Viability-PCR Allows Monitoring Yeast Population Dynamics in Mixed Fermentations Including Viable but Non-Culturable Yeasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Fermentation Procedure
2.3. Population Dynamics by Plating
2.4. Population Dynamics by qPCR
2.4.1. Living/Dead Cell Amplification: Heat Shock and PMAxx Treatment
2.4.2. PMAxx Treatment of Samples from Fermentation
2.4.3. DNA Extraction and qPCR Analysis
2.4.4. Limit of Detection and Quantification by PMAxx-qPCR
2.5. Statistical Analysis
3. Results
3.1. Optimization of PMAxx-qPCR for Yeast Viability Determination
3.2. Monitoring Yeast Population Dynamics of Mixed Fermentation and Differentiation of VBNC Yeasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Target Species | Primer Name | Sequence 5′-3′ | Reference |
|---|---|---|---|
| S. cerevisiae | CESP-F | ATCGAATTTTTGAACGCACATTG | Hierro et al. [61] |
| SCER-R | CGCAGAGAAACCTCTCTTTGGA | ||
| T. delbrueckii | Tods L2 | CAAAGTCATCCAAGCCAGC | García et al. [36] |
| Tods R2 | TTCTCAAACAATCATGTTTGGTAG | ||
| L. thermotolerans | LTH2-F | CGCTCCTTGTGGGTGGGGAT | García et al. [36] |
| LTH2-R | CTGGGCTATAACGCTTCTCC | ||
| M. pulcherrima | MP2-F | AGACACTTAACTGGGCCAGC | García et al. [36] |
| MP2-R | GGGGTGGTGTGGAAGTAAGG | ||
| Total yeast | YEAST-F | GAGTCGAGTTGTTTGGGAATGC | Hierro et al. [34] |
| YEAST-R | TCTCTTTCCAAAGTTCTTTTCATCTTT |
| Living/Dead Mixed Cultures | |||
|---|---|---|---|
| Living Yeast (evaluated concentrations) | Dead Yeast | LoQ (Ct ± SD) | LoD (Ct ± SD) |
| Sc (104 to 10 CFU/mL) | Sc (106 CFU/mL) | 103 (27.907 ± 0.124) | 102 (30.841 ± 0.468) |
| Td (104 to 10 CFU/mL) | Td (106 CFU/mL) | 103 (28.670 ± 0.139) | 102 (30.817 ± 0.273) |
| Lt (104 to 10 CFU/mL) | Lt (106 CFU/mL) | 103 (29.594 ± 0.180) | 102 (32.255 ± 0.483) |
| Mp (104 to 10 CFU/mL) | Mp (106 CFU/mL) | 104 (28.570 ± 0.190) | 103 (32.030 ± 0.940) |
| Living/Living Mixed Cultures | |||
| Living Yeast (evaluated concentrations) | Living Yeast | LoQ (Ct ± SD) | LoD (Ct ± SD) |
| Sc (104 to 10 CFU/mL) | Td (107 CFUs/mL) | 103 (28.057 ± 0.089) | 102 (30.992 ± 0.207) |
| Td (104 to 10 CFU/mL) | Sc (107 CFUs/mL) | 103 (29.922 ± 0.387) | 102 (32.121 ± 0.185) |
| Lt (104 to 10 CFU/mL) | Sc (107 CFUs/mL) | 103 (29.692 ± 0.280) | 102 (31.995 ± 0.759) |
| Mp (104 to 10 CFU/mL) | Sc (107 CFUs/mL) | 104 (29.597 ± 0.495) | 103 (31.173 ± 1.799) |
References
- Liu, P.T.; Lu, L.; Duan, C.Q.; Yan, G.L. The contribution of indigenous non-Saccharomyces wine yeast to improved aromatic quality of Cabernet Sauvignon wines by spontaneous fermentation. LWT Food Sci. Technol. 2016, 71, 356–363. [Google Scholar] [CrossRef]
- Jolly, N.P.; Augustyn, O.P.H.; Pretorius, I.S. The effect of Non-Saccharomyces yeasts on fermentation and wine quality. S. Afr. J. Enol. Vitic. 2003, 24, 55–62. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Wine yeasts for the future. FEMS Yeast Res. 2008, 8, 979–995. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef]
- Clemente-Jimenez, J.M.; Mingorance-Cazorla, L.; Martínez-Rodríguez, S.; Las Heras-Vázquez, F.J.; Rodríguez-Vico, F. Influence of sequential yeast mixtures on wine fermentation. Int. J. Food Microbiol. 2005, 98, 301–308. [Google Scholar] [CrossRef]
