Enhanced Aromatic Profile and Functionality of Cheese Whey Beverages by Incorporation of Probiotic Cells Immobilized on Pistacia terebinthus Resin
Abstract
1. Introduction
2. Materials and Methods
2.1. Immobilized Probiotic Biocatalyst
2.2. Functional Whey Beverages Production
2.3. Whey Beverages Microbiological Profile
2.4. Physicochemical Analysis
2.5. Analysis of Aroma Volatiles by Solid-Phase Microextraction Gas Chromatography–Mass Spectrometry
2.6. Sensory Evaluation
2.7. Experimental Design and Statistical Analysis
3. Results and Discussion
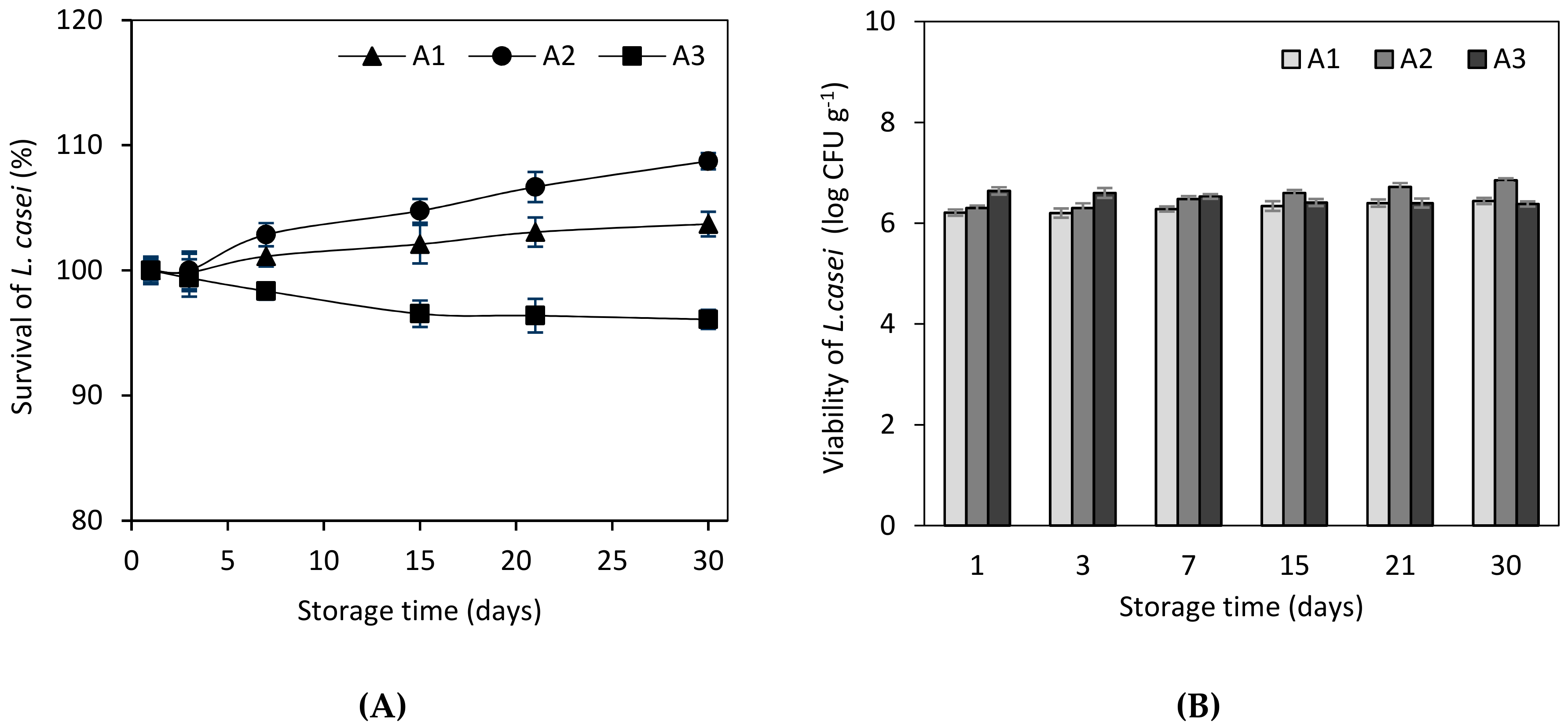
3.1. Microbiological Characteristics and Probiotics Viability
3.2. Physicochemical Characteristics of Whey Beverages
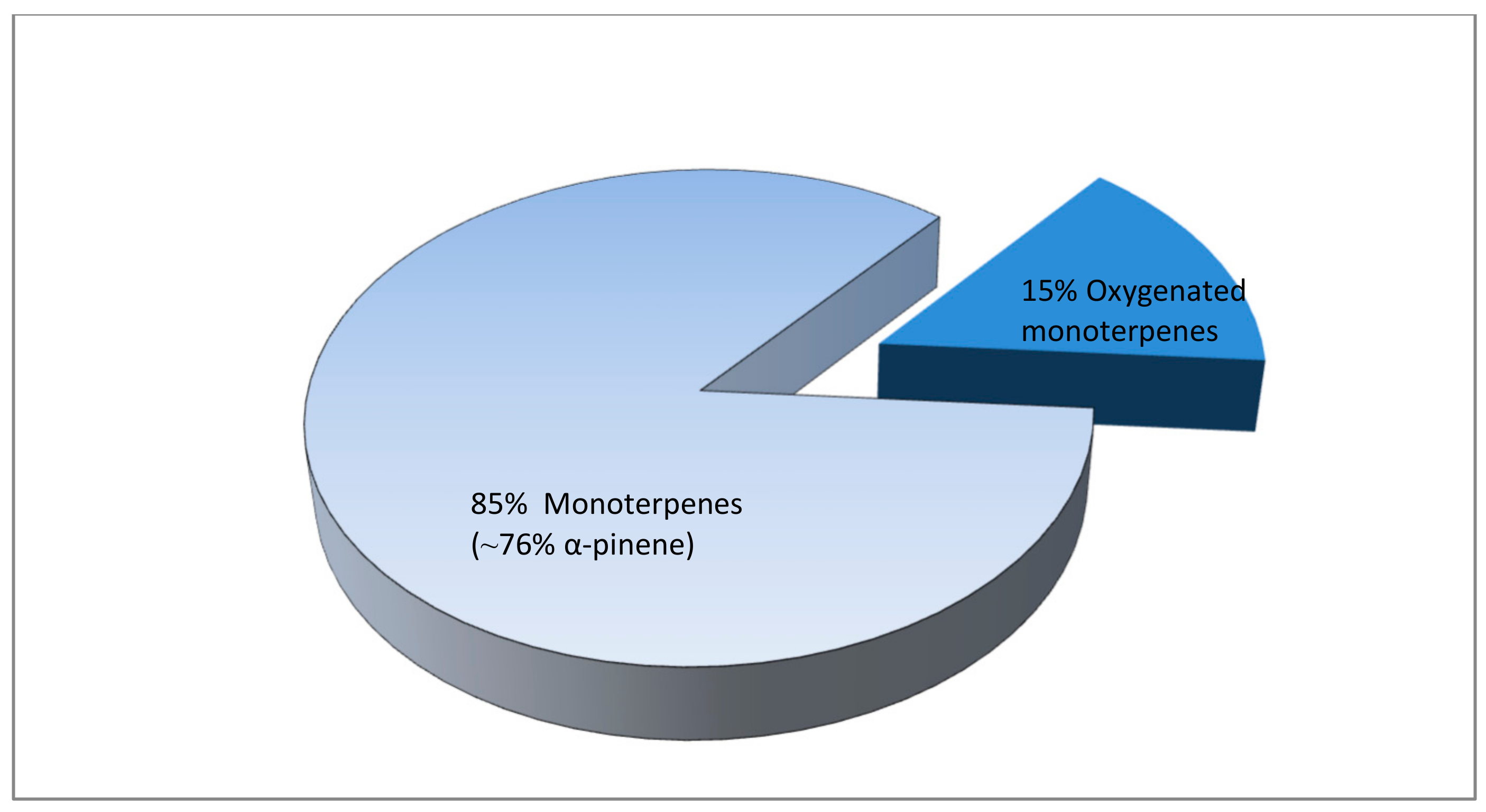
3.3. Effect of Pistacia Terebinthus Resin on Volatile By-Products of Functional Whey Beverages
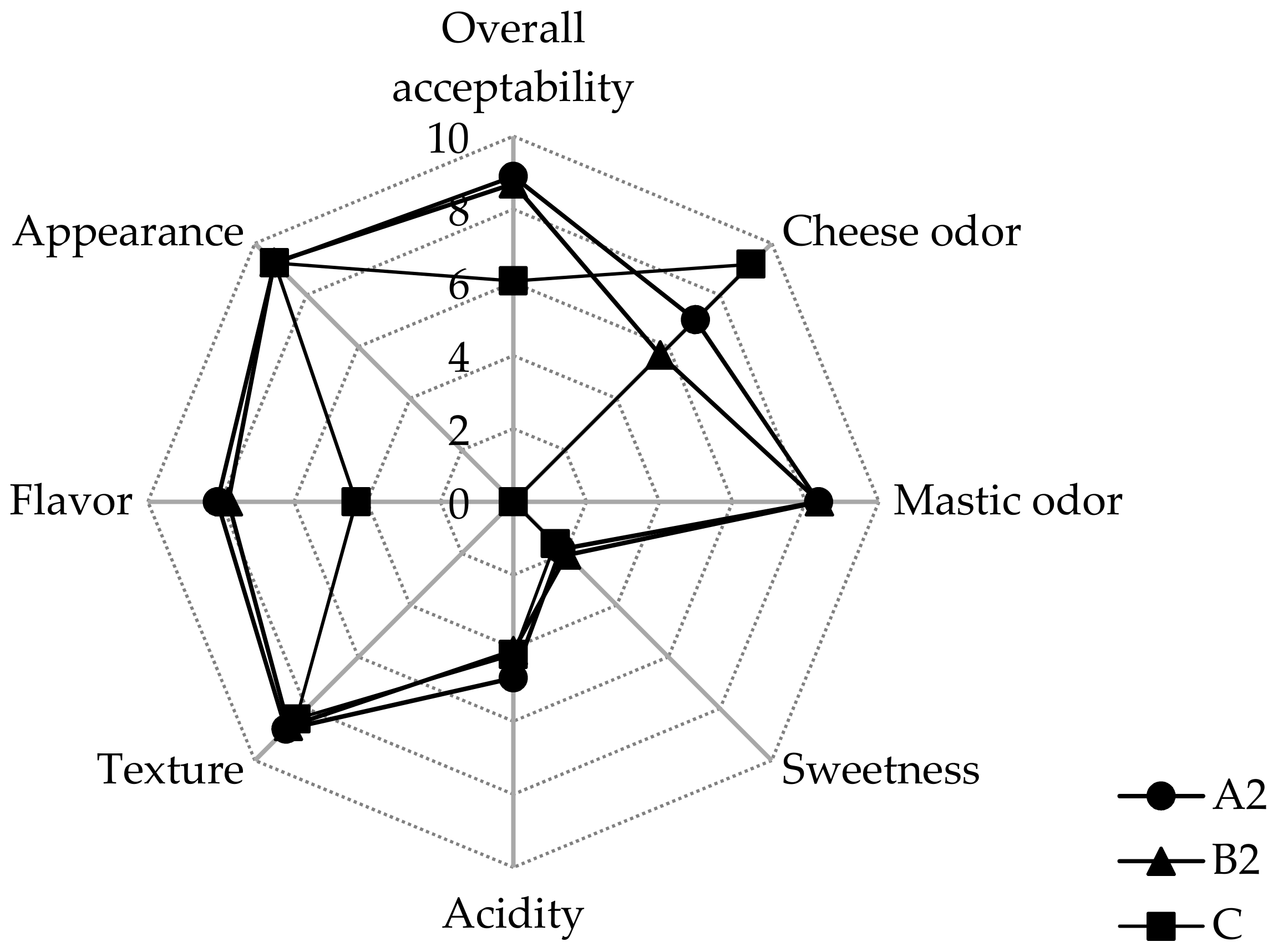
3.4. Effect of Pistacia terebinthus Resin on Sensory Characteristics of Whey Beverages
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hernández-Ledesma, B.; Ramos, M.; Gómez-Ruiz, J.Á. Bioactive components of ovine and caprine cheese whey. Small Rumin. Res. 2011, 101, 196–204. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.S.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef] [PubMed]
- Lappa, I.K.; Papadaki, A.; Kachrimanidou, V.; Terpou, A.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Cheese Whey Processing: Integrated Biorefinery Concepts and Emerging Food Applications. Foods 2019, 8, 347. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D. Therapeutic Potential of Milk Whey. Beverages 2017, 3, 31. [Google Scholar] [CrossRef]
- Panghal, A.; Patidar, R.; Jaglan, S.; Chhikara, N.; Khatkar, S.K.; Gat, Y.; Sindhu, N. Whey valorization: Current options and future scenario—A critical review. Nutr. Food Sci. 2018, 48, 520–535. [Google Scholar] [CrossRef]
- Silva e Alves, A.T.; Spadoti, L.M.; Zacarchenco, P.B.; Trento, F.K.H.S. Probiotic Functional Carbonated Whey Beverages: Development and Quality Evaluation. Beverages 2018, 4, 49. [Google Scholar] [CrossRef]
- Newbold, D.; Koppel, K. Carbonated Dairy Beverages: Challenges and Opportunities. Beverages 2018, 4, 66. [Google Scholar] [CrossRef]
- Cortellino, G.; Rizzolo, A. Storage Stability of Novel Functional Drinks Based on Ricotta Cheese Whey and Fruit Juices. Beverages 2018, 4, 67. [Google Scholar] [CrossRef]
- Jelen, P. 10—Whey-based functional beverages A2—Paquin, Paul. In Functional and Speciality Beverage Technology; Woodhead Publishing: Cambridge, UK, 2009; pp. 259–280. [Google Scholar]
- Janiaski, D.R.; Pimentel, T.C.; Cruz, A.G.; Prudencio, S.H. Strawberry-flavored yogurts and whey beverages: What is the sensory profile of the ideal product? J. Dairy Sci. 2016, 99, 5273–5283. [Google Scholar] [CrossRef] [PubMed]
- Baccouche, A.; Ennouri, M.; Felfoul, I.; Attia, H. A physical stability study of whey-based prickly pear beverages. Food Hydrocol. 2013, 33, 234–244. [Google Scholar] [CrossRef]
