgado, L.; Temelli, F. Characterization of oat beta-glucan and coenzyme Q10-loaded beta-glucan powders generated by the pressurized gas-expanded liquid (PGX) technology. Food Res. Int. 2018, 106, 354–362. [Google Scholar] [CrossRef]
- Couto, R.; Seifried, B.; Moquin, P.; Temelli, F. Coenzyme Q10 solubility in supercritical CO2 using a dynamic system. J. CO2 Util. 2018, 24, 315–320. [Google Scholar] [CrossRef]
- Kröber, H.; Teipel, U. Microencapsulation of particles using supercritical carbon dioxide. In Proceedings of the Chemical Engineering and Processing: Process Intensification; : ; Elsevier: Amsterdam, The Netherlands, 2005; Volume 44, pp. 215–219. [Google Scholar]
- Santos, D.T.; Albarelli, J.Q.; Beppu, M.M.; Meireles, M.A.A. Stabilization of anthocyanin extract from jabuticaba skins by encapsulation using supercritical CO2 as solvent. Food Res. Int. 2013, 50, 617–624. [Google Scholar] [CrossRef]
- Wen, Z.; You, X.; Jiang, L.; Liu, B.; Zheng, Z.; Pu, Y.; Cheng, B. Liposomal incorporation of rose essential oil by a supercritical process. Flavour Fragr. J. 2011, 26, 27–33. [Google Scholar] [CrossRef]
- Wen, Z.; Liu, B.; Zheng, Z.; You, X.; Pu, Y.; Li, Q. Preparation of liposomes entrapping essential oil from Atractylodes macrocephala Koidz by modified RESS technique. Chem. Eng. Res. Des. 2010, 88, 1102–1107. [Google Scholar] [CrossRef]
- Momenkiaei, F.; Raofie, F. Preparation of Curcuma Longa L. Extract Nanoparticles Using Supercritical Solution Expansion. J. Pharm. Sci. 2019, 108, 1581–1589. [Google Scholar] [CrossRef]
- Santos, D.T.; Meireles, M.A.A. Micronization and encapsulation of functional pigments using supercritical carbon dioxide. J. Food Process Eng. 2013, 36, 36–49. [Google Scholar] [CrossRef]
- Mishima, K.; Matsuyama, K.; Tanabe, D.; Yamauchi, S.; Young, T.J.; Johnston, K.P. Microencapsulation of proteins by rapid expansion of supercritical solution with a nonsolvent. AIChE J. 2000, 46, 857–865. [Google Scholar] [CrossRef]
- Miguel, F.; Martín, A.; Mattea, F.; Cocero, M.J. Precipitation of lutein and co-precipitation of lutein and poly-lactic acid with the supercritical anti-solvent process. Chem. Eng. Process. Process Intensif. 2008, 47, 1594–1602. [Google Scholar] [CrossRef]
- Jin, H.; Xia, F.; Jiang, C.; Zhao, Y.; He, L. Nanoencapsulation of Lutein with Hydroxypropylmethyl Cellulose Phthalate by Supercritical Antisolvent. Chin. J. Chem. Eng. 2009, 17, 672–677. [Google Scholar] [CrossRef]
- Martín, A.; Mattea, F.; Gutiérrez, L.; Miguel, F.; Cocero, M.J. Co-precipitation of carotenoids and bio-polymers with the supercritical anti-solvent process. J. Supercrit. Fluids 2007, 41, 138–147. [Google Scholar] [CrossRef]
- Xia, F.; Hu, D.; Jin, H.; Zhao, Y.; Liang, J. Preparation of lutein proliposomes by supercritical anti-solvent technique. Food Hydrocoll. 2012, 26, 456–463. [Google Scholar] [CrossRef]
- Xia, F.; Jin, H.; Zhao, Y.; Guo, X. Supercritical antisolvent-based technology for preparation of vitamin D 3 proliposome and its characteristics. Chin. J. Chem. Eng. 2011, 19, 1039–1046. [Google Scholar] [CrossRef]
- Sosa, M.V.; Rodríguez-Rojo, S.; Mattea, F.; Cismondi, M.; Cocero, M.J. Green tea encapsulation by means of high pressure antisolvent coprecipitation. J. Supercrit. Fluids 2011, 56, 304–311. [Google Scholar] [CrossRef]
- Mezzomo, N.; De Paz, E.; Maraschin, M.; Martín, Á.; Cocero, M.J.; Ferreira, S.R.S. Supercritical anti-solvent precipitation of carotenoid fraction from pink shrimp residue: Effect of operational conditions on encapsulation efficiency. J. Supercrit. Fluids 2012, 66, 342–349. [Google Scholar] [CrossRef]
- Fraile, M.; Buratto, R.; Gómez, B.; Martín, Á.; Cocero, M.J. Enhanced delivery of quercetin by encapsulation in poloxamers by supercritical antisolvent process. Ind. Eng. Chem. Res. 2014, 53, 4318–4327. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Mezzomo, N.; Gomes, C.; Ferreira, S.R.S. Encapsulation of passion fruit seed oil by means of supercritical antisolvent process. J. Supercrit. Fluids 2017, 129, 96–105. [Google Scholar] [CrossRef]
- Aredo, V.; Passalacqua, E.S.; Pratavieira, S.; de Oliveira, A.L. Formation of lycopene-loaded hydrolysed collagen particles by supercritical impregnation. LWT 2019, 110, 158–167. [Google Scholar] [CrossRef]
- Varona, S.; Rodríguez-Rojo, S.; Martín, Á.; Cocero, M.J.; Duarte, C.M.M. Supercritical impregnation of lavandin (Lavandula hybrida) essential oil in modified starch. J. Supercrit. Fluids 2011, 58, 313–319. [Google Scholar] [CrossRef]
