Kinetic Improvement of Bioactive Compounds Extraction from Red Grape (Vitis vinifera Moldova) Pomace by Ultrasonic Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction Technique
2.3. Total Monomeric Anthocyanin Determination
2.4. Total Phenolic Content
2.5. Raman Spectra Analysis
2.6. Statistical Analysis
2.6.1. Analysis of Variance
2.6.2. Response Surface Methodology (RSM)
3. Results and Discussion
3.1. Influence of Extraction Parameters on TMA and TPC Yield
3.1.1. Solvent Type and Concentration
3.1.2. Ultrasonic Frequency
3.1.3. Temperature and Time
3.2. Extraction Modeling
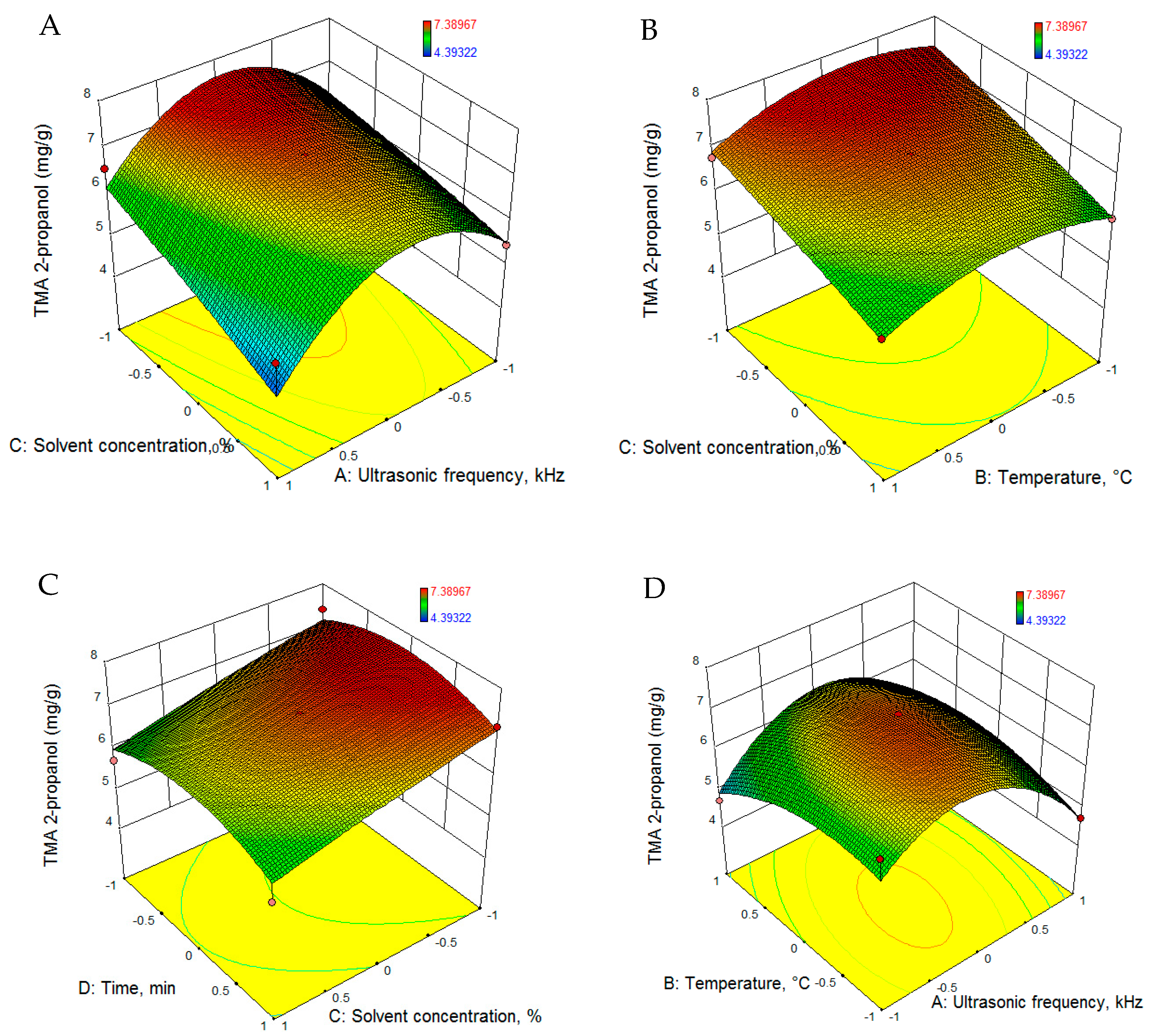
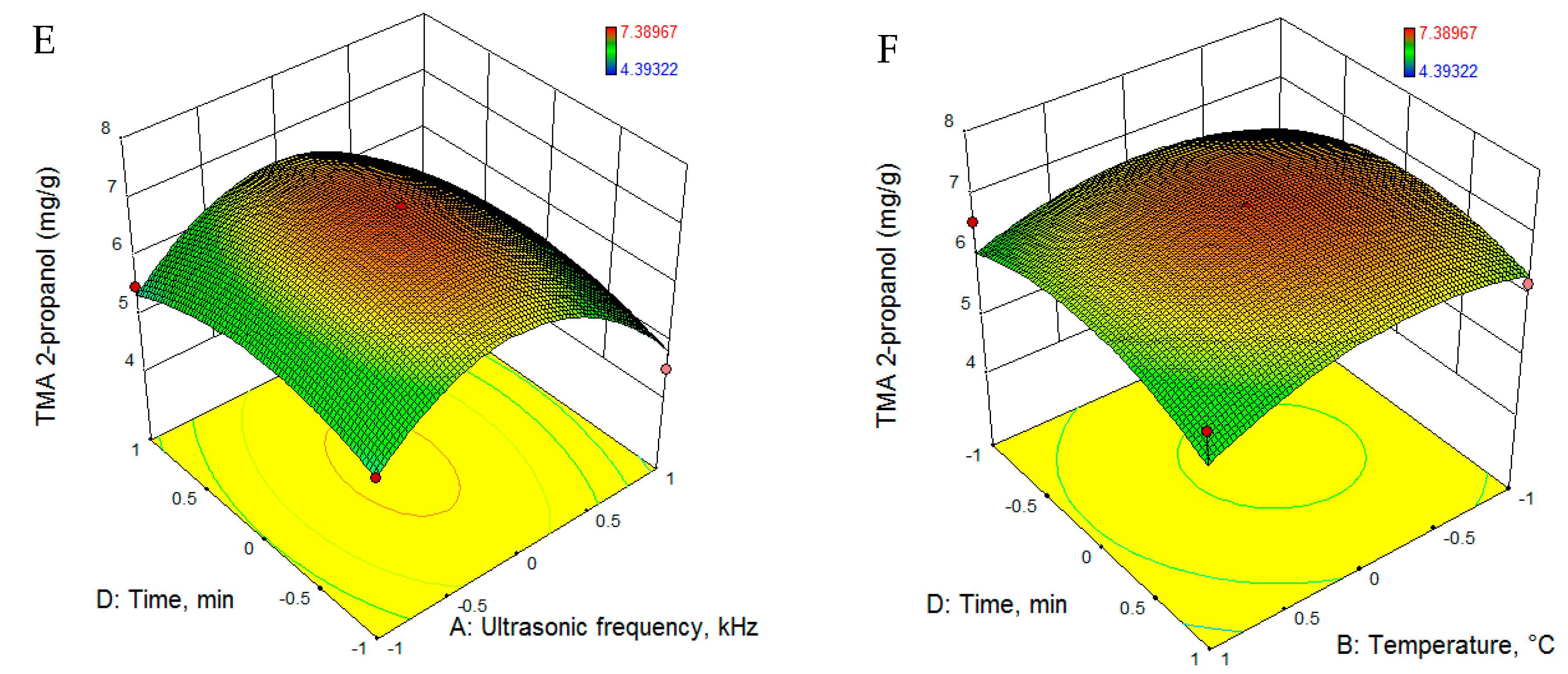
3.2.1. Total Monomeric Anthocyanin
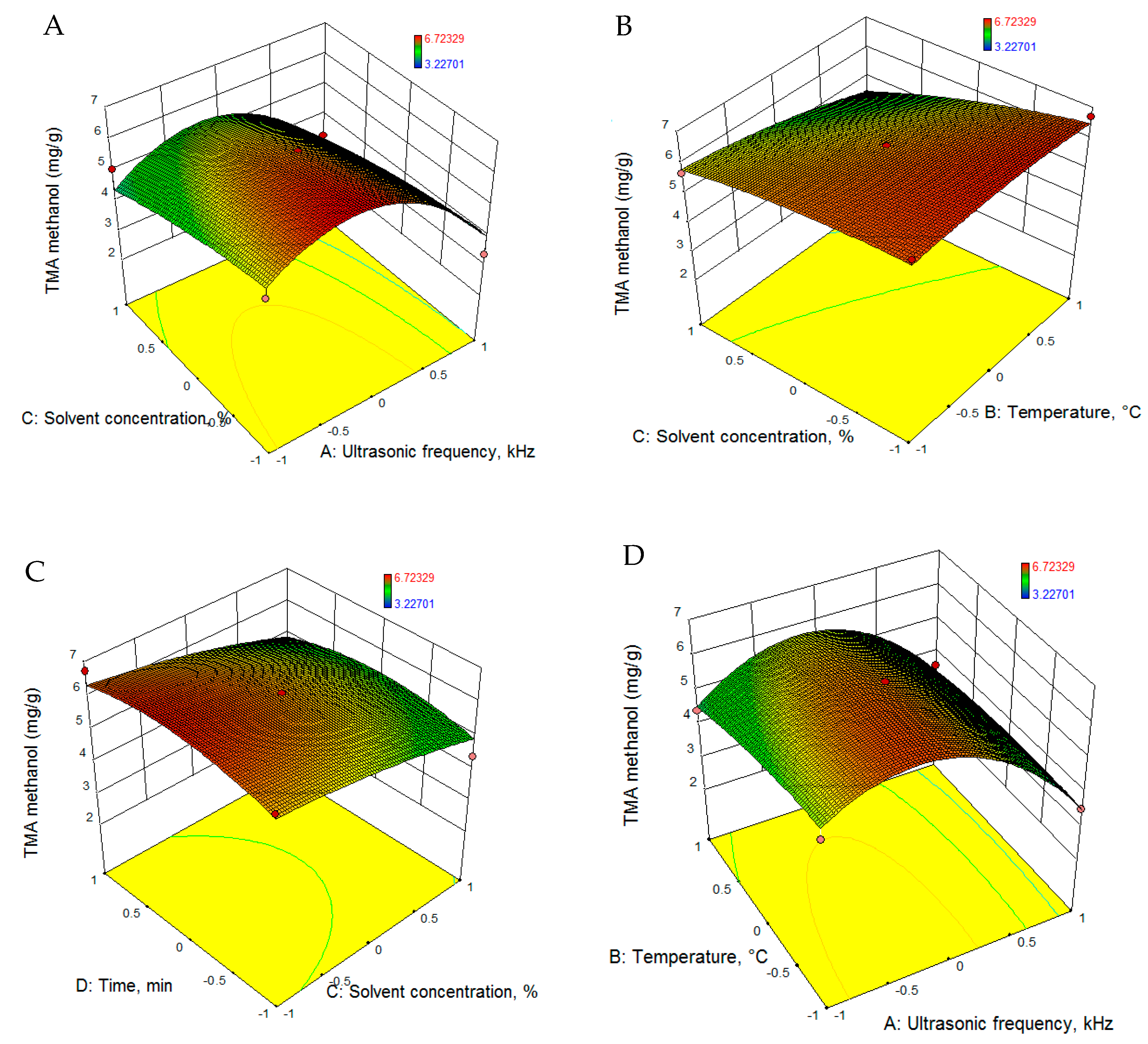
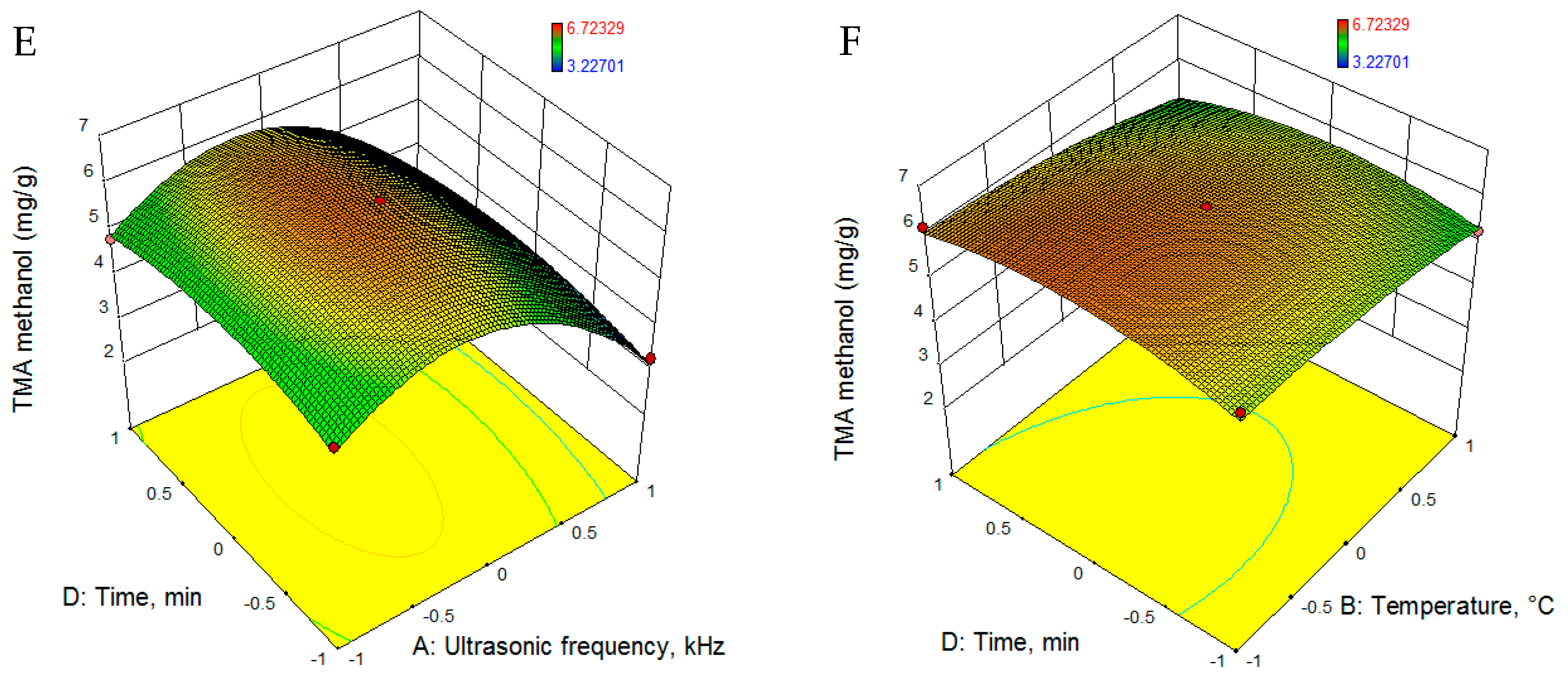
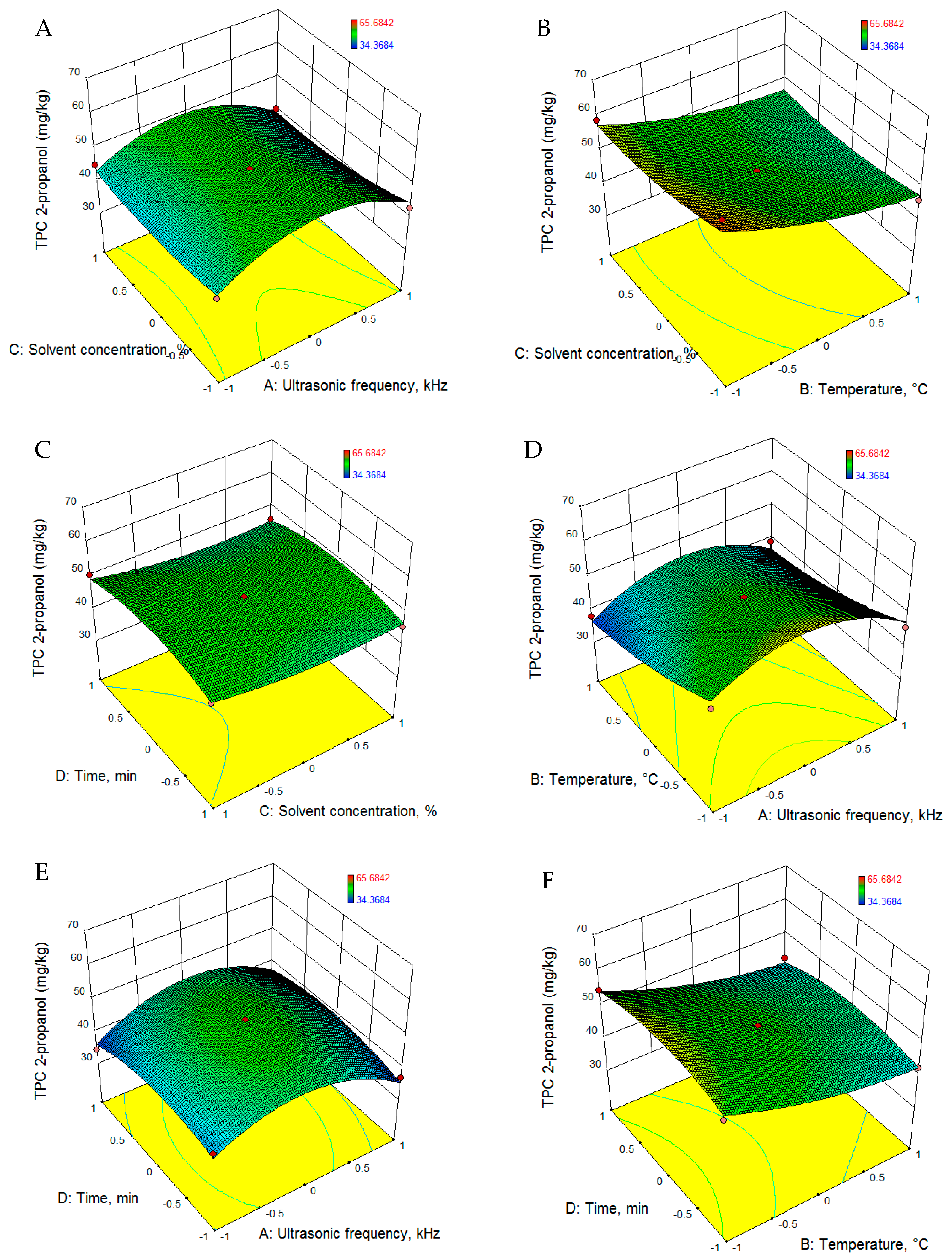
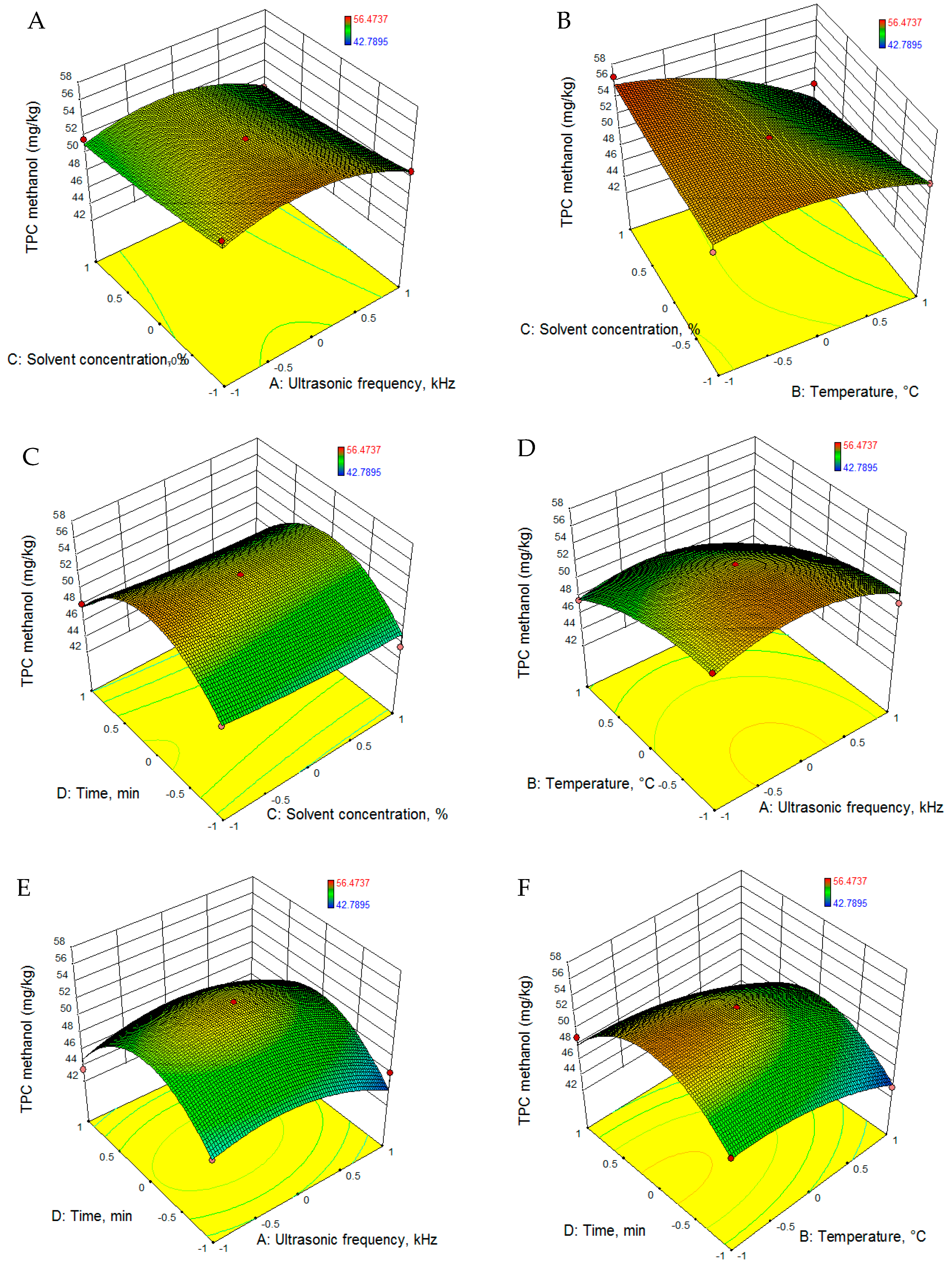
3.2.2. Total Phenolic Content
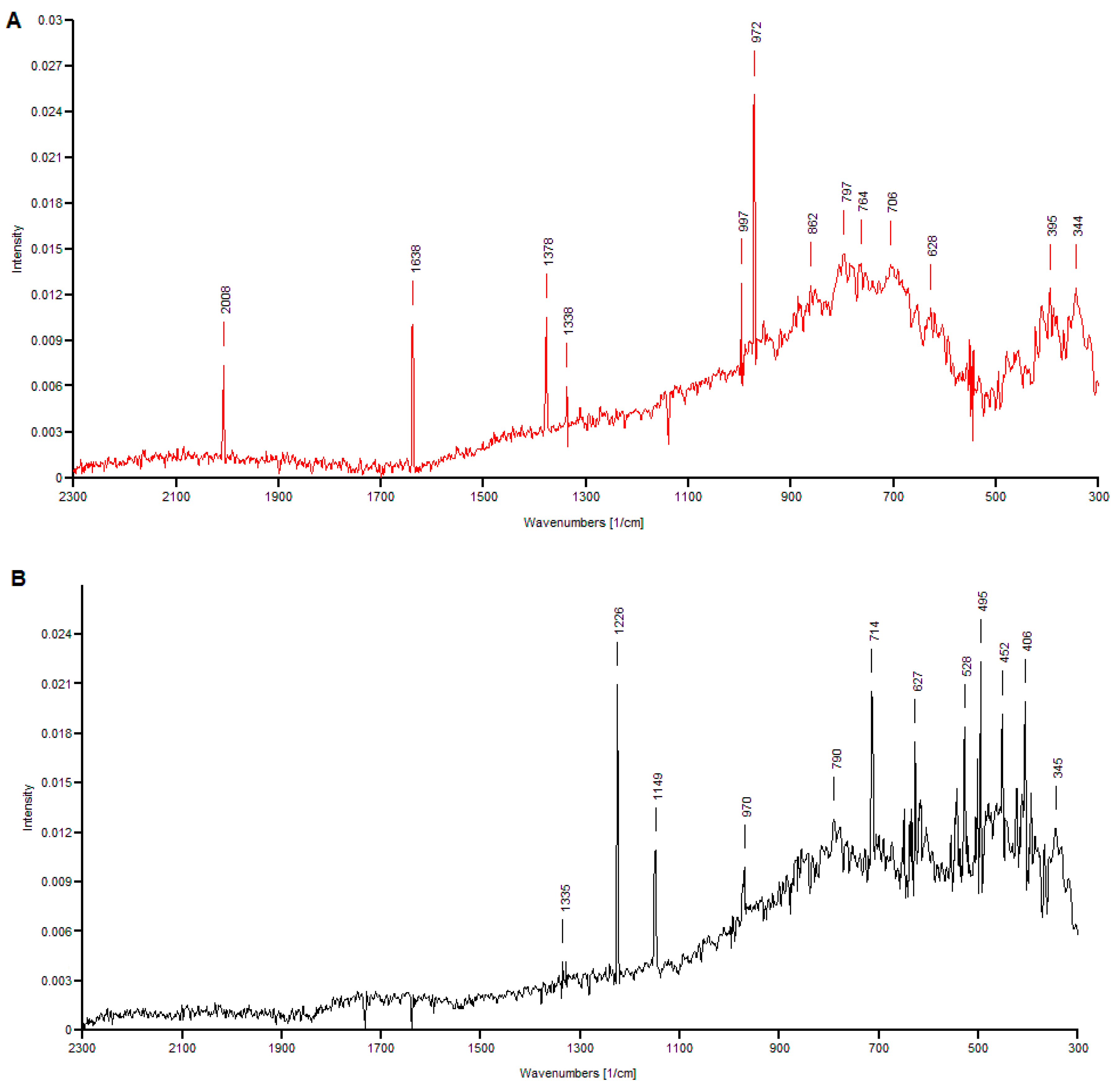
3.3. Raman Spectra Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yi, C.; Shi, J.; Kramer, J.; Xue, S.; Jiang, Y.; Zhang, M.; Ma, Y.; Pohorly, J. Fatty acid composition and phenolic antioxidants of winemaking pomace powder. Food Chem. 2009, 114, 570–576. [Google Scholar] [CrossRef]
- Kierath, T.; Wang, C. The Global Wine Industry Slowly Moving from Balance to Shortage; Morgan Stanley Research: London, UK, 2013. [Google Scholar]
- International Organisation of Vine and Wine. Global Economic Vitiviniculture Data; International Organisation of Vine and Wine: Paris, France, 2015; Available online: http://www.oiv.int/public/medias/2256/en-communique-de-presse-octobre-2015.pdf (accessed on 15 September 2016).
- Wadhwa, M.; Bakshi, M.P.S. Utilization of Fruit and Vegetable Wastes as Livestock Feed and as Substrates for Generation of Other Value-Added Products; RAP Publication; Makkar, H.P.S., Ed.; Food and Agriculture Organization of the United Nations, Regional office for Asia and the Pacific Bangkok: Bangkok, Thailand, 2013; Volume 4, pp. 1–56. [Google Scholar]
- Chouchouli, V.; Kalogeropoulos, N.; Konteles, S.J.; Karvela, E.; Makris, D.P.; Karathanos, V.T. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT Food Sci. Technol. 2013, 53, 522–529. [Google Scholar] [CrossRef]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Dwyer, K.; Hosseinian, F.; Rod, M. The Market Potential of Grape Waste Alternatives. J. Food Res. 2014, 3, 91. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Jiang, Y.; Simonsen, J.; Zhao, Y. Compression-molded biocomposite boards from red and white wine grape pomaces. J. Appl. Polym. Sci. 2011, 119, 2834–2846. [Google Scholar] [CrossRef]
