Antique Traditional Practice: Phenolic Profile of Virgin Olive Oil Obtained from Fruits Stored in Seawater
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Olive Oil Samples Preparation
2.3. Phenolic Compounds
2.3.1. Extraction
2.3.2. HPLC-DAD Analysis
2.3.3. MS Analysis
2.3.4. Spectrophotometric Analysis
Total Phenols (TP)
o-Diphenols
Total Flavonoids (TF)
DPPH Test of Antioxidant Activity
2.4. Statistical Analysis
3. Results and Discussion
3.1. Identification and Quantification of Phenolic Compounds
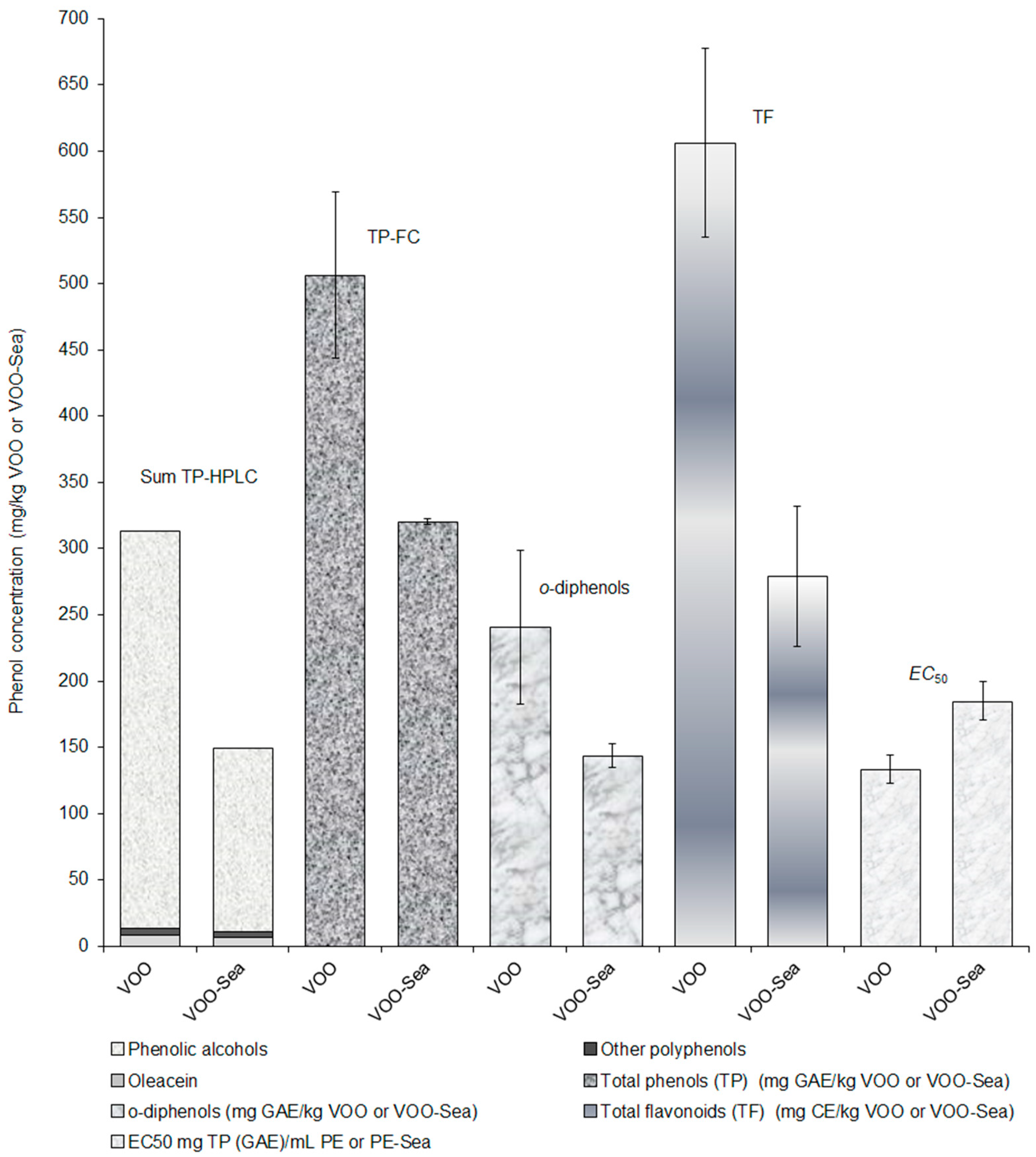
3.2. Effect of Storage of Olive Fruits in the Seawater on the Phenolic Profile of Olive Oil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ilakovac, B. The Reconstruction of the Olive Mill from the Roman Empire Age at Muline, Isle of Ugljan. Works Inst. Hist. Croat. Acad. Sci. Zadar 1998, 40, 1–26. [Google Scholar]
- Torić, J.; Brozović, A.; Baus Lončar, M.; Jakobušić Brala, C.; Karković Marković, A.; Benčić, Đ.; Barbarić, M. Biological activity of phenolic compounds in extra virgin olive oils through their phenolic profile and their combination with anticancer drugs observed in human cervical carcinoma and colon adenocarcinoma cells. Antioxidants 2020, 9, 453. [Google Scholar]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 4, 2001. [Google Scholar]
- Valls, R.M.; Farras, M.; Suarez, M.; Fernandez-Castillejo, S.; Fito, M.; Konstantinidou, V.; Fuentes, F.; López-Miranda, J.; Giralt, M.; Covas, M.I.; et al. Effects of functional olive oil enriched with its own phenolic compounds on endothelial function in hypertensive patients. A randomised controlled trial. Food Chem. 2015, 167, 30–35. [Google Scholar]
- Celano, M.; Maggisano, V.; Lepore, S.M.; Russo, D.; Bulotta, S. Secoiridoids of olive and derivatives as potential coadjuvant drugs in cancer: A critical analysis of experimental studies. Pharmacol. Res. 2019, 142, 77–86. [Google Scholar]