- Priamo, W.L.; De Cezaro, A.M.; Ferreira, S.R.S.; Oliveira, J.V. Precipitation and encapsulation of β-carotene in PHBV using carbon dioxide as anti-solvent. J. Supercrit. Fluids 2010, 54, 103–109. [Google Scholar] [CrossRef]
- Franceschi, E.; De Cesaro, A.M.; Feiten, M.; Ferreira, S.R.S.; Dariva, C.; Kunita, M.H.; Rubira, A.F.; Muniz, E.C.; Corazza, M.L.; Oliveira, J.V. Precipitation of β-carotene and PHBV and co-precipitation from SEDS technique using supercritical CO2. J. Supercrit. Fluids 2008, 47, 259–269. [Google Scholar] [CrossRef]
- Boschetto, D.L.; Dalmolin, I.; de Cesaro, A.M.; Rigo, A.A.; Ferreira, S.R.S.; Meireles, M.A.A.; Batista, E.A.C.; Vladimir Oliveira, J. Phase behavior and process parameters effect on grape seed extract encapsulation by SEDS technique. Ind. Crops Prod. 2013, 50, 352–360. [Google Scholar] [CrossRef]
- Andrade, K.S.; Aguiar, G.P.S.; Rebelatto, E.A.; Lanza, M.; Oliveira, J.V.; Ferreira, S.R.S. Encapsulation of pink pepper extract by SEDS technique: Phase behavior data and process parameters. J. Supercrit. Fluids 2020, 161, 104822. [Google Scholar] [CrossRef]
- Machado, F.R.S.; Reis, D.F.; Boschetto, D.L.; Burkert, J.F.M.; Ferreira, S.R.S.; Oliveira, J.V.; Burkert, C.A.V. Encapsulation of astaxanthin from Haematococcus pluvialis in PHBV by means of SEDS technique using supercritical CO2. Ind. Crops Prod. 2014, 54, 17–21. [Google Scholar] [CrossRef]
- Chen, A.Z.; Li, Y.; Chau, F.T.; Lau, T.Y.; Hu, J.Y.; Zhao, Z.; Mok, D.K.W. Microencapsulation of puerarin nanoparticles by poly(l-lactide) in a supercritical CO2 process. Acta Biomater. 2009, 5, 2913–2919. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.K.; Hassani, L.N.; Calvignac, B.; Beuvier, T.; Hindré, F.; Boury, F. Lysozyme encapsulation within PLGA and CaCO3 microparticles using supercritical CO2 medium. J. Supercrit. Fluids 2013, 79, 159–169. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Silva, L.P.S.; Alves De Rezende, C.; Barbero, G.F.; Martínez, J. Encapsulation of pepper oleoresin by supercritical fluid extraction of emulsions. J. Supercrit. Fluids 2016, 112, 37–43. [Google Scholar] [CrossRef]
- Prieto, C.; Calvo, L. The encapsulation of low viscosity omega-3 rich fish oil in polycaprolactone by supercritical fluid extraction of emulsions. J. Supercrit. Fluids 2017, 128, 227–234. [Google Scholar] [CrossRef]
- Santos, D.T.; Martín, Á.; Meireles, M.A.A.; Cocero, M.J.; Santos, D.T.; Martín, Á.; Meireles, M.A.A.; Cocero, M.J. Production of stabilized sub-micrometric particles of carotenoids using supercritical fluid extraction of emulsions. J. Supercrit. Fluids 2012, 61, 167–174. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F.; Curtis, J.M.; Chen, L. Preparation of liposomes using supercritical carbon dioxide technology: Effects of phospholipids and sterols. Food Res. Int. 2015, 77, 63–72. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F.; Chen, L. Encapsulation of anthocyanin in liposomes using supercritical carbon dioxide: Effects of anthocyanin and sterol concentrations. J. Funct. Foods 2017, 34, 159–167. [Google Scholar] [CrossRef]
- Campardelli, R.; Espirito Santo, I.; Albuquerque, E.C.; De Melo, S.V.; Della Porta, G.; Reverchon, E. Efficient encapsulation of proteins in submicro liposomes using a supercritical fluid assisted continuous process. J. Supercrit. Fluids 2016, 107, 163–169. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Production of liposomes loaded with antioxidants using a supercritical CO2 assisted process. Powder Technol. 2018, 323, 155–162. [Google Scholar] [CrossRef]
- Elvassore, N.; Flaibani, M.; Bertucco, A.; Caliceti, P. Thermodynamic Analysis of Micronization Processes from Gas-Saturated Solution. Ind. Eng. Chem. Res. 2003, 42, 5924–5930. [Google Scholar] [CrossRef]
- Suganya, V.; Anuradha, V. Microencapsulation and Nanoencapsulation: A Review. Int. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Gavara, R.; Lagaron, J.M. Bioactive packaging: Turning foods into healthier foods through biomaterials. Trends Food Sci. Technol. 2006, 17, 567–575. [Google Scholar] [CrossRef]
- Martín, Á.; Pham, H.M.; Kilzer, A.; Kareth, S.; Weidner, E. Phase equilibria of carbon dioxide + poly ethylene glycol + water mixtures at high pressure: Measurements and modelling. Fluid Phase Equilib. 2009, 286, 162–169. [Google Scholar] [CrossRef]
- Weidner, E.; Knez, Z.; Novak, Z. Process for the production of particles or powders. 1997. Available online: https://patents.google.com/patent/US6056791A/en (accessed on 22 September 2020).
