Reutilization of Food Waste: One-Step Extration, Purification and Characterization of Ovalbumin from Salted Egg White by Aqueous Two-Phase Flotation
Abstract
:1. Introduction
2. Materials and Methods
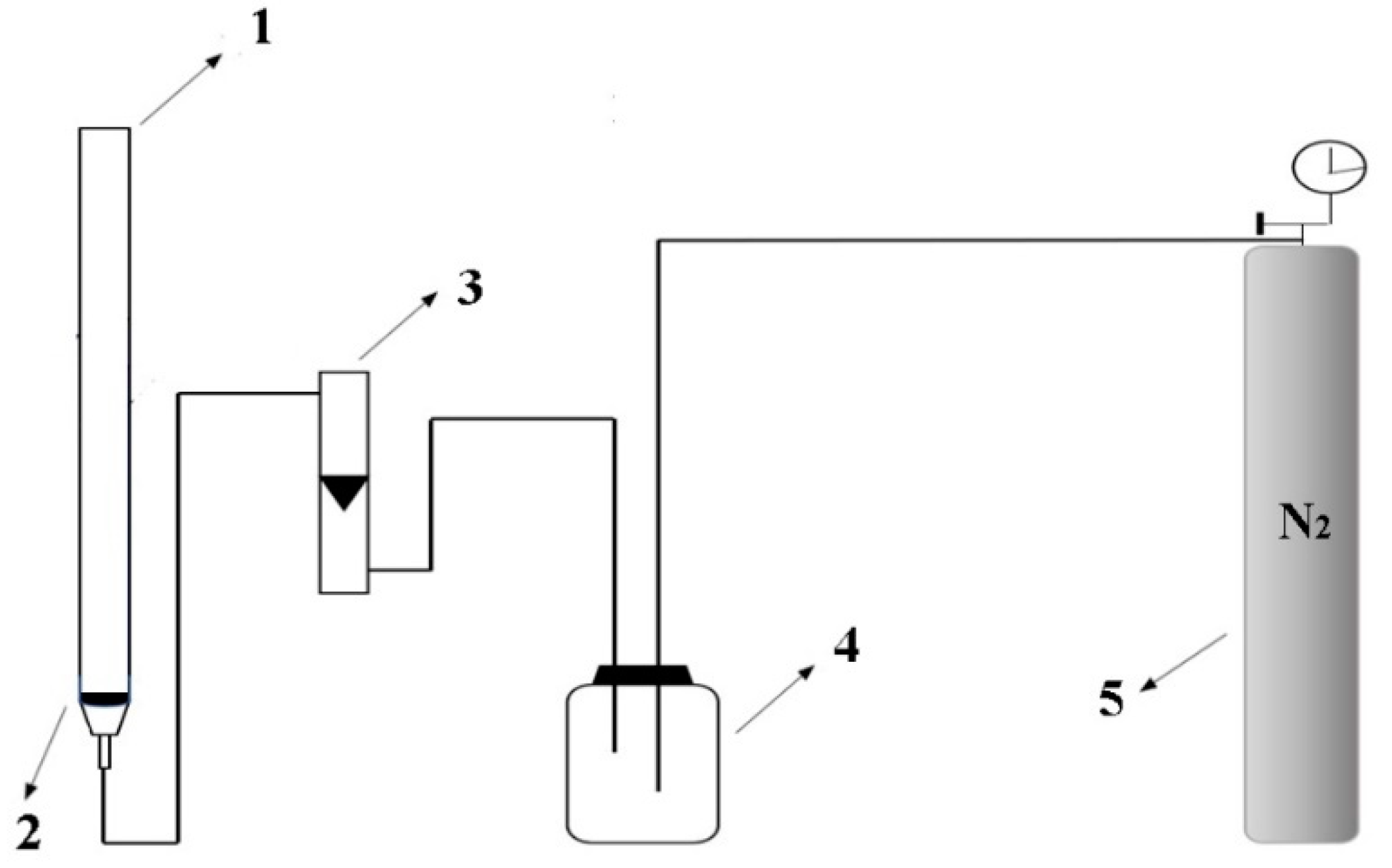
2.1. Instruments
2.2. Materials
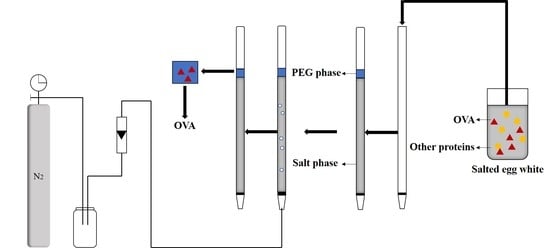
2.3. Preparation of ATPF and Purification
2.4. Experimental Design
2.5. Determining of Protein
2.6. Definition of the Distribution of Protein in ATPF