- Varela, J.; Varela, C. Microbiological strategies to produce beer and wine with reduced ethanol concentration. Curr. Opin. Biotechnol. 2019, 56, 88–96. [Google Scholar] [CrossRef]
- Ciani, M.; Morales, P.; Comitini, F.; Tronchoni, J.; Canonico, L.; Curiel, J.A.; Oro, L.; Rodrigues, A.J.; Gonzalez, R. Non-conventional yeast species for lowering ethanol content of wines. Front. Microbiol. 2016, 7, 642. [Google Scholar] [CrossRef]
- Quirós, M.; Rojas, V.; Gonzalez, R.; Morales, P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food Microbiol. 2014, 181, 85–91. [Google Scholar] [CrossRef]
- Fleet, G.H.; Lafon-Lafourcade, S.; Ribereau-Gayon, P. Evolution of yeasts and lactic acid bacteria during fermentation and storage of Bordeaux wines. Appl. Environ. Microbiol. 1984, 48, 1034–1038. [Google Scholar] [CrossRef]
- Loureiro, V.; Malfeito-Ferreira, M. Spoilage yeasts in the wine industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F. Yeast interactions in multi-starter wine fermentation. Curr. Opin. Food Sci. 2015, 1, 1–6. [Google Scholar] [CrossRef]
- Binati, R.L.; Lemos Junior, W.J.F.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Comitini, F.; Ciani, M. Metschnikowia pulcherrima selected strain for ethanol reduction in wine: Influence of cell immobilization and aeration condition. Foods 2019, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Hidalgo, C.; Schmidt, S.; Henschke, P.A.; Curtin, C.; Varela, C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 2015, 205, 7–15. [Google Scholar] [CrossRef]
- Puškaš, V.S.; Miljić, U.D.; Djuran, J.J.; Vučurović, V.M. The aptitude of commercial yeast strains for lowering the ethanol content of wine. Food Sci. Nutr. 2020, 8, 1489–1498. [Google Scholar] [CrossRef]
- Zhu, X.; Navarro, Y.; Mas, A.; Torija, M.J.; Beltran, G. A rapid method for sSelecting Non-Saccharomyces strains with a lLow ethanol yield. Microorganism 2020, 8, 658. [Google Scholar] [CrossRef]
- Ruiz, J.; Belda, I.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Santos, A.; Benito, S. Analytical impact of Metschnikowia pulcherrima in the volatile profile of Verdejo white wines. Appl. Microbiol. Biotechnol. 2018, 102, 8501–8509. [Google Scholar] [CrossRef]
- Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Bely, M.; Stoeckle, P.; Masneuf-Pomarède, I.; Dubourdieu, D. Impact of mixed Torulaspora delbrueckii-Saccharomyces cerevisiae culture on high-sugar fermentation. Int. J. Food Microbiol. 2008, 122, 312–320. [Google Scholar] [CrossRef] [PubMed]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.M.; Zamora, F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2015, 240, 999–1012. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology: Volume 1, The Microbiology of Wine and Vinifications; John Wiley & Sons Ltd.: West Sussex, UK, 2006; ISBN 0-470-01034-7. [Google Scholar]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. The interaction between Saccharomyces cerevisiae and non-Saccharomyces yeast during alcoholic fermentation is species and strain specific. Front. Microbiol. 