- Maier, T.; Schieber, A.; Kammerer, D.R.; Carle, R. Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem. 2009, 112, 551–559. [Google Scholar] [CrossRef]
- Mirzaei-Aghsaghali, A.; Maheri-Sis, N.; Mansouri, H.; Razeghi, M.E.; Safaei, A.R.; Aghajanzadeh-Golshani, A.; Alipoor, K. Estimation of the nutritive value of tomato pomace for ruminant using in vitro gas production technique. Afr. J. Biotech. 2011, 10, 6251–6256. [Google Scholar]
- Llobera, A.; Cañellas, J. Dietary fibre content and antioxidant activity of Manto Negro red grape (Vitis vinifera): Pomace and stem. Food Chem. 2007, 101, 659–666. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef]
- Rondeau, P.; Gambier, F.; Jolibert, F.; Brosse, N. Compositions and chemical variability of grape pomaces from French vineyard. Ind. Crop. Prod. 2013, 43, 251–254. [Google Scholar] [CrossRef]
- Zacharof, M.-P. Grape Winery Waste as Feedstock for Bioconversions: Applying the Biorefinery Concept. Waste Biomass Valoriz. 2016, 8, 1011–1025. [Google Scholar] [CrossRef] [Green Version]
- López-Vázquez, C.; Bollaín, M.H.; Moser, S.; Orriols, I. Characterization and differentiation of monovarietal grape pomace distillate from native varieties of Galicia. J. Agric. Food Chem. 2010, 58, 9657–9665. [Google Scholar] [CrossRef] [PubMed]
- Arrieta-Garay, Y.; Blanco, P.; López-Vázquez, C.; Rodríguez-Bencomo, J.J.; Pérez-Correa, J.R.; Lopez, F.; Orriols, I. Effects of distillation system and yeast strain on the aroma profile of Albariño (Vitis vinifera L.) grape pomace spirits. J. Agric. Food Chem. 2014, 62, 10552–10560. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.; Barral, M.T.; Cruz, J.; Moldes, A. Valorization of winery waste vs. the costs of not recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Benmeziane, F.; Cadot, Y.; Djamai, R.; Djermoun, L. Determination of major anthocyanin pigments and flavonols in red grape skin of some table grape varieties (Vitis vinifera sp.) by high-performance liquid chromatography–photodiode array detection (HPLC-DAD). OENO One 2016, 50. [Google Scholar] [CrossRef]