- Mattea, F.; Martín, Á.; Cocero, M.J. Carotenoid processing with supercritical fluids. J. Food Eng. 2009, 93, 255–265. [Google Scholar] [CrossRef]
- Esfandiari, N. Production of micro and nano particles of pharmaceutical by supercritical carbon dioxide. J. Supercrit. Fluids 2015, 100, 129–141. [Google Scholar] [CrossRef]
- Wu, J.J.; Shen, L.Y.; Yin, M.C.; Cheng, Y.S. Supercritical carbon dioxide anti-solvent micronization of lycopene extracted and chromatographic purified from Momordica charantia L. aril. J. Taiwan Inst. Chem. Eng. 2017, 80, 64–70. [Google Scholar] [CrossRef]
- Badens, E.; Magnan, C.; Charbit, G. Microparticles of soy lecithin formed by supercritical processes. Biotechnol. Bioeng. 2001, 72, 194–204. [Google Scholar] [CrossRef]
- Prosapio, V.; De Marco, I.; Reverchon, E. Supercritical antisolvent coprecipitation mechanisms. J. Supercrit. Fluids 2018, 138, 247–258. [Google Scholar] [CrossRef]
- Guamán-Balcázar, M.C.; Montes, A.; Pereyra, C.; de la Ossa, E.M. Precipitation of mango leaves antioxidants by supercritical antisolvent process. J. Supercrit. Fluids 2017, 128, 218–226. [Google Scholar] [CrossRef]
- Magnan, C.; Badens, E.; Commenges, N.; Charbit, G. Soy lecithin micronization by precipitation with a compressed fluid antisolvent—Influence of process parameters. J. Supercrit. Fluids 2000, 19, 69–77. [Google Scholar] [CrossRef]
- Chinnarasu, C.; Montes, A.; Ponce, M.T.F.; Casas, L.; Mantell, C.; Pereyra, C.; De La Ossa, E.J.M. Precipitation of antioxidant fine particles from Olea europaea leaves using supercritical antisolvent process. J. Supercrit. Fluids 2015, 97, 125–132. [Google Scholar] [CrossRef]
- Badens, E.; Masmoudi, Y.; Mouahid, A.; Crampon, C. Current situation and perspectives in drug formulation by using supercritical fluid technology. J. Supercrit. Fluids 2018, 134, 274–283. [Google Scholar] [CrossRef]
- Chong, G.H.; Yunus, R.; Choong, T.S.Y.; Abdullah, N.; Spotar, S.Y. Simple guidelines for a self-built laboratory-scale supercritical anti-solvent system. J. Supercrit. Fluids 2011, 60, 69–74. [Google Scholar] [CrossRef]
- Zhao, Z.; Xie, M.; Li, Y.; Chen, A.; Li, G.; Zhang, J.; Hu, H.; Wang, X.; Li, S. Formation of curcumin nanoparticles via solution-enhanced dispersion by supercritical CO2. Int. J. Nanomed. 2015, 10, 3171–3181. [Google Scholar] [CrossRef]
- Nerome, H.; Machmudah, S.; Fukuzato, R.; Higashiura, T.; Youn, Y.S.; Lee, Y.W.; Goto, M. Nanoparticle formation of lycopene/β-cyclodextrin inclusion complex using supercritical antisolvent precipitation. J. Supercrit. Fluids 2013, 83, 97–103. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R.; Campardelli, R.; Della Porta, G.; De Marco, I.; Scognamiglio, M. Supercritical fluids based techniques to process pharmaceutical products difficult to micronize: Palmitoylethanolamide. J. Supercrit. Fluids 2015, 102, 24–31. [Google Scholar] [CrossRef]
- Prieto, C.; Calvo, L. Supercritical fluid extraction of emulsions to nanoencapsulate vitamin E in polycaprolactone. J. Supercrit. Fluids 2017, 119, 274–282. [Google Scholar] [CrossRef]
- Kluge, J.; Fusaro, F.; Casas, N.; Mazzotti, M.; Muhrer, G. Production of PLGA micro- and nanocomposites by supercritical fluid extraction of emulsions: I. Encapsulation of lysozyme. J. Supercrit. Fluids 2009, 50, 327–335. [Google Scholar] [CrossRef]
- Ajiboye, A.L.; Trivedi, V.; Mitchell, J.C. Preparation of polycaprolactone nanoparticles via supercritical carbon dioxide extraction of emulsions. Drug Deliv. Transl. Res. 2018, 8, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Pasquel Reátegui, J.L.; Fernandes, F.P.; dos Santos, P.; Rezende, C.A.; Sartoratto, A.; Queiroga, C.L.; Martínez, J. Production of copaiba (Copaifera officinalis) oleoresin particles by supercritical fluid extraction of emulsions. J. Supercrit. Fluids 2018, 140, 364–371. [Google Scholar] [CrossRef]
- Wang, T.; Deng, Y.; Geng, Y.; Gao, Z.; Zou, J.; Wang, Z. Preparation of submicron unilamellar liposomes by freeze-drying double emulsions. Biochim. Biophys. Acta-Biomembr. 2006, 1758, 222–231. [Google Scholar] [CrossRef]
- Diab, R.; Jaafar-Maalej, C.; Fessi, H.; Maincent, P. Engineered nanoparticulate drug delivery systems: The next frontier for oral administration. AAPS J. 2012, 14, 688–702. [Google Scholar] [CrossRef][Green Version]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J.Y. Emerging Research and Clinical Development Trends of Liposome and Lipid Nanoparticle Drug Delivery Systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef]
- Santo, I.E.; Campardelli, R.; Albuquerque, E.C.; Vieira De Melo, S.A.B.; Reverchon, E.; Porta, G. Della Liposomes Size Engineering by Combination of Ethanol Injection and Supercritical Processing. J. Pharm. Sci. 2015, 104, 3842–3850. [Google Scholar] [CrossRef]
- Coenen, H.; Reimann, K.; Hagen, R. Verfahren und Vorrichtung zur Gewinnung fester Stoffe aus flüssigen Stoffgemischen. 1987. Available online: https://patents.google.com/patent/DE3536622A1/de (accessed on 22 September 2020).
- Solidaridadnetwork. China’s Soy Crushing Industry Impacts on the Global Sustainability Agenda; Isabel Nepstad: Beijing, China, 2017. [Google Scholar]
- NATECO2. Available online: https://www.nateco2.de/en/ (accessed on 30 August 2020).
- Unilever Breakthrough Innovation for Spreads. Available online: https://www.unilever.com/news/news-and-features/Feature-article/2012/breakthrough-innovation-for-spreads.html (accessed on 31 August 2020).
- Seifried PGX Technology. Available online: https://www.ceapro.com/technologies/pgx (accessed on 31 August 2020).