- Silveira, E.O.D.; Lopes Neto, J.H.; Silva, L.A.D.; Raposo, A.E.S.; Magnani, M.; Cardarelli, H.R. The effects of inulin combined with oligofructose and goat cheese whey on the physicochemical properties and sensory acceptance of a probiotic chocolate goat dairy beverage. LWT Food Sci. Technol. 2015, 62, 445–451. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Silva, E.K.; Costa, A.L.R.; Cunha, R.L.; Freitas, M.Q.; Meireles, M.A.A.; Cruz, A.G. Manufacturing a prebiotic whey beverage exploring the influence of degree of inulin polymerization. Food Hydrocol. 2018, 77, 787–795. [Google Scholar] [CrossRef]
- Huang, S.; Vignolles, M.-L.; Chen, X.D.; Le Loir, Y.; Jan, G.; Schuck, P.; Jeantet, R. Spray drying of probiotics and other food-grade bacteria: A review. Trends Food Sci. Technol. 2017, 63, 1–17. [Google Scholar] [CrossRef]
- Fiocco, D.; Longo, A.; Arena, M.P.; Russo, P.; Spano, G.; Capozzi, V. How probiotics face food stress: They get by with a little help. Crit. Rev. Food Sci. Nutr. 2019. [Google Scholar] [CrossRef]
- Terpou, A.; Mantzourani, I.; Galanis, A.; Kanellaki, M.; Bezirtzoglou, E.; Bekatorou, A.; Koutinas, A.A.; Plessas, S. Employment of L. paracasei K5 as a Novel Potentially Probiotic Freeze-Dried Starter for Feta-Type Cheese Production. Microorganisms 2018, 7, 3. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Bosnea, L.; Kanellaki, M.; Kopsahelis, N. Novel frozen yogurt production fortified with sea buckthorn berries and probiotics. LWT Food Sci. Technol. 2019, 105, 242–249. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Schoina, V.; Terpou, A.; Angelika-Ioanna, G.; Koutinas, A.; Kanellaki, M.; Bosnea, L. Use of Pistacia terebinthus resin as immobilization support for Lactobacillus casei cells and application in selected dairy products. J. Food Sci. Technol. 2015, 52, 5700–5708. [Google Scholar] [CrossRef]
- Schoina, V.; Terpou, A.; Bosnea, L.; Kanellaki, M.; Nigam, P.S. Entrapment of Lactobacillus casei ATCC393 in the viscus matrix of Pistacia terebinthus resin for functional myzithra cheese manufacture. LWT Food Sci. Technol. 2018, 89, 441–448. [Google Scholar] [CrossRef]
- Terpou, A.; Nigam, P.S.; Bosnea, L.; Kanellaki, M. Evaluation of Chios mastic gum as antimicrobial agent and matrix forming material targeting probiotic cell encapsulation for functional fermented milk production. LWT Food Sci. Technol. 2018, 97, 109–116. [Google Scholar] [CrossRef]
- Bosnea, L.A.; Moschakis, T.; Biliaderis, C.G. Microencapsulated cells of Lactobacillus paracasei subsp. paracasei in biopolymer complex coacervates and their function in a yogurt matrix. Food Funct. 2017, 8, 554–562. [Google Scholar]
- Kallis, M.; Sideris, K.; Kopsahelis, N.; Bosnea, L.; Kourkoutas, Y.; Terpou, A.; Kanellaki, M. Pistacia terebinthus Resin as Yeast Immobilization Support for Alcoholic Fermentation. Foods 2019, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Patel, S.; Uddin, G.; Siddiqui, B.S.; Ahmad, B.; Muhammad, N.; Mabkhot, Y.N.; Hadda, T.B. Phytochemical, ethnomedicinal uses and pharmacological profile of genus Pistacia. Biomed. Pharmacother. 2017, 86, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Morkhade, D.M. Evaluation of gum mastic (Pistacia lentiscus) as a microencapsulating and matrix forming material for sustained drug release. Asian J. Pharm. Sci. 2017, 12, 424–432. [Google Scholar] [CrossRef]