- Luan, L.Y.; Zhang, Z.W.; Xi, Z.M.; Huo, S.S.; Ma, L.N. Brassinosteroids regulate anthocyanin biosynthesis in the ripening of grape berries. S. Afr. J. Enol. Vitic. 2013, 34, 196–203. [Google Scholar] [CrossRef]
- Amico, V.; Chillemi, R.; Mangiafico, S.; Spatafora, C.; Tringali, C. Polyphenol-enriched fractions from Sicilian grape pomace: HPLC–DAD analysis and antioxidant activity. Bioresour. Technol. 2008, 99, 5960–5966. [Google Scholar] [CrossRef]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.L. Wine by-products: Phenolic characterization and antioxidant activity evaluation of grapes and grape pomaces from six different French grape varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef] [PubMed]
- Ratnasooriya, C.C.; Rupasinghe, H.V. Extraction of phenolic compounds from grapes and their pomace using β-cyclodextrin. Food Chem. 2012, 134, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Francis, F.J. Anthocyanins in grapes and grape products. Crit. Rev. Food Sci. Nutr. 1995, 35, 341–371. [Google Scholar] [CrossRef] [PubMed]
- Anđelković, M.; Radovanović, B.; Anđelković-Milenković, A.; Radovanović, V.; Zarubica, A.; Stojković, N.; Nikolić, V. The determination of bioactive ingredients of grape pomace (Vranac variety) for potential use in food and pharmaceutical industries. Adv. Technol. 2015, 4, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef] [PubMed]
- Sousa, E.C.; Uchôa-Thomaz, A.M.A.; Carioca, J.O.B.; Morais, S.M.D.; Lima, A.D.; Martins, C.G.; Alexandrino, C.D.; Ferreira, P.A.T.; Rodrigues, A.L.M.; Rodrigues, S.P.; et al. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol. 2014, 34, 135–142. [Google Scholar] [CrossRef] [Green Version]
- El Darra, N.; Grimi, N.; Vorobiev, E.; Louka, N.; Maroun, R. Extraction of polyphenols from red grape pomace assisted by pulsed ohmic heating. Food Biol. Technol. 2013, 6, 1281–1289. [Google Scholar] [CrossRef]
- Pinelo, M.; Ruiz-Rodríguez, A.; Sineiro, J.; Señoráns, F.J.; Reglero, G.; Núñez, M.J. Supercritical fluid and solid–liquid extraction of phenolic antioxidants from grape pomace: A comparative study. Eur. Food Res. Technol. 2007, 226, 199–205. [Google Scholar] [CrossRef]