- Wani, T.A.; Masoodi, F.A.; Gani, A.; Baba, W.N.; Rahmanian, N.; Wani, I.A.; Ahmad, M. Olive oil and its principal bioactive compound: Hydroxytyrosol—A review of the recent literature. Trends Food Sci. Technol. 2018, 77, 77–90. [Google Scholar]
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.L.; Smith, A.B.; Breslin, P.A.S. Ibuprofen-like activity in extra-virgin olive oil: Phytochemistry. Nature 2005, 437, 45–46. [Google Scholar]
- Parkinson, L.; Keast, R. Oleocanthal, a Phenolic Derived from Virgin Olive Oil: A Review of the Beneficial Effects on Inflammatory Disease. Int. J. Mol. Sci. 2014, 15, 12323–12334. [Google Scholar]
- Miljković, I. Modern Fruit Growing; Znanje: Zagreb, Croatia, 1991. [Google Scholar]
- Jerman Klen, T.; Mozetič Vodopivec, B. Optimisation of olive oil phenol extraction conditions using a high-power probe ultrasonication. Food Chem. 2012, 134, 2481–2488. [Google Scholar]
- Jakobušić Brala, C.; Benčić, D.; Šindrak, Z.; Barbarić, M.; Uršić, S. Labeled extra virgin olive oil as food supplement; phenolic compounds in oils from some autochthonous Croatian olives. Grasas Aceites 2015, 66, e099. [Google Scholar]
- Pampaloni, B.; Mavilia, C.; Fabbri, S.; Romani, A.; Ieri, F.; Tanini, A.; Tonelli, F.; Brandi, M.L. In Vitro Effects of Extracts of Extra Virgin Olive Oil on Human Colon Cancer Cells. Nutr. Cancer 2014, 66, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Gutfinger, T. Polyphenols in olive oils. J. Am. Oil Chem. Soc. 1981, 58, 966–968. [Google Scholar]
- Mateos, R.; Espartero, J.L.; Trujillo, M.; Ríos, J.J.; León-Camacho, M.; Alcudia, F.; Cert, A. Determination of phenols, flavones, and lignans in virgin olive oils by solid-phase extraction and high-performance liquid chromatography with diode array ultraviolet detection. J. Agric. Food Chem. 2001, 49, 2185–2192. [Google Scholar]