- Zorzan, M.; Collazuol, D.; Ribaudo, G.; Ongaro, A.; Scaroni, C.; Zagotto, G.; Armanini, D.; Barollo, S.; Galeotti, F.; Volpi, N.; et al. Biological effects and potential mechanisms of action of Pistacia lentiscus Chios mastic extract in Caco-2 cell model. J. Funct. Foods 2019, 54, 92–97. [Google Scholar] [CrossRef]
- European Union Herbal Monograph on Pistacia lentiscus L. Resin (Mastix). Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/draft-european-union-herbal-monograph-pistacia-lentiscus-l-resin-mastix_en.pdf (accessed on 4 December 2019).
- Kang, J.S.; Wanibuchi, H.; Salim, E.I.; Kinoshita, A.; Fukushima, S. Evaluation of the toxicity of mastic gum with 13 weeks dietary administration to F344 rats. Food Chem. Toxicol. 2007, 45, 494–501. [Google Scholar] [CrossRef]
- Paraschos, S.; Magiatis, P.; Mitakou, S.; Petraki, K.; Kalliaropoulos, A.; Maragkoudakis, P.; Mentis, A.; Sgouras, D.; Skaltsounis, A.L. In vitro and in vivo activities of chios mastic gum extracts and constituents against Helicobacter pylori. Antimicrob. Agents Chemother. 2007, 51, 551–559. [Google Scholar] [CrossRef]
- Hadjimbei, E.; Botsaris, G.; Goulas, V.; Gekas, V. Health-Promoting Effects of Pistacia Resins: Recent Advances, Challenges, and Potential Applications in the Food Industry. Food Rev. Int. 2015, 31, 1–12. [Google Scholar] [CrossRef]
- Bozorgi, M.; Memariani, Z.; Mobli, M.; Salehi Surmaghi, M.H.; Shams-Ardekani, M.R.; Rahimi, R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): A Review of Their Traditional Uses, Phytochemistry, and Pharmacology. Sci. World J. 2013, 2013, 33. [Google Scholar] [CrossRef] [PubMed]
- Favaro-Trindade, C.S.; Grosso, C.R. Microencapsulation of L. acidophilus (La-05) and B. lactis (Bb-12) and evaluation of their survival at the pH values of the stomach and in bile. J. Microencapsul. 2002, 19, 485–494. [Google Scholar]
- Terpou, A.; Bosnea, L.; Kanellaki, M.; Plessas, S.; Bekatorou, A.; Bezirtzoglou, E.; Koutinas, A.A. Growth Capacity of a Novel Potential Probiotic Lactobacillus paracasei K5 Strain Incorporated in Industrial White Brined Cheese as an Adjunct Culture. J. Food Sci. 2018, 83, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A.; Nigam, P. Enhanced probiotic viability and aromatic profile of yogurts produced using wheat bran (Triticum aestivum) as cell immobilization carrier. Process. Biochem. 2017, 55, 1–10. [Google Scholar] [CrossRef]
- Terpou, A.; Gialleli, A.-I.; Bosnea, L.; Kanellaki, M.; Koutinas, A.A.; Castro, G.R. Novel cheese production by incorporation of sea buckthorn berries (Hippophae rhamnoides L.) supported probiotic cells. LWT Food Sci. Technol. 2017, 79, 616–624. [Google Scholar] [CrossRef]
- Terpou, A.; Bosnea, L.; Kanellaki, M. Effect of Mastic Gum (Pistacia Lentiscus Via Chia) as a Probiotic Cell Encapsulation Carrier for Functional Whey Beverage Production. SCIOL Biomed. 2017, 1, 1–10. [Google Scholar]
- Bosnea, L.A.; Kopsahelis, N.; Kokkali, V.; Terpou, A.; Kanellaki, M. Production of a novel probiotic yogurt by incorporation of L. casei enriched fresh apple pieces, dried raisins and wheat grains. Food Bioprod. Process. 2017, 102, 62–71. [Google Scholar] [CrossRef]