- Casas, L.; Mantell, C.; Rodríguez, M.; De La Ossa, E.M.; Roldan, A.; De Ory, I.; Caro, I.; Blandino, A.; Cardoso, L.C. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J. Food Eng. 2010, 96, 304–308. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Ormazabal, M.; Vallejo, A.; Olivares, M.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Optimization of supercritical fluid consecutive extractions of fatty acids and polyphenols from Vitis vinifera grape wastes. J. Food Sci. 2015, 80, 101–107. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Li, Y.; Han, L.; Ma, R.; Xu, X.; Zhao, C.; Wang, Z.; Chen, F.; Hu, X. Effect of energy density and citric acid concentration on anthocyanins yield and solution temperature of grape peel in microwave-assisted extraction process. J. Food Eng. 2012, 109, 274–280. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Orsat, V.; Gariépy, Y.; Thangavel, K. Optimization of microwave-assisted extraction of phenolic antioxidants from grape seeds (Vitis vinifera). Food Biol. Technol. 2013, 6, 441–455. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Corrales, M.; García, A.F.; Butz, P.; Tauscher, B. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J. Food Eng. 2009, 90, 415–421. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Z.; Sun, D.W. Kinetic modeling of ultrasound-assisted extraction of phenolic compounds from grape marc: Influence of acoustic energy density and temperature. Ultrason. Sonochem. 2014, 21, 1461–1469. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Comas-Serra, F.; Femenia, A.; Rosselló, C.; Simal, S. Effect of power ultrasound application on aqueous extraction of phenolic compounds and antioxidant capacity from grape pomace (Vitis vinifera L.): Experimental kinetics and modeling. Ultrason. Sonochem. 2015, 22, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; García-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [Green Version]
- Rabino, I.; Mancinelli, A.L. Light, Temperature, and Anthocyanin Production. Plant Physiol. 1986, 81, 922–924. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Cheok, C.Y.; Chin, N.L.; Yusof, Y.A.; Talib, R.A.; Law, C.L. Optimization of total monomeric anthocyanin (TMA) and total phenolic content (TPC) extractions from mangosteen (Garciniamangostana Linn.) hull using ultrasonic treatments. Ind. Crop. Prod. 2013, 50, 1–7. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Ortega-Regules, A.; Romero-Cascales, I.; Ros Garcia, J.M.; Bautista-Ortin, A.B.; López-Roca, J.M.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Anthocyanins and tannins in four grape varieties (Vitis vinifera L.). Evolution of their content and extractability. OENO One 2008, 42, 147–156. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Bart, J.C.J. Additives in Polymers Industrial Analysis and Applications; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Budiul, M.; Albulescu, M. Comparative study on content of total phenols and flavonoids in grape pomace extracts. New Front. Chem. 2013, 22, 101–110. [Google Scholar]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crop. Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Cheng, V.J.; Bekhit, A.E.-D.A.; McConnell, M.; Mros, S.; Zhao, J. Effect of extraction solvent, waste fraction and grape variety on the antimicrobial and antioxidant activities of extracts from wine residue from cool climate. Food Chem. 2012, 134, 474–482. [Google Scholar] [CrossRef]
- Benmeziane, F.; Djamai, R.; Cadot, Y.; Seridi, R. Optimization of extraction parameters of phenolic compounds from Algerian fresh table grapes, (Vitis vinifera). Int. Food Res. J. 2014, 21, 1061–1065. [Google Scholar]
- Vardanega, R.; Santos, D.T.; Meireles, M.A.A. Intensification of bioactive compounds extraction from medicinal plants using ultrasonic irradiation. Pharmacogn. Rev. 2014, 8, 88–95. [Google Scholar] [Green Version]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G.; Lovillo, M.P. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef]
- Ghafoor, K.; Choi, Y.H. Optimization of Ultrasound Assisted Extraction of Phenolic Compounds and Antioxidants from Grape Peel through Response Surface Methodology. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 295–300. [Google Scholar] [CrossRef]
- Buckow, R.; Kastell, A.; Terefe, N.S.; Versteeg, C. Pressure and Temperature Effects on Degradation Kinetics and Storage Stability of Total Anthocyanins in Blueberry Juice. J. Agric. Food Chem. 2010, 58, 10076–10084. [Google Scholar] [CrossRef]
- Danisman, G.; Arslan, E.; Toklucu, A.K. Kinetic analysis of anthocyanin degradation and polymeric colour formation in grape juice during heating. Food Chem. 2015, 33, 103–108. [Google Scholar]
- Xu, D.P.; Zhou, Y.; Zheng, J.; Li, S.; Li, A.N.; Li, H.B. Optimization of ultrasound-assisted extraction of natural antioxidants from the flower of Jatrophaintegerrima by response surface methodology. Molecules 2015, 21, 18. [Google Scholar] [CrossRef]