- Liu, N.; Nguyen, H.; Wismer, W.; Temelli, F. Development of an orange-flavoured functional beverage formulated with beta-glucan and coenzyme Q10-impregnated beta-glucan. J. Funct. Foods 2018, 47, 397–404. [Google Scholar] [CrossRef]
- Couto, R.; Seifried, B.; Yépez, B.; Moquin, P.; Temelli, F. Adsorptive precipitation of co-enzyme Q10 on PGX-processed β-glucan powder. J. Supercrit. Fluids 2018, 141, 157–165. [Google Scholar] [CrossRef]
- De Paz, E.; Rodríguez, S.; Kluge, J.; Martín, Á.; Mazzotti, M.; Cocero, M.J. Solubility of β-carotene in poly-(ε-caprolactone) particles produced in colloidal state by Supercritical Fluid Extraction of Emulsions (SFEE). J. Supercrit. Fluids 2013, 84, 105–112. [Google Scholar] [CrossRef]
- Kluge, J.; Mazzotti, M.; Muhrer, G. Solubility of Ketoprofen in colloidal PLGA. Int. J. Pharm. 2010, 399, 163–172. [Google Scholar] [CrossRef] [PubMed]
| 1 | Poly(hydroxybutirate-co-hydroxyvalerate) |

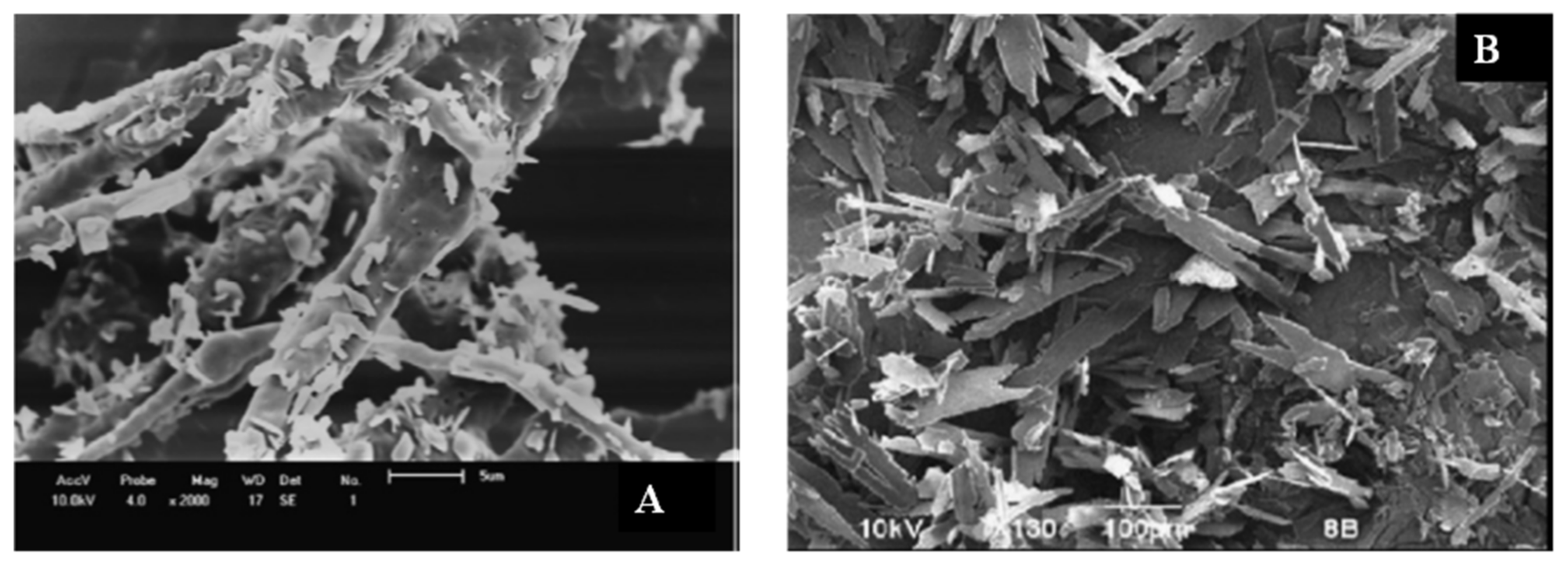
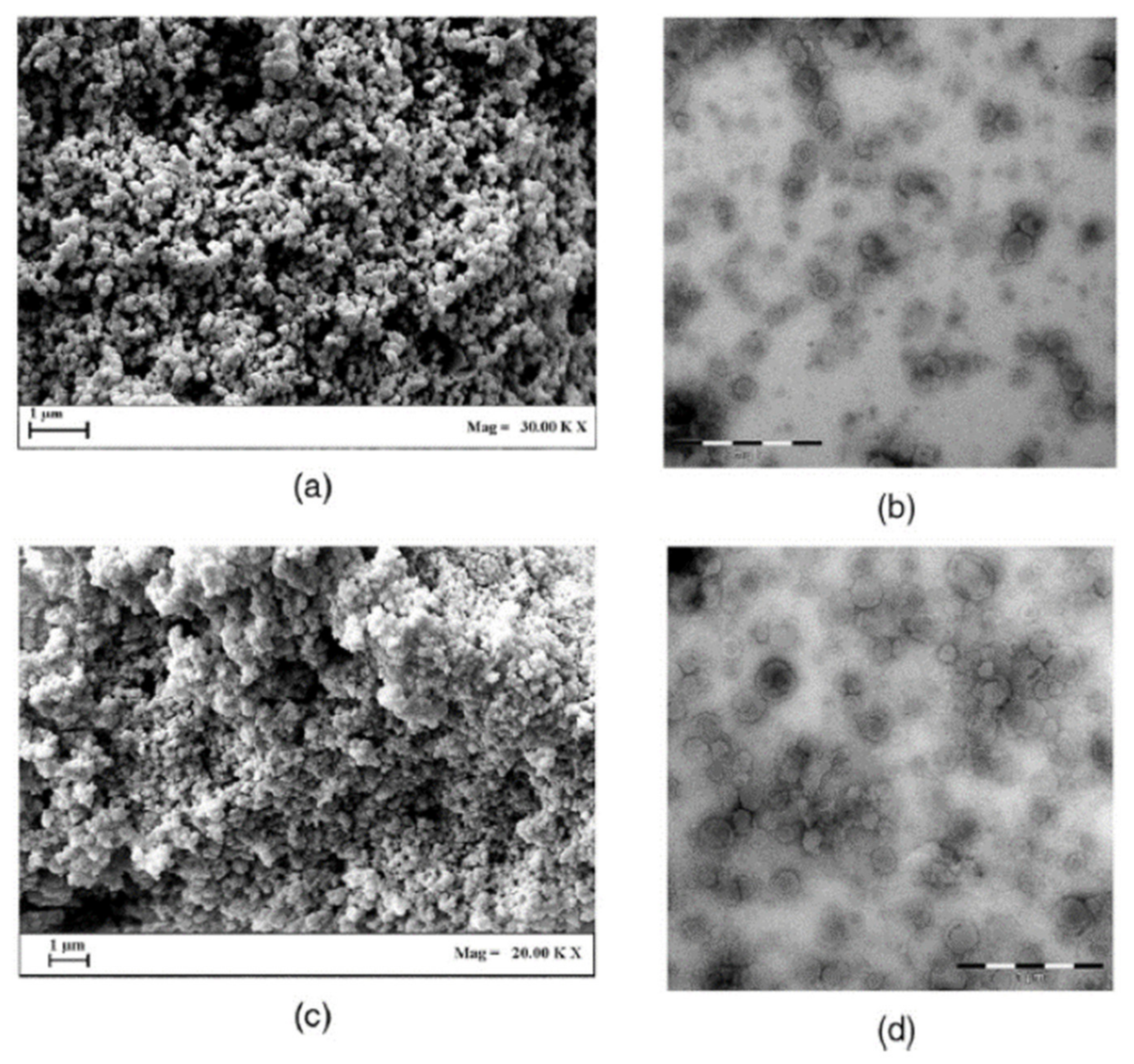

| Bioactive Compound | Basic Structure | Reference |
|---|---|---|
| Curcumin | 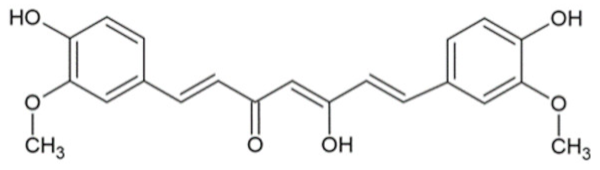 | [28,29] |
| β-carotene |  | [30] |
| Astaxanthin | 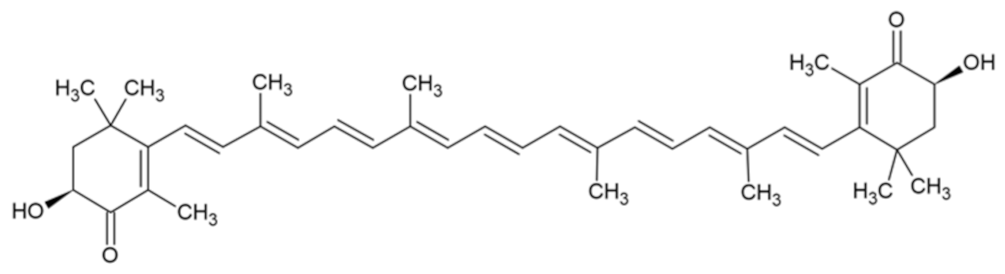 | [31] |
| Fucoxanthin | 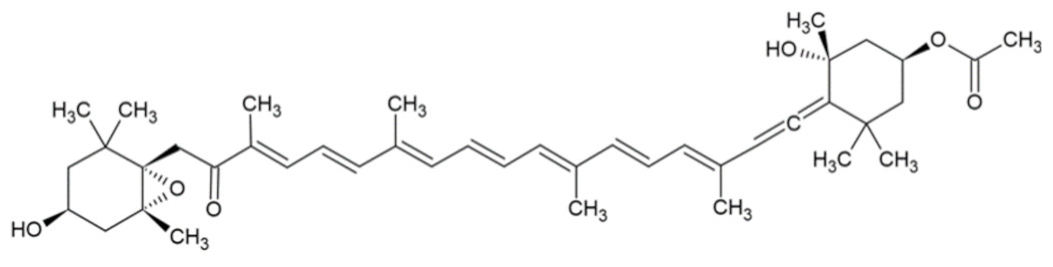 | [32] |
| Theophylline | 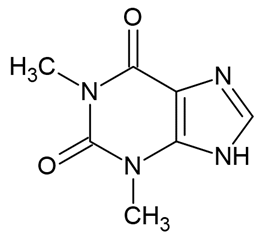 | [33] |
| Caffeine | 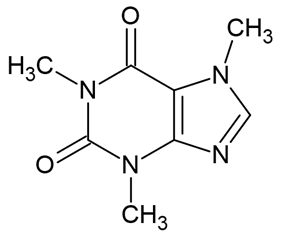 | [34,35] |
| Vitamin B2 |  | [36] |
| Vitamin A | 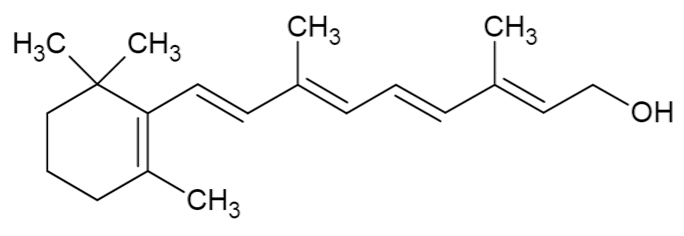 | [37] |
| Linalool | 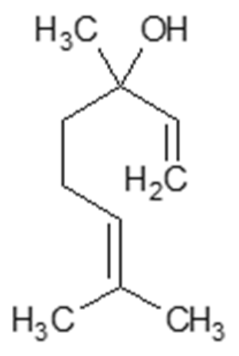 | [38,39] |
| Coenzyme-Q10 | 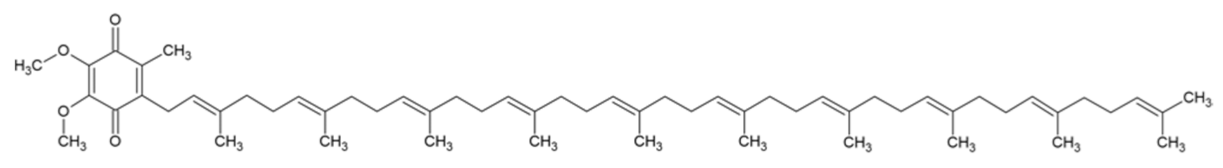 | [40,41] |
| Omega-3 PUFAs1 | 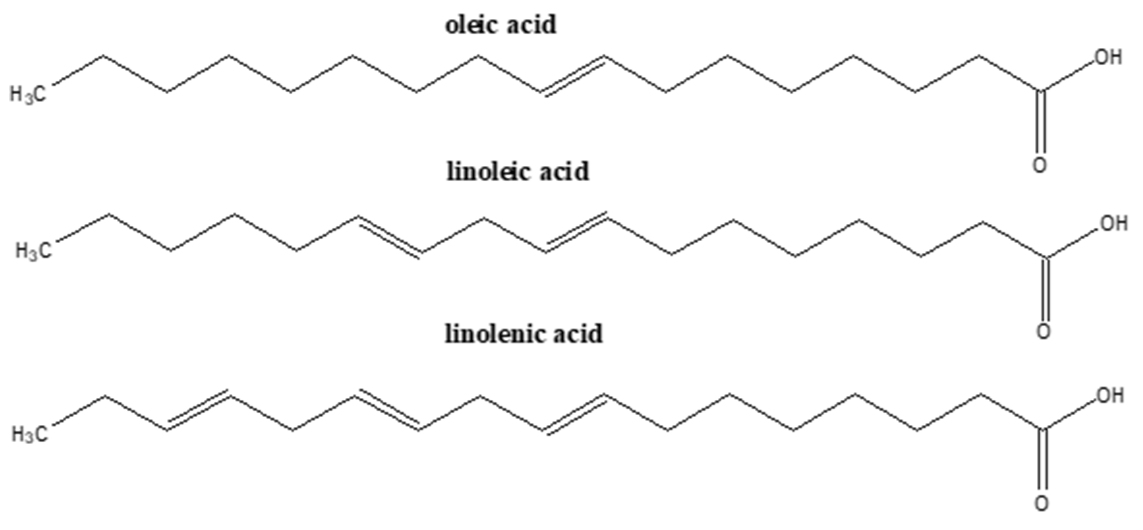 | [31,42,43] |
| Eugenol | 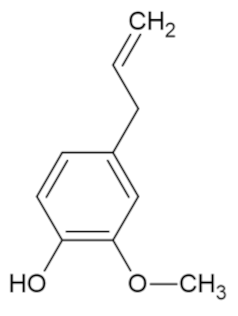 | [44] |
| Thymol | 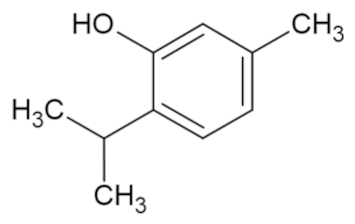 | [44] |
| Technology | Bioactive Compound | Carrier Material | Process Conditions | Encapsulation Efficiency | Reference |
|---|---|---|---|---|---|
| PGSS 1 | Theophylline | Hydrogenated palm oil | Pressure: 12–18 MPa Temperature: 60 °C | 0.5–3.5% | [33] |
| PGSS 1 | Caffeine | Glyceryl monostearate | Pressure: 13 MPa Temperature: 62 °C Time: 1 h | 140 mg/g | [34] |
| PGSS 1 | No bioactive | Rapeseed 70 | Pressure: 7–18 MPa Temperature: 60–100 °C | - 9 | [56] |
| PGSS 1 | Anthocyanin concentrates from grape residues | Starch and silica | Pressure: 10–18 MPa Temperature: 25 °C | - 9 | [57] |
| PGSS 1 | Caffeine, Glutathione, Ketoprofen, silanized TiO2 | Glyceryl monostearate, Hydrogenated castor oil | Pressure: 13 MPa Temperature: 72 °C Time: 1 h | - 9 | [35] |
| PGSS 1 | Lavandin essential oil | Polyethylene glycol | Pressure: 5.4–8.5 MPa Temperature: 76–84 °C | 14–66% | [38] |
| PGSS 1 | Cydia pomonella granulovirus | Palm oil-based fat and lecithin-based surfactant | Pressure: 10 MPa Temperature: 65 °C | - 9 | [58] |