- Saxami, G.; Ypsilantis, P.; Sidira, M.; Simopoulos, C.; Kourkoutas, Y.; Galanis, A. Distinct adhesion of probiotic strain Lactobacillus casei ATCC 393 to rat intestinal mucosa. Anaerobe 2012, 18, 417–420. [Google Scholar] [CrossRef]
- Lye, H.-S.; Rahmat-Ali, G.R.; Liong, M.-T. Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int. Dairy J. 2010, 20, 169–175. [Google Scholar] [CrossRef]
- Cordeiro, M.A.; Souza, E.L.S.; Arantes, R.M.E.; Balthazar, C.F.; Guimarães, J.T.; Scudino, H.; Silva, H.L.A.; Rocha, R.S.; Freitas, M.Q.; Esmerino, E.A.; et al. Fermented whey dairy beverage offers protection against Salmonella enterica ssp. enterica serovar Typhimurium infection in mice. J. Dairy Sci. 2019, 102, 6756–6765. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; de Alencar, S.M.; de Sousa, R.L.M.; Moreno, A.M.; Da Gloria, E.M. Antimicrobial activity of several essential oils on pathogenic and beneficial bacteria. Ind. Crops Prod. 2017, 97, 128–136. [Google Scholar] [CrossRef]
- Hernández, A.; Pérez-Nevado, F.; Ruiz-Moyano, S.; Serradilla, M.J.; Villalobos, M.C.; Martín, A.; Córdoba, M.G. Spoilage yeasts: What are the sources of contamination of foods and beverages? Int. J. Food Microbiol. 2018, 286, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Khorshidian, N.; Yousefi, M.; Khanniri, E.; Mortazavian, A.M. Potential application of essential oils as antimicrobial preservatives in cheese. Innov. Food Sci. Emerg. Technol. 2018, 45, 62–72. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Marei, G.I.K.; Rabea, E.I.; Taktak, N.E.M. Antimicrobial and antioxidant activities of hydrocarbon and oxygenated monoterpenes against some foodborne pathogens through in vitro and in silico studies. Pesticide Biochem. Physiol. 2019, 158, 185–200. [Google Scholar] [CrossRef]
- Mantzourani, I.; Terpou, A.; Alexopoulos, A.; Kimbaris, A.; Bezirtzoglou, E.; Koutinas, A.A.; Plessas, S. Production of a Potentially Synbiotic Pomegranate Beverage by Fermentation with Lactobacillus plantarum ATCC 14917 Adsorbed on a Prebiotic Carrier. Appl. Biochem. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Adams, T.B.; Gavin, C.L.; McGowen, M.M.; Waddell, W.J.; Cohen, S.M.; Feron, V.J.; Marnett, L.J.; Munro, I.C.; Portoghese, P.S.; Rietjens, I.M.C.M.; et al. The FEMA GRAS assessment of aliphatic and aromatic terpene hydrocarbons used as flavor ingredients. Food Chem. Toxicol. 2011, 49, 2471–2494. [Google Scholar] [CrossRef]
- Rodríguez-López, M.I.; Mercader-Ros, M.T.; Pellicer, J.A.; Gómez-López, V.M.; Martínez-Romero, D.; Núñez-Delicado, E.; Gabaldón, J.A. Evaluation of monoterpene-cyclodextrin complexes as bacterial growth effective hurdles. Food Control 2019. [Google Scholar] [CrossRef]
- Ameh, S.J.; Obodozie-Ofoegbu, O. Chapter 11—Essential Oils as Flavors in Carbonated Cola and Citrus Soft Drinks. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 111–121. [Google Scholar]
- Duru, M.E.; Cakir, A.; Kordali, S.; Zengin, H.; Harmandar, M.; Izumi, S.; Hirata, T. Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia 2003, 74, 170–176. [Google Scholar] [CrossRef]