- Zhu, Z.; Guan, Q.; Guo, Y.; He, J.; Liu, G.; Li, S.; Barba, F.J.; Jaffrin, M.Y. Green ultrasound-assisted extraction of anthocyanin and phenolic compounds from purple sweet potato using response surface methodology. Int. Agrophys. 2016, 30, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Goula, A.M.; Thymiatis, K.; Kaderides, K. Valorization of grape pomace: Drying behavior and ultrasound extraction of phenolics. Food Bioprod. Process. 2016, 100, 132–144. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Wang, L.J.; Li, D.; Jiao, S.S.; Chen, X.D.; Mao, Z.H. Ultrasound-assisted extraction of oil from flaxseed. Sep. Purif. Technol. 2008, 62, 192–198. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—A response surface approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar] [CrossRef]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds, Antioxidants, and Anthocyanins from Grape (Vitis vinifera) Seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef]
- Meneghini, C.; Caron, S.; Poulin, A.C.J.; Proulx, A.; Emond, F.; Paradis, P.; Pare, C.; Fougeres, A. Determination of Ethanol Concentration by Raman Spectroscopy in Liquid-Core Microstructured Optical Fiber. IEEE Sens. J. 2008, 8, 1250–1255. [Google Scholar] [CrossRef]
- Gallego, Á.L.; Guesalaga, A.R.; Bordeu, E.; González, Á.S. Rapid Measurement of Phenolics Compounds in Red Wine Using Raman Spectroscopy. IEEE Trans. Instrum. Meas. 2011, 60, 507–512. [Google Scholar] [CrossRef]
- Teslova, T.; Corredor, C.; Livingstone, R.; Spataru, T.; Birke, R.L.; Lombardi, J.R.; Cañamares, M.V.; Leona, M. Raman and surface-enhanced Raman spectra of flavone and several hydroxy derivatives. J. Raman Spectrosc. 2007, 38, 802–818. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sánchez-Cortés, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef]
| Factor | −1 | 0 | 1 |
|---|---|---|---|
| Concentration (%), C | 50 | 70 | 90 |
| Ultrasonic frequency (kHz), U | 12.5 | 25 | 37.5 |
| Temperature (°C), T | 50 | 60 | 70 |
| Time (min), t | 15 | 30 | 45 |
| Parameter | TMA (mg/g)–Mean Values | TPC (mg/g)–Mean Values | |
|---|---|---|---|
| Solvent type | 2-Propanol | 5.37 | 42.54 |
| Methanol | 4.58 | 46.53 | |
| ANOVA F-ratio | 215.32*** | 158.92*** | |
| Solvent concentration (%) | 50 | 5.40 | 45.72 |
| 70 | 5.01 | 44.65 | |
| 90 | 4.51 | 43.24 | |
| ANOVA F-ratio | 92.78*** | 20.72*** | |
| Ultrasonic frequency (kHz) | 12.5 | 4.92 | 43.52 |
| 25.0 | 6.03 | 48.92 | |
| 37.5 | 3.97 | 41.17 | |
| ANOVA F-ratio | 493.90*** | 210.32*** | |
| Temperature (°C) | 50 | 5.08 | 48.76 |
| 60 | 5.17 | 45.05 | |
| 70 | 4.68 | 39.80 | |