- Kim, D. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar]
- Antonini, E.; Farina, A.; Leone, A.; Mazzara, E.; Urbani, S.; Selvaggini, R.; Servili, M.; Ninfali, P. Phenolic compounds and quality parameters of family farming versus protected designation of origin (PDO) extra-virgin olive oils. J. Food Compost. Anal. 2015, 43, 75–81. [Google Scholar]
- Jerman Klen, T.; Golc Wondra, A.; Vrhovšek, U.; Mozetič Vodopivec, B. Phenolic profiling of olives and olive oil process-derived matrices using UPLC-DAD-ESI-QTOF-HRMS analysis. J. Agric. Food Chem. 2015, 63, 3859–3872. [Google Scholar]
- Becerra-Herrera, M.; Velez-Martin, A.; Ramos-Merchante, A.; Richter, P.; Beltran, R.; Sayago, A. Characterization and evaluation of phenolic profiles and color as potential discriminating features among Spanish extra virgin olive oils with protected designation of origin. Food Chem. 2018, 241, 328–337. [Google Scholar]
- Fanali, C.; Della Posta, S.; Vilmercati, A.; Dugo, L.; Russo, M.; Petitti, T.; Mondello, L.; de Gara, L. Extraction, analysis, and antioxidant activity evaluation of phenolic compounds in different italian extra-virgin olive oils. Molecules 2018, 23, 3249. [Google Scholar]
- Kulišić Bilušić, T.; Melliou, E.; Giacometti, J.; Čaušević, A.; Čorbo, S.; Landeka, M.; Magiatis, P. Phenolics, fatty acids, and biological potential of selected Croatian EVOOs: Characterization of selected Croatian EVOOs. Eur. J. Lipid Sci. Technol. 2017, 119, 1700108. [Google Scholar]