- Fink, J.K. 12—Terpene Resins. In Reactive Polymers: Fundamentals and Applications, 3rd ed.; Fink, J.K., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 403–415. [Google Scholar]
- Curioni, P.M.G.; Bosset, J.O. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Sun, L.; Liao, D.; Yang, Z.; Chen, X.; Tong, Z. Measurement and correlation of (vapor+liquid) equilibrium data for {α-pinene+p-cymene+(S)-(−)-limonene} ternary system at atmospheric pressure. J. Chem. Thermodynam. 2013, 58, 416–421. [Google Scholar] [CrossRef]
- Risner, D.; Tomasino, E.; Hughes, P.; Meunier-Goddik, L. Volatile aroma composition of distillates produced from fermented sweet and acid whey. J. Dairy Sci. 2019, 102, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Vichi, S.; Riu-Aumatell, M.; Mora-Pons, M.; Guadayol, J.M.; Buxaderas, S.; López-Tamames, E. HS-SPME coupled to GC/MS for quality control of Juniperus communis L. berries used for gin aromatization. Food Chem. 2007, 105, 1748–1754. [Google Scholar] [CrossRef]
- Petrović, J.; Stojković, D.; Soković, M. Terpene core in selected aromatic and edible plants: Natural health improving agents. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2019; Volume 90, pp. 423–451. [Google Scholar]
- Memariani, Z.; Sharifzadeh, M.; Bozorgi, M.; Hajimahmoodi, M.; Farzaei, M.H.; Gholami, M.; Siavoshi, F.; Saniee, P. Protective effect of essential oil of Pistacia atlantica Desf. on peptic ulcer: Role of α-pinene. J. Tradit. Chin. Med. 2017, 37, 57–63. [Google Scholar] [CrossRef]
- Rodrigues, K.A.d.F.; Amorim, L.V.; Dias, C.N.; Moraes, D.F.C.; Carneiro, S.M.P.; Carvalho, F.A.D.A. Syzygium cumini (L.) Skeels essential oil and its major constituent α-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. J. Ethnopharmacol. 2015, 160, 32–40. [Google Scholar] [CrossRef]
- Ebmeyer, F. Theoretical investigations towards an understanding of the α-pinene/camphene rearrangement. J. Molec. Struct. Theochem. 2002, 582, 251–255. [Google Scholar] [CrossRef]
- Girola, N.; Figueiredo, C.R.; Farias, C.F.; Azevedo, R.A.; Ferreira, A.K.; Teixeira, S.F.; Capello, T.M.; Martins, E.G.A.; Matsuo, A.L.; Travassos, L.R.; et al. Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo. Biochem. Biophys. Res. Com. 2015, 467, 928–934. [Google Scholar] [CrossRef]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Rønhave Laursen, R.; Ouwehand, A.C. The Production and Delivery of Probiotics: A Review of a Practical Approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef]
- Mantzourani, I.; Kazakos, S.; Terpou, A.; Mallouchos, A.; Kimbaris, A.; Alexopoulos, A.; Bezirtzoglou, E.; Plessas, S. Assessment of Volatile Compounds Evolution, Antioxidant Activity, and Total Phenolics Content during Cold Storage of Pomegranate Beverage Fermented by Lactobacillus paracasei K5. Fermentation 2018, 4, 95. [Google Scholar] [CrossRef]
- Capozzi, V.; Yener, S.; Khomenko, I.; Farneti, B.; Cappellin, L.; Gasperi, F.; Scampicchio, M.; Biasioli, F. PTR-ToF-MS Coupled with an Automated Sampling System and Tailored Data Analysis for Food Studies: Bioprocess Monitoring, Screening and Nose-space Analysis. J. Vis. Exp. 2017, 23, 54075. [Google Scholar] [CrossRef]