2016, 7, 502. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; García-Fernández, D.; González, B.; Izidoro, I.; Esteve-Zarzoso, B.; Beltran, G.; Mas, A. Yeast biodiversity from DOQ priorat uninoculated fermentations. Front. Microbiol. 2016, 7, 930. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.; Viana, T.; Albergaria, H.; Arneborg, N. Antimicrobial peptides (AMPs) produced by Saccharomyces cerevisiae induce alterations in the intracellular pH, membrane permeability and culturability of Hanseniaspora guilliermondii cells. Int. J. Food Microbiol. 2015, 205, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Albergaria, H.; Arneborg, N. Dominance of Saccharomyces cerevisiae in alcoholic fermentation processes: Role of physiological fitness and microbial interactions. Appl. Microbiol. Biotechnol. 2016, 100, 2035–2046. [Google Scholar] [CrossRef]
- Pérez-Nevado, F.; Albergaria, H.; Hogg, T.; Girio, F. Cellular death of two non-Saccharomyces wine-related yeasts during mixed fermentations with Saccharomyces cerevisiae. Int. J. Food Microbiol. 2006, 108, 336–345. [Google Scholar] [CrossRef]
- Nissen, P.; Nielsen, D.; Arneborg, N. Viable Saccharomyces cerevisiae cells at high concentrations cause early growth arrest of non-Saccharomyces yeasts in mixed cultures by a cell—cell contact-mediated mechanism. Yeast 2003, 20, 331–341. [Google Scholar] [CrossRef]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. Interaction between Hanseniaspora uvarum and Saccharomyces cerevisiae during alcoholic fermentation. Int. J. Food Microbiol. 2015, 206, 67–74. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Giacosa, S.; Río Segade, S.; Rolle, L.; Cocolin, L. Cell-to-cell contact mechanism modulates Starmerella bacillaris death in mixed culture fermentations with Saccharomyces cerevisiae. Int. J. Food Microbiol. 2019, 289, 106–114. [Google Scholar] [CrossRef]
- Renault, P.E.; Albertin, W.; Bely, M. An innovative tool reveals interaction mechanisms among yeast populations under oenological conditions. Appl. Microbiol. Biotechnol. 2013, 97, 4105–4119. [Google Scholar] [CrossRef] [PubMed]
- Pallmann, C.L.; Brown, J.A.; Olineka, T.L.; Cocolin, L.; Mills, D.A.; Bisson, L.F. Use of WL medium to profile native flora fermentations. Am. J. Enol. Vitic. 2001, 52, 198–203. [Google Scholar]
- Hierro, N.; Esteve-Zarzoso, B.; González, Á.; Mas, A.; Guillamón, J.M. Real-time quantitative PCR (QPCR) and reverse transcription-QPCR for detection and enumeration of total yeasts in wine. Appl. Environ. Microbiol. 2006, 72, 7148–7155. [Google Scholar] [CrossRef]
- Díaz, C.; Molina, A.M.; Nähring, J.; Fischer, R. Characterization and dynamic behavior of wild yeast during spontaneous wine fermentation in steel tanks and amphorae. Biomed Res. Int. 2013, 2013, 540465. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Esteve-Zarzoso, B.; Crespo, J.; Cabellos, J.M.; Arroyo, T. Yeast monitoring of wine mixed or sequential fermentations made by native strains from D.O. “Vinos de Madrid” using real-time quantitative PCR. Front. Microbiol. 2017, 8, 2520. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Glawe, D.A.; Weller, D.M.; Okubara, P.A. Real-time PCR assays for the quantification of native yeast DNA in grape berry and fermentation extracts. J. Microbiol. Methods 2020, 168, 105794. [Google Scholar] [CrossRef] [PubMed]