- Šarolić, M.; Gugić, M.; Friganović, E.; Tuberoso, C.; Jerković, I. Phytochemicals and other characteristics of croatian monovarietal extra virgin olive oils from oblica, lastovka and levantinka varieties. Molecules 2015, 20, 4395–4409. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.N.; Galeano-Diaz, T.; Sanchez, J.; de Miguel, C.; Martin-Vertedor, D. Antioxidant capacity of the phenolic fraction and its effect on the oxidative stability of olive oil varieties grown in the southwest of Spain. Grasas Aceites 2014, 65, e004. [Google Scholar]
- Rotondi, A.; Fabbri, A.; Tommaso, G. Sensory and chemical properties of extra virgin olive oils produced in two different Italian regions: Tuscany and Emilia-Romagna. J. Food Agric. Environ. 2008, 6, 71–77. [Google Scholar]
- Kosma, I.; Vatavali, K.; Kontakos, S.; Kontominas, M.; Kiritsakis, A.; Badeka, A. Geographical differentiation of Greek extra virgin olive oil from late-harvested Koroneiki cultivar fruits. J. Am. Oil Chem. Soc. 2017, 94, 1373–1384. [Google Scholar] [CrossRef]
- Ocakoglu, D.; Tokatli, F.; Ozen, B.; Korel, F. Distribution of simple phenols, phenolic acids and flavonoids in Turkish monovarietal extra virgin olive oils for two harvest years. Food Chem. 2009, 113, 401–410. [Google Scholar] [CrossRef]
- Rigane, G.; Ayadi, M.; Boukhris, M.; Sayadi, S.; Bouaziz, M. Characterisation and phenolic profiles of two rare olive oils from southern Tunisia: Dhokar and Gemri-Dhokar cultivars. J. Sci. Food Agric. 2013, 93, 527–534. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef]
- Cuyas, E.; Verdura, S.; Lozano-Sanchez, J.; Viciano, I.; Llorach-Pares, L.; Nonell-Canals, A.; Bosch-Barrerab, J.; Brunet, J.; Segura-Carreteroc, A.; Sanchez-Martineze, M.; et al. The extra virgin olive oil phenolic oleacein is a dual substrate-inhibitor of catechol-O-methyltransferase. Food Chem. Toxicol. 2019, 128, 35–45. [Google Scholar] [CrossRef]