- Aderinola, T.A. Nutritional, Antioxidant and Quality Acceptability of Smoothies Supplemented with Moringa oleifera Leaves. Beverages 2018, 4, 104. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Garcia, C.; Fessard, A.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M.; Remize, F. Nutritional and Microbiological Quality of Tiger Nut Tubers (Cyperus esculentus), Derived Plant-Based and Lactic Fermented Beverages. Fermentation 2018, 5, 3. [Google Scholar] [CrossRef]
- Mallouchos, A.; Paul, L.; Argyro, B.; Koutinas, A.; Komaitis, M. Ambient and low temperature winemaking by immobilized cells on brewer’s spent grains: Effect on volatile composition. Food Chem. 2007, 104, 918–927. [Google Scholar] [CrossRef]
- Kopsahelis, N.; Panas, P.; Kourkoutas, Y.; Koutinas, A.A. Evaluation of thermally-dried immobilized cells of Lactobacillus delbrueckii subsp. bulgaricus on apple pieces as a potent starter culture. J. Agri. Food Chem. 2007, 55, 9829–9836. [Google Scholar]
- Terpou, A.; Gialleli, A.; Bekatorou, A.; Dimitrellou, D.; Ganatsios, V.; Barouni, E.; Koutinas, A.A.; Kanellaki, M. Sour milk production by wheat bran supported probiotic biocatalyst as starter culture. Food Bioprod. Process. 2017, 101, 184–192. [Google Scholar] [CrossRef]
- Dan, T.; Ren, W.; Liu, Y.; Tian, J.; Chen, H.; Li, T.; Liu, W. Volatile Flavor Compounds Profile and Fermentation Characteristics of Milk Fermented by Lactobacillus delbrueckii subsb. bulgaricus. Front. Microbiol. 2019, 10, 2183. [Google Scholar] [CrossRef]
- Karagul-Yuceer, Y.; Drake, M.A.; Cadwallader, K.R. Aroma-active Components of Liquid Cheddar Whey. J. Food Sci. 2003, 68, 1215–1219. [Google Scholar] [CrossRef]
- Lee, C.W.; Richard, J. Catabolism of L-phenylalanine by some microorganisms of cheese origin. J. Dairy Res. 1984, 51, 461–469. [Google Scholar] [CrossRef]
- Quach, M.L.; Chen, X.D.; Stevenson, R.J. Headspace sampling of whey protein concentrate solutions using solid-phase microextraction. Food Res. Int. 1999, 31, 371–379. [Google Scholar] [CrossRef]
- Bergamaschi, M.; Bittante, G. From milk to cheese: Evolution of flavor fingerprint of milk, cream, curd, whey, ricotta, scotta, and ripened cheese obtained during summer Alpine pasture. J. Dairy Sci. 2018, 101, 3918–3934. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Levićc, S.; Petrovićc, T.; Ivanovićd, S.; Nedovićc, V.; Kourkoutas, Y. Encapsulation of Lactobacillus casei ATCC 393 in alginate capsules for probiotic fermented milk production. LWT Food Sci. Technol. 2019, 116, 108501. [Google Scholar] [CrossRef]
- Sattin, E.; Andreani, N.A.; Carraro, L.; Lucchini, R.; Fasolato, L.; Telatin, A.; Balzan, S.; Novelli, E.; Simionati, B.; Cardazzo, B. A Multi-Omics Approach to Evaluate the Quality of Milk Whey Used in Ricotta Cheese Production. Front. Microbiol. 2016, 7, 1272. [Google Scholar] [CrossRef] [PubMed]
| Whey Beverage | Storage Time (Days) | pH | Total Acidity 1 | Glucose 2 | Galactose 2 | Lactose 2 |
|---|---|---|---|---|---|---|
| C | 1 | 3.92 ± 0.02 a | 0.6 ± 0.04 a | 0.30 ± 0.01 a | 0.18 ± 0.01 a | 1.82 ± 0.01 a |
| 15 | 3.93 ± 0.02 a | 0.6 ± 0.04 a | 0.27 ± 0.02 a | 0.18 ± 0.01 a | 1.80 ± 0.01 a | |