| PGSS 1 | Co-enzyme Q10 | Polyethylene glycol | Pressure: 10–25 MPa Temperature: 75–80 °C Time: 30 min | - 9 | [40] |
| PGSS 1 | Garlic essential oil | Polyethylene glycol | Pressure: 15.76–20.34 MPa Temperature: 50–62 °C | 26.10–48.93% | [59] |
| PGSS 1 | Mackerel lecithin | Polyethylene glycol | Pressure: 15–30 MPa Temperature: 40–50 °C Time: 1 h Stirring speed: 250 rpm | - 9 | [60] |
| PGSS 1 | β-carotene | Poly-(ε-caprolactone) | Pressure: 11 and 15 MPa Temperature: 50–70 °C Time: 240 min | 306–336 ppm | [30] |
| PGSS 1 | Linalool and lavandin essential oil | Poly-(ε-caprolactone) | Pressure: 6–11 MPa Temperature: 50–70 °C Time: 2 h | 11–50% for linalool 13–45% for lavandin oil | [39] |
| PGSS 1 | Squid lecithin | Polyethylene glycol | Pressure: 20–30 MPa Temperature: 40–50 °C Stirring speed: 200–400 rpm Time: 1 h | - 9 | [61] |
| PGSS 1 | Hydroxytyrosol-rich concentrate | Glycerol monostearate | Pressure: 13 MPa Temperature: 62 °C Time: 30 min | - 9 | [62] |
| PGSS 1 | Curcuminoids extract | Polyethylene glycol | Pressure: 16 MPa Temperature: 50 °C Time: 120 min | - 9 | [63] |
| PGSS 1 | Wheat germ oil | Polyethylene glycol | Pressure: 10–30 MPa Temperature: 40–50 °C Time: 1 h | - 9 | [64] |
| CO2-expanded lipid mixture | Peppermint essential oil | Fully hydrogenated soybean oil | Pressure: 20 MPa Temperature: 57 °C Stirring speed: 1000 rpm | 39–47.5% | [65] |
| PGSS 1 | Mackerel reaction oil | Polyethylene glycol | Pressure: 10, 15, 20 MPa Temperature: 45–55 °C | - 99 | [66] |
| PGSS 1 | Coffee oil flavor | Polyethylene glycol | Pressure: 20–30 MPa Temperature: 40–50 °C Stirring speed: 300 rpm Time: 1 h | 79.78% | [67] |
| PGSS 1 | Anthocyanins from Elderberry (Sambucus nigra) | Palm fat | Pressure: 10 MPa Temperature: 60 °C Time: 2 h | - 9 | [68] |
| PGSS 1 | Spearmint essential oil | Fully hydrogenated canola oil | Pressure: 12.2 MPa Temperature: 60 °C Stirring speed: 20 Hz Time: 1 h | 96% | [69] |
| PGSS 1 | Menthol | Beeswax | Pressure: 6–20 MPa Temperature: 60 °C | 60% | [70] |
| Modified PGSS | Vitamin B2 | Fully Hydrogenated canola oil | Pressure: 10–25 MPa Temperature: 65 °C Time: 1 h | 12–48% | [36] |
| PGSS 1 | Citrus oil | Polyethylene glycol | Pressure: 20–40 MPa Temperature: 40–50 °C Stirring speed: 400 rpm Time: 1 h | 43.95–83.87% | [71] |
| PGSS 1 | Omega-3 PUFAs and astaxanthin-rich salmon oil | Polyethylene glycol | Pressure: 15–25 MPa Temperature: 45–55 °C Time: 1 h | 62.19–79.20% | [31] |
| PGSS 1 | Fucoxanthin-rich oil | Polyethylene glycol | Pressure: 10–30 MPa Temperature: 45–65 °C Stirring speed: 400 rpm Time: 1 h | 62.41–81.85% | [32] |
| PGSS 1 | Eucalyptol | Polyethylene glycol Polycaprolactone | Pressure: 8 MPa Temperature: 45 °C Stirring speed: 150 rpm Time: 1 h | 60.69–77.36% | [72] |