- Andorrà, I.; Esteve-Zarzoso, B.; Guillamón, J.M.; Mas, A. Determination of viable wine yeast using DNA binding dyes and quantitative PCR. Int. J. Food Microbiol. 2010, 144, 257–262. [Google Scholar] [CrossRef]
- Maturano, Y.P.; Mestre, M.V.; Combina, M.; Toro, M.E.; Vazquez, F.; Esteve-Zarzoso, B. Culture-dependent and independent techniques to monitor yeast species during cold soak carried out at different temperatures in winemaking. Int. J. Food Microbiol. 2016, 237, 142–149. [Google Scholar] [CrossRef]
- Andorrà, I.; Landi, S.; Mas, A.; Guillamón, J.M.; Esteve-Zarzoso, B. Effect of oenological practices on microbial populations using culture-independent techniques. Food Microbiol. 2008, 25, 849–856. [Google Scholar] [CrossRef]
- Zott, K.; Claisse, O.; Lucas, P.; Coulon, J.; Lonvaud-Funel, A.; Masneuf-Pomarede, I. Characterization of the yeast ecosystem in grape must and wine using real-time PCR. Food Microbiol. 2010, 27, 559–567. [Google Scholar] [CrossRef]
- Vendrame, M.; Iacumin, L.; Manzano, M.; Comi, G. Use of propidium monoazide for the enumeration of viable Oenococcus oeni in must and wine by quantitative PCR. Food Microbiol. 2013, 42, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Torija, M.J.; Mateo, E.; Guillamón, J.M.; Mas, A. Identification and quantification of acetic acid bacteria in wine and vinegar by TaqMan-MGB probes. Food Microbiol. 2010, 27, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Esteve-Zarzoso, B.; Cocolin, L.; Mas, A.; Rantsiou, K. Viable and culturable populations of Saccharomyces cerevisiae, Hanseniaspora uvarum and Starmerella bacillaris (synonym Candida zemplinina) during Barbera must fermentation. Food Res. Int. 2015, 78, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, M.; Manzano, M.; Comi, G.; Bertrand, J.; Iacumin, L. Use of propidium monoazide for the enumeration of viable Brettanomyces bruxellensis in wine and beer by quantitative PCR. Food Microbiol. 2014, 42, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Ko, G. Using propidium monoazide to distinguish between viable and nonviable bacteria, MS2 and murine norovirus. Lett. Appl. Microbiol. 2012, 55, 182–188. [Google Scholar] [CrossRef]
- Nogva, H.K.; Dromtorp, S.M.; Nissen, H.; Rudi, K. Ethidium monoazide for DNA-based differentiation of viable and dead bacteria by 5′-nuclease PCR. Biotechniques 2003, 34, 804–813. [Google Scholar] [CrossRef]
- Nocker, A.; Camper, A.K. Selective removal of DNA from dead cells of mixed bacterial communities by use of ethidium monoazide. Appl. Environ. Microbiol. 2006, 72, 1997–2004. [Google Scholar] [CrossRef]
- Rudi, K.; Naterstad, K.; Drømtorp, S.M.; Holo, H. Detection of viable and dead Listeria monocytogenes on gouda-like cheeses by real-time PCR. Lett. Appl. Microbiol. 2005, 40, 301–306. [Google Scholar] [CrossRef]