| 30 | 4.02 ± 0.02 b | 0.5 ± 0.04 b | 0.22 ± 0.01 b | 0.11 ± 0.01 b | 1.80 ± 0.01 a | |
| A1 | 1 | 3.90 ± 0.02 a | 0.7 ± 0.04 a | 0.16 ± 0.01 a | 0.20 ± 0.02 a | 1.80 ± 0.01 a |
| 15 | 3.69 ± 0.02 b | 0.7 ± 0.03 a | 0.13 ± 0.01 b | 0.21 ± 0.02 a | 1.79 ± 0.01 a | |
| 30 | 3.67 ± 0.02 b | 0.7 ± 0.03 a | 0.11 ± 0.01 b | 0.23 ± 0.02 a | 1.70 ± 0.01 b | |
| A2 | 1 | 3.90 ± 0.02 a | 0.7 ± 0.04 a | 0.17 ± 0.01 a | 0.20 ± 0.02 a | 1.78 ± 0.01 a |
| 15 | 3.71 ± 0.02 b | 0.7 ± 0.03 a | 0.13 ± 0.01 b | 0.22 ± 0.02 a | 1.70 ± 0.01 b | |
| 30 | 3.64 ± 0.02 c | 0.7 ± 0.04 a | 0.10 ± 0.02 b | 0.21 ± 0.02 a | 1.65 ± 0.02 c | |
| A3 | 1 | 3.90 ± 0.02 a | 0.7 ± 0.03 a | 0.17 ± 0.01 a | 0.25 ± 0.02 a | 1.81 ± 0.01 a |
| 15 | 3.71 ± 0.02 b | 0.7 ± 0.04 a | 0.14 ± 0.02 b | 0.23 ± 0.02 a | 1.80 ± 0.01 a | |
| 30 | 3.71 ± 0.02 b | 0.7 ± 0.04 a | 0.13 ± 0.05 b | 0.24 ± 0.02 a | 1.77 ± 0.01 b | |
| B1 | 1 | 3.93 ± 0.02 a | 0.6 ± 0.03 a | 0.33 ± 0.02 a | 0.18 ± 0.01 a | 1.83 ± 0.01 a |
| 15 | 3.91 ± 0.02 a | 0.6 ± 0.04 a | 0.31 ± 0.02 a | 0.18 ± 0.02 a | 1.83 ± 0.01 a | |
| 30 | 3.88 ± 0.02 a | 0.6 ± 0.04 a | 0.32 ± 0.02 a | 0.15 ± 0.03 a | 1.83 ± 0.01 a | |
| B2 | 1 | 3.93 ± 0.02 a | 0.6 ± 0.04 a | 0.31 ± 0.02 a | 0.17 ± 0.01 a | 1.80 ± 0.01 a |
| 15 | 3.91 ± 0.03 a | 0.6 ± 0.04 a | 0.32 ± 0.02 a | 0.15 ± 0.03 a | 1.80 ± 0.01 a | |
| 30 | 3.90 ± 0.02 a | 0.6 ± 0.04 a | 0.33 ± 0.02 a | 0.15 ± 0.02 a | 1.81 ± 0.02 a | |
| B3 | 1 | 3.94 ± 0.02 a | 0.6 ± 0.04 a | 0.33 ± 0.02 a | 0.18 ± 0.01 a | 1.83 ± 0.01 a |
| 15 | 3.92 ± 0.02 a | 0.6 ± 0.04 a | 0.34 ± 0.02 a | 0.17 ± 0.01 a | 1.82 ± 0.01 a | |
| 30 | 3.91 ± 0.02 a | 0.6 ± 0.04 a | 0.32 ± 0.02 a | 0.18 ± 0.02 a | 1.82 ± 0.01 a |
| Compounds | ID * | C | A2 | B2 |
|---|---|---|---|---|
| Esters | ||||
| ethyl acetate | RT, KI, MS | 1.4 | 0.7 | 1.3 |
| ethyl butanoate | RT, KI, MS | 2.7 | 1.3 | 2.1 |
| propyl butanoate | MS | Nd | 0.1 | 0.2 |
| ethyl pentanoate | MS | Nd | 0.1 | 0.1 |
| butyl butanoate | MS | 1.3 | 0.8 | 1.2 |
| ethyl hexanoate | RT, KI, MS | 2.5 | 1.5 | 1.9 |
| ethyl heptanoate | RT, MS | Nd | Tr | 0.2 |
| ethyl octanoate | RT, KI, MS | 3.4 | 1.7 | 3.1 |
| ethyl nonanoate | RT, MS | Nd | 0.1 | 0.1 |
| ethyl decanoate | RT, KI, MS | 1.2 | 0.8 | 0.8 |
| ethyl dodecanoate | KI, MS | 0.8 | 0.5 | 0.5 |
| ethyl tetradecanoate | KI, MS | Nd | 0.1 | Nd |
| Sum esters/Sum total compounds | 13.2 | 7.7 | 11.5 | |
| Organic acids | ||||
| boutanoic acid | RT, MS | 4.1 | 2.7 | 3.3 |
| hexanoic acid | RT, MS | 11.6 | 11.6 | 10.6 |
| 2-methyl-butanoic acid | KI, MS | Nd | 0.6 | Nd |
| 3-methyl-butanoic acid | KI, MS | Nd | 0.9 | 0.1 |
| octanoic acid | RT, KI, MS | 13.5 | 16.2 | 10.3 |
| nonanoic acid | RT, KI, MS | 2.8 | 1.5 | 2.1 |
| decanoic acid | RT, KI, MS | 8.7 | 14.5 | 7.4 |
| Sum acids/Sum total compounds | 40.6 | 48.1 | 33.7 | |
| Alcohols | ||||
| 2-pentanol | Nd | 0.3 | Nd | |
| 2-methyl-1-butanol | RT, MS | Nd | 0.3 | Nd |
| 3-methyl-1-butanol | RT, MS | 1.6 | Nd | Nd |
| 2-heptanol | RT, MS | Nd | 0.2 | Nd |
| 1-hexanol | RT, KI, MS | 9.6 | 2.4 | 6.6 |
| 1-octen-3-ol | RT, KI, MS | 2.5 | 1.5 | 3.1 |
| 1-heptanol | RT, KI, MS | 1.2 | 1.4 | Nd |
| 2-ethyl-1-hexanol | RT, KI, MS | 1.8 | 1.3 | 1.7 |