| PGSS 1 | Limonene | Modified starches | Pressure: 10–12 MPa Temperature: 50–60 °C Stirring speed: 1250 rpm Time: 45 min | 86% | [73] |
| PGSS 1 | Nimodipine Fenofibrate o-vanillin | Polyethylene glycol Polyoxyethylene stearyl ether | Pressure: 10–25 MPa Temperature: 45–60 °C Time: 1 h | Nimodipine: 59.7–98.82 Fenofibrate: 67–93.67% o-vanillin: 68.78–99.31 | [74] |
| PGSS 1 | Brewer’s spent grain oil | Polyethylene glycol | Pressure: 10–20–30–35 MPa Temperature: 45–55 °C Time: 1 h | 73.5% | [75] |
| PGSS-drying | Green tea extracts | - 9 | Pressure: 5.9–10 MPa Pre-expansion temperature: 130 °C | - 9 | [76] |
| PGSS-drying | Lavandin oil | Modified OSA-starch from waxy maize | Pressure: 9–12.4 MPa Pre-expansion temperature: 100–131 °C Spray tower temperature: 60–75 °C | 6–55% | [38] |
| PGSS-drying | Lavandin essential oil | Soybean lecithin | Pressure: 6–10.3 MPa Pre-expansion temperature: 103–128 °C Spray tower temperature: 38–52 °C | 6–14.5% | [77] |
| PGSS-drying | β-carotene | Soybean lecithin | Pressure: 8.1–10.3 MPa Pre-expansion temperature: 102–132 °C Spray tower temperature: 55 °C Time: 60 min | 29–58% | [78] |
| PGSS-drying | Fish oil | Chitosan, Maltodextrin | Pressure: 11–25.7 MPa Spray tower temperature: 64–119 °C | - 9 | [79] |
| PGSS-drying | Resveratrol | β-glucans Soy-bean lecithin | Pressure: 9.5 MPa Pre-expansion temperature: 125 °C Spray tower temperature: 65–70 °C | - 9 | [80] |
| PGSS-drying | Epigallocatechin gallate | Octenyl-succinic-anhydride modified starch Soybean lecithin β-glucan (Glucagel™) | Pressure: 9.5 MPa Pre-expansion temperature: 124.85 °C Spray tower temperature: 69.85 °C | OSA-starch: 80.5% Lecithin: 75.8% β-glucan: 77.4% | [81] |
| PGSS-drying | Quercetin | Poly-(ethylene glycol)-block-poly-(propylene glycol)-block- poly-(ethylene glycol) Soy-bean lecithin | Pressure: 7.68–11.77 MPa Pre-expansion temperature: 109.4–132.5 °C Spray tower temperature: 64.7–75.1 °C | - 9 | [82] |
| PGSS-drying | Omega-3 | Octenyl-succinic-anhydride modified starch | Pressure: 10 MPa Pre-expansion temperature: 110 °C Spray tower temperature: 55 °C | 97.9% | [83] |
| PGSS-drying | Rice bran oil | Pea protein isolate (PPI) and Maltodextrin (MD) | Pressure: 10 MPa Pre-expansion temperature: 105 °C Spray tower temperature: 55 °C | 53% | [84] |
| PGX 2 | Co-enzyme Q10 | β-glucan | Pressure: 10–30 MPa Temperature: 32–50 °C | - 9 | [85,86] |
| RESS 3 | Glass beads | Stearyl alcohol (1-Octadecanol) | Pressure: 8 MPa Temperature: 55 °C | - 9 | [87] |
| RESS 3 | Anthocyanin extract obtained from jabuticaba (Myrciaria cauliflora) skins | Polyethylene glycol | Pressure: 10–35 MPa Temperature: 40–50 °C Time: 30 min | 79.78% | [88] |
| RESS 3 | Rose essential oil | Phosphatidylcholine and cholesterol | Pressure: 20–30 MPa Temperature: 60–70 °C Time: 2 h | 73.16–90.28% | [89] |