- Miotto, M.; Barretta, C.; Ossai, S.O.; da Silva, H.S.; Kist, A.; Vieira, C.R.W.; Parveen, S. Optimization of a propidium monoazide-qPCR method for Escherichia coli quantification in raw seafood. Int. J. Food Microbiol. 2020, 318, 108467. [Google Scholar] [CrossRef]
- Ravindran, V.B.; Shahsavari, E.; Soni, S.K.; Ball, A.S. Viability determination of Ascaris ova in raw wastewater: A comparative evaluation of culture-based, BacLight Live/Dead staining and PMA-qPCR methods. Water Sci. Technol. 2019, 80, 817–826. [Google Scholar] [CrossRef]
- Dorn-In, S.; Gareis, M.; Schwaiger, K. Differentiation of live and dead Mycobacterium tuberculosis complex in meat samples using PMA qPCR. Food Microbiol. 2019, 84, 103275. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Breidt, F. Enumeration of viable Listeria monocytogenes cells by real-time PCR with propidium monoazide and ethidium monoazide in the presence of dead cells. Appl. Environ. Microbiol. 2007, 73, 8028–8031. [Google Scholar] [CrossRef] [PubMed]
- Nocker, A.; Cheung, C.Y.; Camper, A.K. Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J. Microbiol. Methods 2006, 67, 310–320. [Google Scholar] [CrossRef]
- Sicard, A.; Merfa, M.V.; Voeltz, M.; Zeilinger, A.R.; De La Fuente, L.; Almeida, R.P.P. Discriminating between viable and membrane-damaged cells of the plant pathogen Xylella fastidiosa. PLoS ONE 2019, 14, e0221119. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, W.; López-Gálvez, F.; Allende, A.; Aznar, R.; Sánchez, G. Evaluation of viability PCR performance for assessing norovirus infectivity in fresh-cut vegetables and irrigation water. Int. J. Food Microbiol. 2016, 229, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Jiang, N.; Lv, Q.; Kan, Y.; Hao, J.; Li, J.; Luo, L. Detection of Clavibacter michiganensis subsp. michiganensis in viable but nonculturable state from tomato seed using improved qPCR. PLoS ONE 2018, 13, e0196525. [Google Scholar] [CrossRef]
- Shi, H.; Xu, W.; Trinh, Q.; Luo, Y.; Liang, Z.; Li, Y.; Huang, K. Establishment of a viable cell detection system for microorganisms in wine based on ethidium monoazide and quantitative PCR. Food Control 2012, 27, 81–86. [Google Scholar] [CrossRef]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburu, F.; Querol, A. Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Bacteriol. 1999, 49, 329–337. [Google Scholar] [CrossRef]
- Beltran, G.; Novo, M.; Rozès, N.; Mas, A.; Guillamón, J.M. Nitrogen catabolite repression in Saccharomyces cerevisiae during wine fermentations. FEMS Yeast Res. 2004, 4, 625–632. [Google Scholar] [CrossRef]
- Hierro, N.; Esteve-Zarzoso, B.; Mas, A.; Guillamón, J.M. Monitoring of Saccharomyces and Hanseniaspora populations during alcoholic fermentation by real-time quantitative PCR. FEMS Yeast Res. 2007, 7, 1340–1349. [Google Scholar] [CrossRef]
- Contreras, A.; Curtin, C.; Varela, C. Yeast population dynamics reveal a potential ‘collaboration’ between Metschnikowia pulcherrima and Saccharomyces uvarum for the production of reduced alcohol wines during Shiraz fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; Zulian, L.; Ferreres, À.; Pastor, R.; Esteve-Zarzoso, B.; Beltran, G.; Mas, A. Sequential inoculation of native non-Saccharomyces and Saccharomyces cerevisiae strains for wine making. Front. Microbiol. 2017, 8, 1293. [Google Scholar] [CrossRef]