| 1-octanol | RT, KI, MS | 1.0 | 1.0 | 0.2 |
| phenylethyl alcohol | RT, KI, MS | 0.9 | 2.2 | 2.4 |
| Sum alcohols/Sum total compounds | 18.7 | 10.5 | 14.0 | |
| Aldehydes | ||||
| 3-methyl butanal | RT, MS | 1.6 | 1.0 | 2.3 |
| hexanal | RT, KI, MS | 6.0 | 2.4 | 3.9 |
| heptanal | RT, KI, MS | 2.9 | 1.7 | 3.4 |
| octanal | RT, KI, MS | 1.1 | 0.9 | Nd |
| 2-pentenal | RT, MS | Nd | 0.7 | Nd |
| 2-heptenal | RT, KI, MS | Nd | Nd | 0.1 |
| 2-octenal | RT, MS | 1.3 | 0.8 | 2.2 |
| nonanal | RT, MS | Nd | 0.2 | Nd |
| decanal | RT, KI, MS | 1.2 | 0.5 | 1.1 |
| benzaldehyde | RT, KI, MS | 1.1 | 0.7 | 1.3 |
| Sum aldehydes/Sum total compounds | 15.4 | 8.9 | 14.4 | |
| Ketones | ||||
| 2-butanone | RT, KI, MS | 3.7 | 4.6 | 3.1 |
| 2-pentanone | RT, MS | 2.7 | 3.1 | 3.3 |
| 2-heptanone | RT, MS | 1.9 | 3.3 | 2.5 |
| 2-nonanone | RT, MS | 2.8 | 3.9 | 1.9 |
| 2,3-butanedione | RT, KI, MS | 1.0 | 3.0 | Nd |
| Sum ketones/Sum total compounds | 12.1 | 17.9 | 10.7 | |
| Monoterpenes | ||||
| a-pinene | KI, MS | Tr | 5.3 | 11.6 |
| camphere | KI, MS | Nd | 0.1 | 0.2 |
| β-pinene | KI, MS | Nd | 0.3 | 0.7 |
| 3-carene | KI, MS | Nd | Tr | 0.1 |
| β-myrcene | KI, MS | Nd | Tr | 0.1 |
| 2-carene | KI, MS | Nd | Nd | 0.0 |
| D-limonene | KI, MS | Tr | 0.1 | 0.3 |
| Beta-phellandrene | KI, MS | Nd | Tr | 0.0 |
| o-cymene | KI, MS | Nd | 0.1 | 0.2 |
| Sum monoterpenes/Sum total | Tr | 5.9 | 13.2 | |
| Oxygenated Monoterpenes | ||||
| eucalyptol | KI, MS | Nd | 0.1 | 0.1 |
| terpinolene | KI, MS | Nd | 0.2 | 0.5 |
| linalool | KI, MS | Nd | 0.1 | 0.2 |
| 4-terpineol | KI, MS | Nd | 0.1 | 0.2 |
| pinocarveol | KI, MS | Nd | 0.1 | 0.2 |
| verbenol | KI, MS | Nd | Tr | 0.1 |
| α-terpineol | KI, MS | Nd | 0.3 | 0.8 |
| melilotal | KI, MS | Nd | Tr | 0.1 |
| myrtenol | KI, MS | Nd | Tr | 0.1 |
| p-cymene-8-ol | KI, MS | Nd | 0.1 | 0.2 |
| Sum oxyg. monoterpenes/Sum total | Tr | 1.0 | 2.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoina, V.; Terpou, A.; Papadaki, A.; Bosnea, L.; Kopsahelis, N.; Kanellaki, M. Enhanced Aromatic Profile and Functionality of Cheese Whey Beverages by Incorporation of Probiotic Cells Immobilized on Pistacia terebinthus Resin. Foods 2020, 9, 13. https://doi.org/10.3390/foods9010013
Schoina V, Terpou A, Papadaki A, Bosnea L, Kopsahelis N, Kanellaki M. Enhanced Aromatic Profile and Functionality of Cheese Whey Beverages by Incorporation of Probiotic Cells Immobilized on Pistacia terebinthus Resin. Foods. 2020; 9(1):13. https://doi.org/10.3390/foods9010013
Chicago/Turabian StyleSchoina, Vasiliki, Antonia Terpou, Aikaterini Papadaki, Loulouda Bosnea, Nikolaos Kopsahelis, and Maria Kanellaki. 2020. "Enhanced Aromatic Profile and Functionality of Cheese Whey Beverages by Incorporation of Probiotic Cells Immobilized on Pistacia terebinthus Resin" Foods 9, no. 1: 13. https://doi.org/10.3390/foods9010013
APA StyleSchoina, V., Terpou, A., Papadaki, A., Bosnea, L., Kopsahelis, N., & Kanellaki, M. (2020). Enhanced Aromatic Profile and Functionality of Cheese Whey Beverages by Incorporation of Probiotic Cells Immobilized on Pistacia terebinthus Resin. Foods, 9(1), 13. https://doi.org/10.3390/foods9010013