| RESS 3 | Essential oil of Atractylodes macrocephala Koidz | Phosphatidylcholine and cholesterol | Pressure: 15–30 MPa Temperature: 65 °C Time: 1 h | 82.18% | [90] |
| RESS 3 | Curcuma Longa L. extracts | - 9 | Pressure: 8–35 MPa Temperature: 50 °C Time: 10–30 min | - 9 | [91] |
| RESS 3 | Rutin and anthocyanin-rich extract | Polyethylene glycol | Pressure: 20 MPa Temperature: 40 °C Time: 30 min | 44.2% | [92] |
| RESS-N | Lysozyme and lipase | Polyethylene glycol Poly(methyl methacrylate) Poly(L-lactic acid) Poly(DL-lactide-co-glycolide) | Pressure: 20 MPa Temperature: 35 °C | - 9 | [93] |
| SAS 4 | Bixin (annatto seed extract) | Polyethylene glycol | Pressure: 10 MPa Temperature: 40 °C | 62% | [92] |
| SAS 4 | Lutein | Polylactic acid | Pressure: 8–10 MPa Temperature: 35–45 °C | 90% | [94] |
| SAS 4 | Lutein | Hydroxypropylmethyl cellulose phthalate | Pressure: 11–15 MPa Temperature:40–50 °C | 88.41% | [95] |
| SAS 4 | Lutein β-carotene | Polyethylene glycol | Pressure: 8–10 MPa Temperature: 15 °C | - 9 | [96] |
| SAS 4 | Lutein | Hydrogenated phosphatidylcholine | Pressure: 8–16 MPa Temperature: 35–55 °C | >90% | [97] |
| SAS 4 | Vitamin D3 | Hydrogenated phosphatidylcholine | Pressure: 8–12 MPa Temperature: 35–55 °C | 98% | [98] |
| SAS 4 | Polyphenols (green tea extract) | Poly(epsilon-caprolactone) | Pressure: 8–12 MPa Temperature: 11–34 °C | - 9 | [99] |
| SAS 4 | Rosemary antioxidants | Pluronic® F 88Pluronic® F 127 | Pressure: 8–10 MPa Temperature: 25–50 °C | 100% | [53] |
| SAS 4 | Astaxanthin (Shrimp extract) | Pluronic® F 127 | Pressure: 10–12 MPa Temperature: 35–40 °C | 74% | [100] |
| SAS 4 | Quercetin | Pluronic® F 127 | Pressure: 10 MPa Temperature: 40 °C | 35–56% | [101] |
| SAS 4 | Passion fruit seeds oil | Poly(lactic-co-glycolic) acid | Pressure: 9–11 MPa Temperatures: 35 and 45 °C | 67.8–91% | [102] |
| GAS 5 | Rosemary extract | Polycaprolactone | Pressure: 20–30 MPa Temperature: 40 °C | 82.8% | [54] |
| Supercritical impregnation | Lycopene | Hydrolysed collagen | Pressure: 15–25 MPa Temperature: 50–60 °C | 84–94% | [103] |
| Supercritical impregnation | Lavandin essential oil | Modified OSA starch derived from waxy maize | Pressure: 10–12 MPa Temperature: 40–50 °C Time: 2 h | Lavandin oil: 22% Linalool: 22% Linalyl acetate: 51% | [104] |
| SEDS 6 | Lutein | Zein | Pressure: 10–15 MPa Temperature: 32–45 °C | 34.44–83.15% | [55] |
| SEDS 6 | β-Carotene | Poly(hydroxybutirate-co-hydroxyvalerate) | Pressure: 8 MPa Temperature: 40 °C | 7.75–55.54% | [105] |
| SEDS 6 | β-Carotene | Poly(hydroxybutirate-co-hydroxyvalerate) | Pressure: 8–12 MPa Temperature: 30–70 °C | 80% | [106] |
| SEDS 6 | Grape seed extract | Poly(hydroxybutirate-co-hydroxyvalerate) | Pressure: 8–12 MPa Temperature: 35–45 °C | 66.01% | [107] |
| SEDS 6 | Pink pepper extract (PPE) | Poly(hydroxybutirate-co-hydroxyvalerate) | Pressure: 8–12.5 MPa Temperature: 35–55 °C | 20.2–95.1% | [108] |