- Capusoni, C.; Arioli, S.; Donzella, S.; Guidi, B.; Serra, I.; Compagno, C. Hyper-osmotic stress elicits membrane depolarization and decreased permeability in halotolerant marine Debaryomyces hansenii strains and in Saccharomyces cerevisiae. Front. Microbiol. 2019, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Du Toit, M.; Du Toit, W.J. The effects of copper and high sugar concentrations on growth, fermentation efficiency and volatile acidity production of different commercial wine yeast strains. Aust. J. Grape Wine Res. 2006, 12, 50–56. [Google Scholar] [CrossRef]
- Larue, F.; Lafon-Lafourcade, S.; Ribereau-Gayon, P. The various functions of steroids on the yeast metabolism in grape must during fermentation: The notion of survival factor. Ann. Microbiol. 1979, 130, 231–243. [Google Scholar]
- Attfield, P.V. Stress tolerance: The key to effective strains of industrial baker’s yeast. Nat. Biotechnol. 1997, 15, 1351–1357. [Google Scholar] [CrossRef]
- Pérez-Torrado, R.; Carrasco, P.; Aranda, A.; Gimeno-Alcañiz, J.; Pérez-Ortín, J.E.; Matallana, E.; Del Olmo, M. Study of the first hours of microvinification by the use of osmotic stress-response genes as probes. Syst. Appl. Microbiol. 2002, 25, 153–161. [Google Scholar] [CrossRef]
- Colwell, R.R. Global climate and infectious disease: The cholera paradigm. Science 1996, 274, 2025–2031. [Google Scholar] [CrossRef]
- Overney, A.; Jacques-André-Coquin, J.; Ng, P.; Carpentier, B.; Guillier, L.; Firmesse, O. Impact of environmental factors on the culturability and viability of Listeria monocytogenes under conditions encountered in food processing plants. Int. J. Food Microbiol. 2017, 244, 74–81. [Google Scholar] [CrossRef]
- Ayrapetyan, M.; Oliver, J.D. The viable but non-culturable state and its relevance in food safety. Curr. Opin. Food Sci. 2016, 8, 127–133. [Google Scholar] [CrossRef]
- Millet, V.; Lonvaud-Funel, A. The viable but non-culturable state of wine micro-organisms during storage. Lett. Appl. Microbiol. 2000, 30, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Križanović, S.; Tomašević, M.; Režek Jambrak, A.; Ćurko, N.; Gracin, L.; Lukić, K.; Kovačević Ganić, K. Effect of thermosonication and physicochemical properties of wine on culturability, viability, and metabolic activity of Brettanomyces bruxellensis yeast in red wines. J. Agric. Food Chem. 2019, 68, 3302–3311. [Google Scholar] [CrossRef] [PubMed]
- Hommel, B.; Sturny-Leclère, A.; Volant, S.; Veluppillai, N.; Duchateau, M.; Yu, C.H.; Hourdel, V.; Varet, H.; Matondo, M.; Perfect, J.R.; et al. Cryptococcus neoformans resists to drastic conditions by switching to viable but non-culturable cell phenotype. PLoS Pathog. 2019, 15, e1007945. [Google Scholar] [CrossRef]
- Salma, M.; Rousseaux, S.; Sequeira-Le Grand, A.; Divol, B.; Alexandre, H. Characterization of the viable but nonculturable (VBNC) state in Saccharomyces cerevisiae. PLoS ONE 2013, 8, e77600. [Google Scholar] [CrossRef] [PubMed]