- Bilušić, T.; Žanetić, M.; Ljubenkov, I.; Generalić Mekinić, I.; Štambuk, S.; Bojović, V.; Soldo, B.; Magiatis, P. Molecular characterization of Dalmatian cultivars and the influence of the olive fruit harvest period on chemical profile, sensory characteristics and oil oxidative stability. Eur. Food Res. Technol. 2018, 244, 281–289. [Google Scholar] [CrossRef]
- Hachicha Hbaieb, R.; Kotti, F.; García-Rodríguez, R.; Gargouri, M.; Sanz, C.; Pérez, A.G. Monitoring endogenous enzymes during olive fruit ripening and storage: Correlation with virgin olive oil phenolic profiles. Food Chem. 2015, 174, 240–247. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Delcuratolo, D.; Gomes, T.; Colelli, G. Effect of different temperatures and storage atmospheres on Coratina olive oil quality. Food Chem. 2007, 102, 571–576. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Procida, G.; Bencic, D.; Zelinotti, T. Effect of olive fruits storage in sea water on oil quality. Food Technol. Biotechnol. 1999, 37, 209–214. [Google Scholar]
- Koprivnjak, O.; Procida, G.; Zelinotti, T. Changes in the volatile components of virgin olive oil during fruit storage in aqueous media. Food Chem. 2000, 70, 377–384. [Google Scholar] [CrossRef]
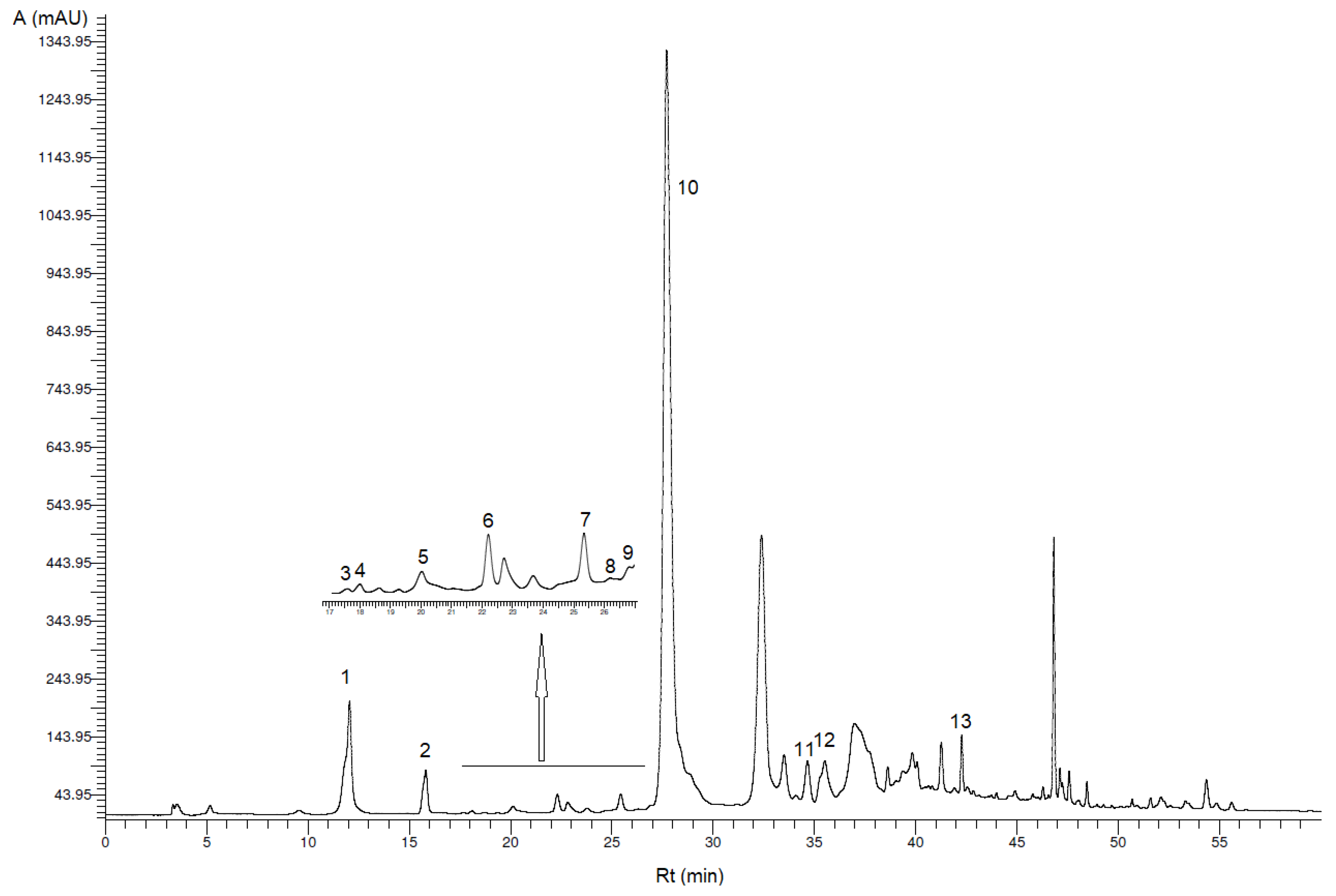
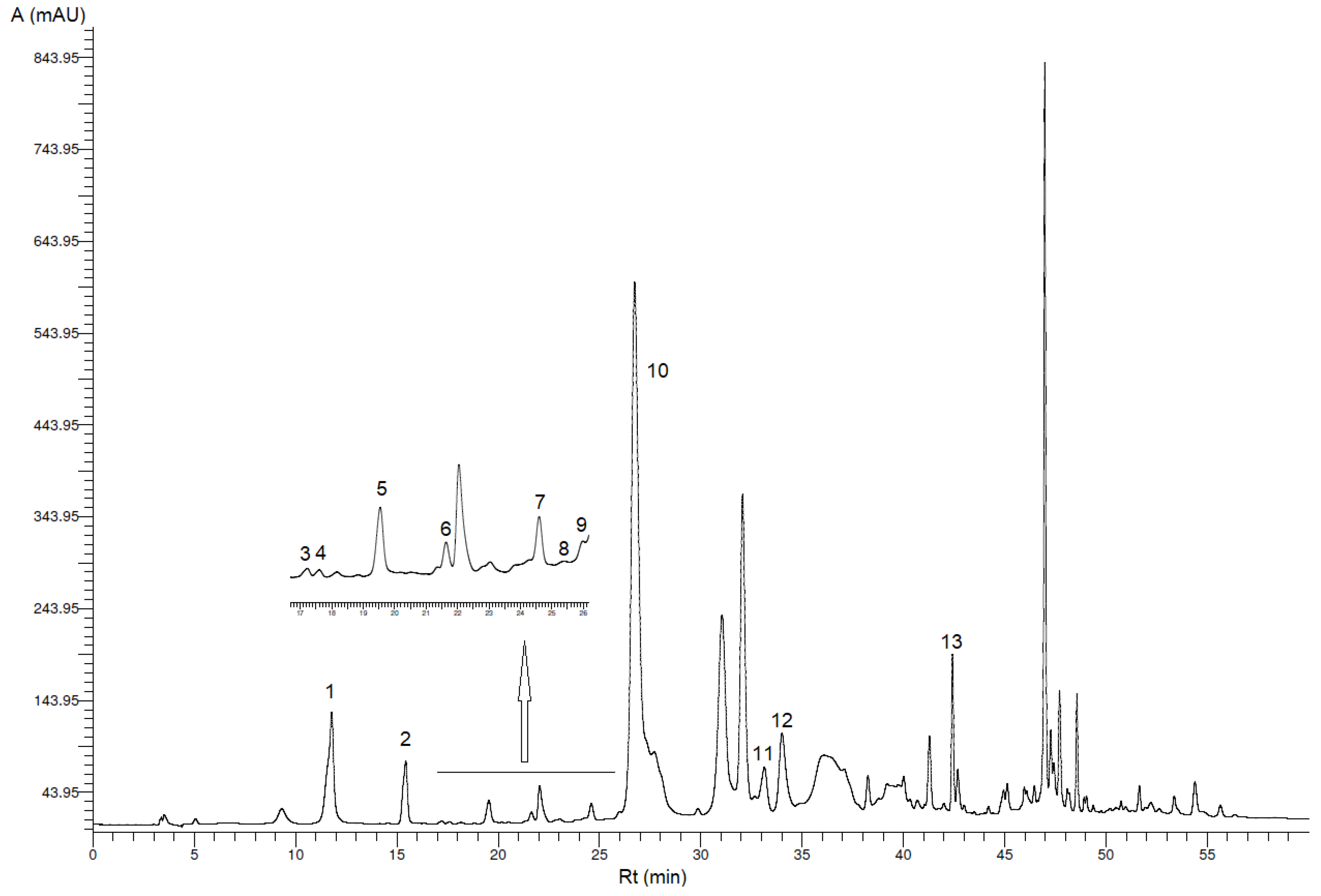
| Peak * | Phenolic Compound | VOO (mg/kg VOO ± SD) | VOO-Sea (mg/kg VOO-Sea ± SD) |
|---|---|---|---|
| 1 | Hydroxytyrosol | 18.2 b ± 3.0 | 12.9 a ± 1.0 |
| 3,4-Dihydroxybenzoic acid | n.d. | n.d. | |
| 2 | Tyrosol | 8.10 b ± 0.34 | 7.20 a ± 0.28 |
| 3 | p-Hydroxybenzoic acid | 0.10 ± 0.02 ** | 0.11 ± 0.00 ** |
| 4 | Homovanillyl alcohol | 0.38 ± 0.04 ** | 0.22 ± 0.01 ** |
| 5 | Vanillic acid | 0.64 a ± 0.20 | 0.97 b ± 0.03 |
| Syringic acid | n.d. | n.d. | |
| 6 | Vanillin | 0.53 ± 0.05 | 0.23 ± 0.01 ** |
| 7 | p-Coumaric acid | 0.43 ± 0.04 ** | 0.30 ± 0.04 ** |