| ANOVA F-ratio | 31.88*** | 269.92*** | |
| Time (min) | 15 | 4.84 | 43.38 |
| 30 | 5.24 | 48.18 | |
| 45 | 4.84 | 42.05 | |
| ANOVA F-ratio | 24.73*** | 138.47*** |
| Process Parameters Interactions | TMA (mg/g) | TPC (mg/g) |
|---|---|---|
| Solvent type × concentration | 2.92ns | 0.08ns |
| Solvent type × ultrasonic frequency | 27.74*** | 71.76*** |
| Solvent type × temperature | 4.40* | 21.54*** |
| Solvent type × time | 0.31ns | 0.45ns |
| Concentration × ultrasonic frequency | 12.80*** | 3.37* |
| Concentration × temperature | 0.68ns | 6.58*** |
| Concentration × time | 0.24ns | 0.38ns |
| Ultrasonic frequency × temperature | 5.82*** | 1.17ns |
| Ultrasonic frequency × time | 0.62ns | 1.15ns |
| Temperature × time | 1.12ns | 0.47ns |
| Run | Standard Order | Ultrasonic Frequency, U | Temperature, T | Solvent Concentration, C | Time, t | ||||
|---|---|---|---|---|---|---|---|---|---|
| Coded | Experimental (kHz) | Coded | Experimental (°C) | Coded | Experimental (%) | Coded | Experimental (min) | ||
| 1 | 1 | −1 | 12.5 | −1 | 50 | 0 | 70 | 0 | 30 |
| 2 | 10 | 0 | 25 | 0 | 60 | 0 | 70 | 0 | 30 |
| 3 | 7 | 0 | 25 | 0 | 60 | −1 | 50 | 1 | 45 |
| 4 | 4 | 1 | 37.5 | 1 | 70 | 0 | 70 | 0 | 30 |
| 5 | 5 | 0 | 25 | 0 | 60 | −1 | 50 | −1 | 15 |
| 6 | 2 | 1 | 37.5 | −1 | 50 | 0 | 70 | 0 | 30 |
| 7 | 9 | 0 | 25 | 0 | 60 | 0 | 70 | 0 | 30 |
| 8 | 6 | 0 | 25 | 0 | 60 | 1 | 90 | −1 | 15 |
| 9 | 3 | −1 | 12.5 | 1 | 70 | 0 | 70 | 0 | 30 |
| 10 | 8 | 0 | 25 | 0 | 60 | 1 | 90 | 1 | 45 |
| 11 | 18 | 0 | 25 | 1 | 70 | 1 | 90 | 0 | 30 |
| 12 | 13 | −1 | 12.5 | 0 | 60 | 0 | 70 | 1 | 45 |
| 13 | 15 | 0 | 25 | −1 | 50 | −1 | 50 | 0 | 30 |
| 14 | 11 | −1 | 12.5 | 0 | 60 | 0 | 70 | −1 | 15 |
| 15 | 20 | 0 | 25 | 0 | 60 | 0 | 70 | 0 | 30 |
| 16 | 19 | 0 | 25 | 0 | 60 | 0 | 70 | 0 | 30 |
| 17 | 16 | 0 | 25 | 1 | 70 | −1 | 50 | 0 | 30 |
| 18 | 12 | 1 | 37.5 | 0 | 60 | 0 | 70 | −1 | 15 |
| 19 | 14 | 1 | 37.5 | 0 | 60 | 0 | 70 | 1 | 45 |
| 20 | 17 | 0 | 25 | −1 | 50 | 1 | 90 | 0 | 30 |
| 21 | 29 | 0 | 25 | 0 | 60 | 0 | 70 | 0 | 30 |
| 22 | 28 | 0 | 25 | 1 | 70 | 0 | 70 | 1 | 45 |
| 23 | 21 | −1 | 12.5 | 0 | 60 | −1 | 50 | 0 | 30 |
| 24 | 27 | 0 | 25 | −1 | 50 | 0 | 70 | 1 | 45 |
| 25 | 23 | −1 | 12.5 | 0 | 60 | 1 | 90 | 0 | 30 |
| 26 | 30 | 0 | 25 | 0 | 60 | 0 | 70 | 0 | 30 |
| 27 | 22 | 1 | 37.5 | 0 | 60 | −1 | 50 | 0 | 30 |
| 28 | 26 | 0 | 25 | 1 | 70 | 0 | 70 | −1 | 15 |