- Munna, M.S.; Humayun, S.; Noor, R. Influence of heat shock and osmotic stresses on the growth and viability of Saccharomyces cerevisiae SUBSC01. BMC Res. Notes 2015, 8, 369. [Google Scholar] [CrossRef]
- Petitgonnet, C.; Klein, G.L.; Roullier-Gall, C.; Schmitt-Kopplin, P.; Quintanilla-Casas, B.; Vichi, S.; Julien-David, D.; Alexandre, H. Influence of cell-cell contact between L. thermotolerans and S. cerevisiae on yeast interactions and the exo-metabolome. Food Microbiol. 2019, 83, 122–133. [Google Scholar] [CrossRef]
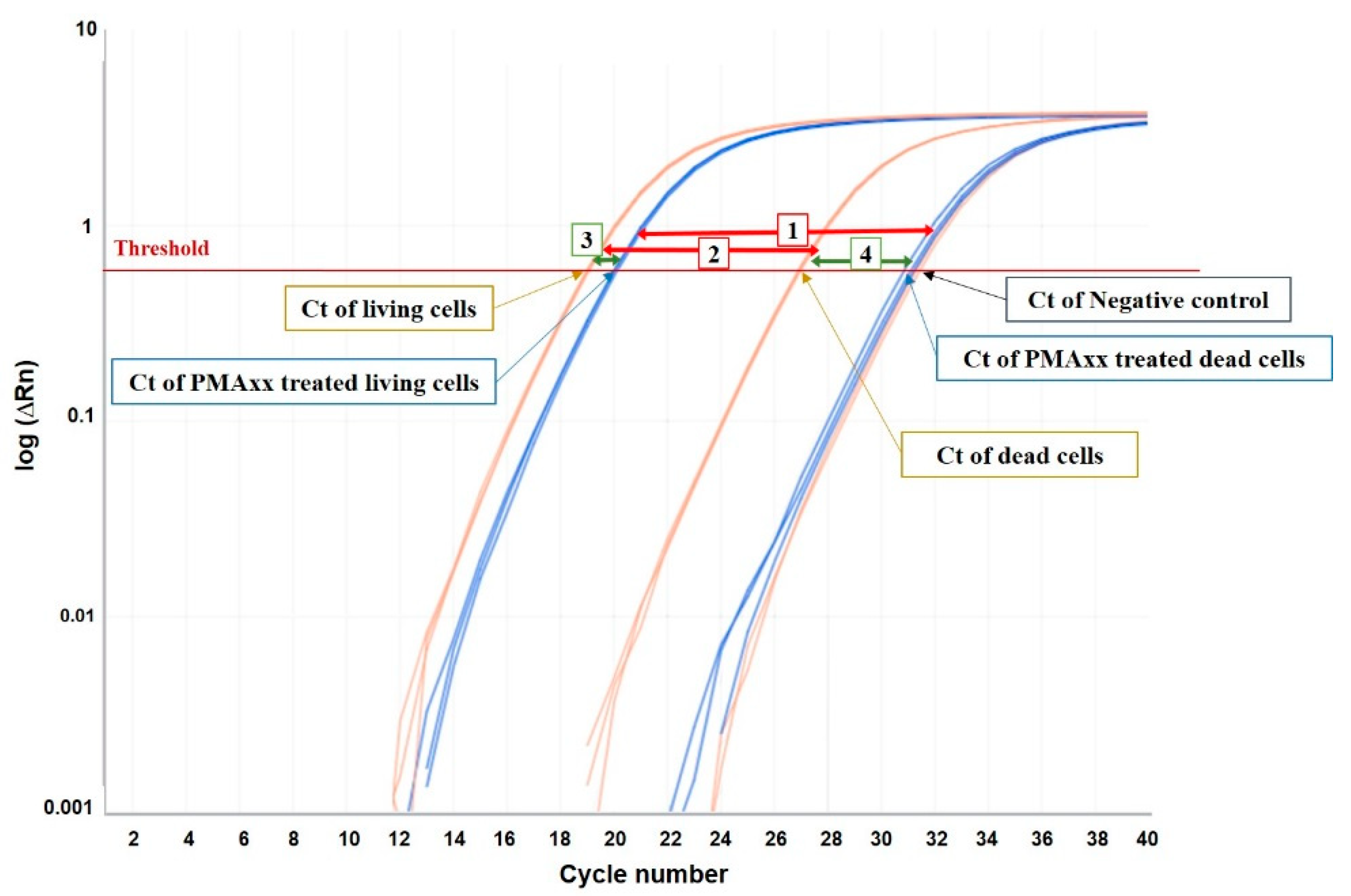


| (a) ΔCt (PMAxx Treated–Untreated Cells) | (b) ΔCt (Dead–Living Cells) | |||||
|---|---|---|---|---|---|---|
| Living Cells | Dead Cells | p Value | PMAxx | non-PMAxx | p Value | |
| S. cerevisiae | 2.003 ± 0.428 | 10.4 ± 1.558 | 0.001 | 15.42 ± 0.888 | 7.76 ± 1.447 | 0.018 |
| T. delbrueckii | 1.631 ± 1.040 | 5.566 ± 1.498 | 0.029 | 11.33 ± 1.736 | 7.392 ± 1.099 | 0.020 |
| L. thermotolerans | 2.171 ± 0.395 | 8.22 ± 0.310 | 0.010 | 13.46 ± 1.708 | 8.814 ± 0.3917 | 0.003 |
| M. pulcherrima | 0.5525 ± 0.665 | 4.834 ± 0.984 | 0.041 | 11.83 ± 1.170 | 7.548 ± 0.4837 | 0.036 |
| qPCR | |||||||
|---|---|---|---|---|---|---|---|
| Slope | Y-Intersection | R2 | Efficiency (%) | Error | LoQ | LoD | |
| S. cerevisiae | −3.275 | 37.526 | 0.999 | 102 | 0.041 | 102 | 10 |
| T. delbrueckii | −3.149 | 37.174 | 1 | 107.77 | 0.027 | 102 | 10 |
| L. thermotolerans | −3.226 | 38.466 | 1 | 104.17 | 0.02 | 103 | 102 |
| M. pulcherrima | −3.389 | 44.651 | 0.996 | 97.287 | 0.071 | 104 | 103 |
| Total yeast | −3.539 | 40.168 | 0.995 | 91.657 | 0.085 | ND | ND |
| PMAxx-qPCR | |||||||
| Slope | Y-Intersection | R2 | Efficiency (%) | Error | LoQ | LoD | |
| S. cerevisiae | −3.353 | 38.009 | 0.999 | 98.719 | 0.011 | 103 | 102 |
| T. delbrueckii | −3.542 | 38.1 | 0.998 | 91.552 | 0.011 | 103 | 102 |
| L. thermotolerans | −3.24 | 39.945 | 0.998 | 103.5 | 0.015 | 103 | 102 |
| M. pulcherrima | −3.34 | 41.802 | 0.999 | 99.214 | 0.023 | 104 | 103 |
| Total yeast | −3.129 | 37.672 | 0.994 | 108.73 | 0.074 | ND | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, Y.; Torija, M.-J.; Mas, A.; Beltran, G. Viability-PCR Allows Monitoring Yeast Population Dynamics in Mixed Fermentations Including Viable but Non-Culturable Yeasts. Foods 2020, 9, 1373. https://doi.org/10.3390/foods9101373
Navarro Y, Torija M-J, Mas A, Beltran G. Viability-PCR Allows Monitoring Yeast Population Dynamics in Mixed Fermentations Including Viable but Non-Culturable Yeasts. Foods. 2020; 9(10):1373. https://doi.org/10.3390/foods9101373
Chicago/Turabian StyleNavarro, Yurena, María-Jesús Torija, Albert Mas, and Gemma Beltran. 2020. "Viability-PCR Allows Monitoring Yeast Population Dynamics in Mixed Fermentations Including Viable but Non-Culturable Yeasts" Foods 9, no. 10: 1373. https://doi.org/10.3390/foods9101373
APA StyleNavarro, Y., Torija, M.-J., Mas, A., & Beltran, G. (2020). Viability-PCR Allows Monitoring Yeast Population Dynamics in Mixed Fermentations Including Viable but Non-Culturable Yeasts. Foods, 9(10), 1373. https://doi.org/10.3390/foods9101373