| 8 | Benzoic acid | 0.22 ± 0.10 ** | 0.24 ± 0.07 ** |
| 9 | Ferulic acid | 0.27 ± 0.04 ** | 0.22 ± 0.02 ** |
| 10 | Oleacein | 300 b ± 32 | 138 a ± 5 |
| o-Coumaric acid | n.d. | n.d. | |
| 11 | Pinoresinol | 4.02 b ± 0.12 | 2.64 a ± 0.07 |
| 12 | Cinnamic acid | 0.84 a ± 0.17 | 0.93 b ± 0.06 |
| 13 | Apigenin | 0.98 a ± 0.03 | 1.68 b ± 0.10 |
| VOO | VOO-Sea | |
|---|---|---|
| Total phenols * | 506 b ± 63 | 320 a ± 2 |
| o-Diphenols * | 241 b ± 58 | 144 a ± 9 |
| Total flavonoids ** | 606 b ± 71 | 279 a ± 53 |
| EC50 *** | 133 a ± 11 | 185 b ± 15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torić, J.; Barbarić, M.; Uršić, S.; Jakobušić Brala, C.; Karković Marković, A.; Zebić Avdičević, M.; Benčić, Đ. Antique Traditional Practice: Phenolic Profile of Virgin Olive Oil Obtained from Fruits Stored in Seawater. Foods 2020, 9, 1347. https://doi.org/10.3390/foods9101347
Torić J, Barbarić M, Uršić S, Jakobušić Brala C, Karković Marković A, Zebić Avdičević M, Benčić Đ. Antique Traditional Practice: Phenolic Profile of Virgin Olive Oil Obtained from Fruits Stored in Seawater. Foods. 2020; 9(10):1347. https://doi.org/10.3390/foods9101347
Chicago/Turabian StyleTorić, Jelena, Monika Barbarić, Stanko Uršić, Cvijeta Jakobušić Brala, Ana Karković Marković, Maja Zebić Avdičević, and Đani Benčić. 2020. "Antique Traditional Practice: Phenolic Profile of Virgin Olive Oil Obtained from Fruits Stored in Seawater" Foods 9, no. 10: 1347. https://doi.org/10.3390/foods9101347
APA StyleTorić, J., Barbarić, M., Uršić, S., Jakobušić Brala, C., Karković Marković, A., Zebić Avdičević, M., & Benčić, Đ. (2020). Antique Traditional Practice: Phenolic Profile of Virgin Olive Oil Obtained from Fruits Stored in Seawater. Foods, 9(10), 1347. https://doi.org/10.3390/foods9101347