| 29 | 25 | 0 | 25 | −1 | 50 | 0 | 70 | −1 | 15 |
| 30 | 24 | 1 | 37.5 | 0 | 60 | 1 | 90 | 0 | 30 |
| Response | Polynomial Equation |
|---|---|
| Total Monomeric Anthocyanin Content | |
| TMA2-p (mg/g) | 7.104 − 0.282 · U− 0.172 · T − 0.570 · C − 0.026 · t − 1.420 · U2 − 0.506 · T2 − 0.052 · C2 − 0.514 · t2 + 0.373 · U · T − 0.182 · U · C + 0.056 · U · t + 0.047 · T · C − 0.130 · T · t + 0.014 · C · t |
| TMAm (mg/g) | 6.169 − 0.814 · U − 0.300 · T − 0.632 · C + 0.053 · t − 1.701 · U2 − 0.187 · T2 − 0.179 · C2 − 0.438 · t2 + 0.246 · U · T + 0.084 · U · C + 0.014 · U · t − 0.347 · T · C − 0.129 · T · t − 0.074 · C · t |
| Total Phenolic Content | |
| TPC2-p (mg/g) | 49315.79 − 763.15 · U − 6265.81 · T − 1541.22 · C − 421.12 · t − 8689.46 · U2 + 2227.60 · T2 + 2069.74 · C2 − 3465.81 · t2 + 802.63 · U · T − 1157.89 · U · C + 1407.89 · U · t + 1060.52 · T · C − 118.64 · T · t − 184.21 · C · t |
| TPCm (mg/g) | 53105.26 − 1027.19 · U − 2798.24 · T − 863.94 · C − 570.35 · t − 2406.97 · U2 − 1550.39 · T2 + 316.57 · C2 − 5811.05 · t2 − 381.57 · U · T + 2.63 · U · C + 523.68 · U · t − 1802.63 · T · C − 105.26 · T · t + 24.21 · C · t |
| Response | Sum of Squares | Mean Square | F-Value | R2 | Lack of Fit |
|---|---|---|---|---|---|
| TMA2-p (mg/g) | 21.38 | 1.53 | 7.95*** | 0.8954 | 2.50 |
| TMAm (mg/g) | 34.94 | 2.50 | 16.75*** | 0.9475 | 1.94 |
| TPC2-p (mg/g) | 1254.03 | 89.57 | 17.99*** | 0.8981 | 64.72 |
| TPCm (mg/g) | 399.26 | 28.52 | 19.94*** | 0.9555 | 18.59 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dranca, F.; Oroian, M. Kinetic Improvement of Bioactive Compounds Extraction from Red Grape (Vitis vinifera Moldova) Pomace by Ultrasonic Treatment. Foods 2019, 8, 353. https://doi.org/10.3390/foods8080353
Dranca F, Oroian M. Kinetic Improvement of Bioactive Compounds Extraction from Red Grape (Vitis vinifera Moldova) Pomace by Ultrasonic Treatment. Foods. 2019; 8(8):353. https://doi.org/10.3390/foods8080353
Chicago/Turabian StyleDranca, Florina, and Mircea Oroian. 2019. "Kinetic Improvement of Bioactive Compounds Extraction from Red Grape (Vitis vinifera Moldova) Pomace by Ultrasonic Treatment" Foods 8, no. 8: 353. https://doi.org/10.3390/foods8080353
APA StyleDranca, F., & Oroian, M. (2019). Kinetic Improvement of Bioactive Compounds Extraction from Red Grape (Vitis vinifera Moldova) Pomace by Ultrasonic Treatment. Foods, 8(8), 353. https://doi.org/10.3390/foods8080353