| SEDS 6 | Astaxanthin | Poly(hydroxybutirate-co-hydroxyvalerate) | Pressure: 8–10 MPa Temperature: 35 °C | 20.93–48.25% | [109] |
| SEDS 6 | Puerarin | Poly(L-lactide) | Pressure: 12 MPa Temperature: 33 °C | 39.4% | [110] |
| SFEE 7 | Lysozyme | Poly(lactic-co-glycolic) Calcium carbonate | Pressure: 8–10 MPa Temperature: 33–40 °C Mixing speed: 1500 rpm Time: 25 min | 60% | [111] |
| SFEE 7 | Astaxanthin (Shrimp extract) | Pluronic® F 127 | Pressure: 10 MPa Temperature: 40 °C | 93% | [100] |
| SFEE 7 | Oleoresin of Capsicum frutescens pepper | Hi-Cap 100 modified starch | Pressure: 9–11 MPa Temperature: 40 °C | 100% | [112] |
| SFEE 7 | Low viscosity omega-3 rich fish oil | Polycaprolactone | Pressure: 8 MPa Temperature: 40 °C | 12–43% | [113] |
| SFEE 7 | β-caroteneLycopene | Octenyl succinyl modified starch | Pressure: 9–13 MPa Temperature: 80 °C | 34–89% | [114] |
| SFEE 7 | Quercetin | Poly-(ethylene glycol)-block-poly-(propylene glycol)-block- poly-(ethylene glycol)Soy-bean lecithin | Pressure: 7.91–10.48 MPa Temperature: 34.6–40.3 °C Time: 75–104 min Mixing speed: 1500 rpm | 80.1–98.5% | [82] |
| SuperLip 8 | Phospholipids | Soy lecithin | Pressure: 30 MPa Temperature: 40–50 °C Time: 60 min | -9 | [115] |
| SuperLip 8 | Lutein | Soy lecithin | Pressure: 3–30 MPa Temperature: 40–65 °C Mixing speed: 550 rpm Time: 60 min | 56.7–97.0% | [46] |
| SuperLip 8 | Anthocyanin | Soy lecithin | Pressure: 30 MPa Temperature: 50 °C Mixing speed: 550 rpm Time: 60 min | 50.6% | [116] |
| SuperLip 8 | BSA | Soybean phosphatidylcholine and phosphatidyl glycerol | Pressure: 12.5–17.5 MPa Temperature: 40–70 °C Mixing speed: 400 rpm Time: 30 min | 92–98% | [117] |
| SuperLip 8 | Eugenol α-lipoic acid | Lipids and lipophilic compounds | Pressure: 10 MPa Temperature: 35–40 °C | 68.1–94.2% | [118] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klettenhammer, S.; Ferrentino, G.; Morozova, K.; Scampicchio, M. Novel Technologies Based on Supercritical Fluids for the Encapsulation of Food Grade Bioactive Compounds. Foods 2020, 9, 1395. https://doi.org/10.3390/foods9101395
Klettenhammer S, Ferrentino G, Morozova K, Scampicchio M. Novel Technologies Based on Supercritical Fluids for the Encapsulation of Food Grade Bioactive Compounds. Foods. 2020; 9(10):1395. https://doi.org/10.3390/foods9101395
Chicago/Turabian StyleKlettenhammer, Stefan, Giovanna Ferrentino, Ksenia Morozova, and Matteo Scampicchio. 2020. "Novel Technologies Based on Supercritical Fluids for the Encapsulation of Food Grade Bioactive Compounds" Foods 9, no. 10: 1395. https://doi.org/10.3390/foods9101395
APA StyleKlettenhammer, S., Ferrentino, G., Morozova, K., & Scampicchio, M. (2020). Novel Technologies Based on Supercritical Fluids for the Encapsulation of Food Grade Bioactive Compounds. Foods, 9(10), 1395. https://doi.org/10.3390/foods9101395