2.7. Characterization of Ovalbumin Structure
2.7.1. Electrophoresis
2.7.2. Nano LC-ESI-MS/MS
2.7.3. Spectrum Analysis
2.8. Determination of Functional Properties of Ovalbumin
2.8.1. Oil Binding Capacity (OBC)
2.8.2. Viscosity
2.8.3. Emulsibility
2.8.4. Foam Capacity
3. Results and Discussion
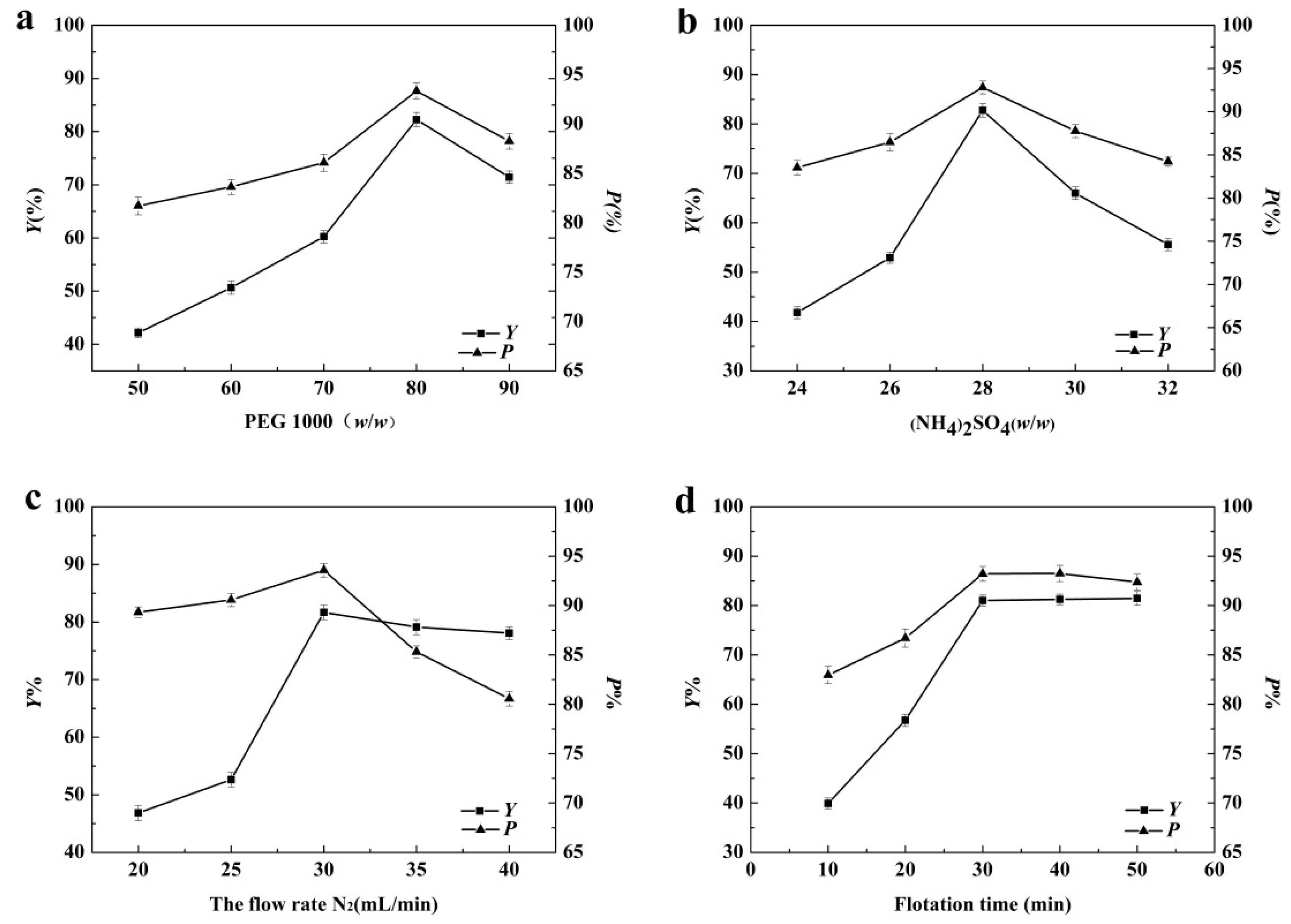
3.1. Single-Factor Variable Analysis
3.2. Response Surface Analysis
3.2.1. Statistical Analysis and Model Fitting
3.2.2. Analysis of Variance
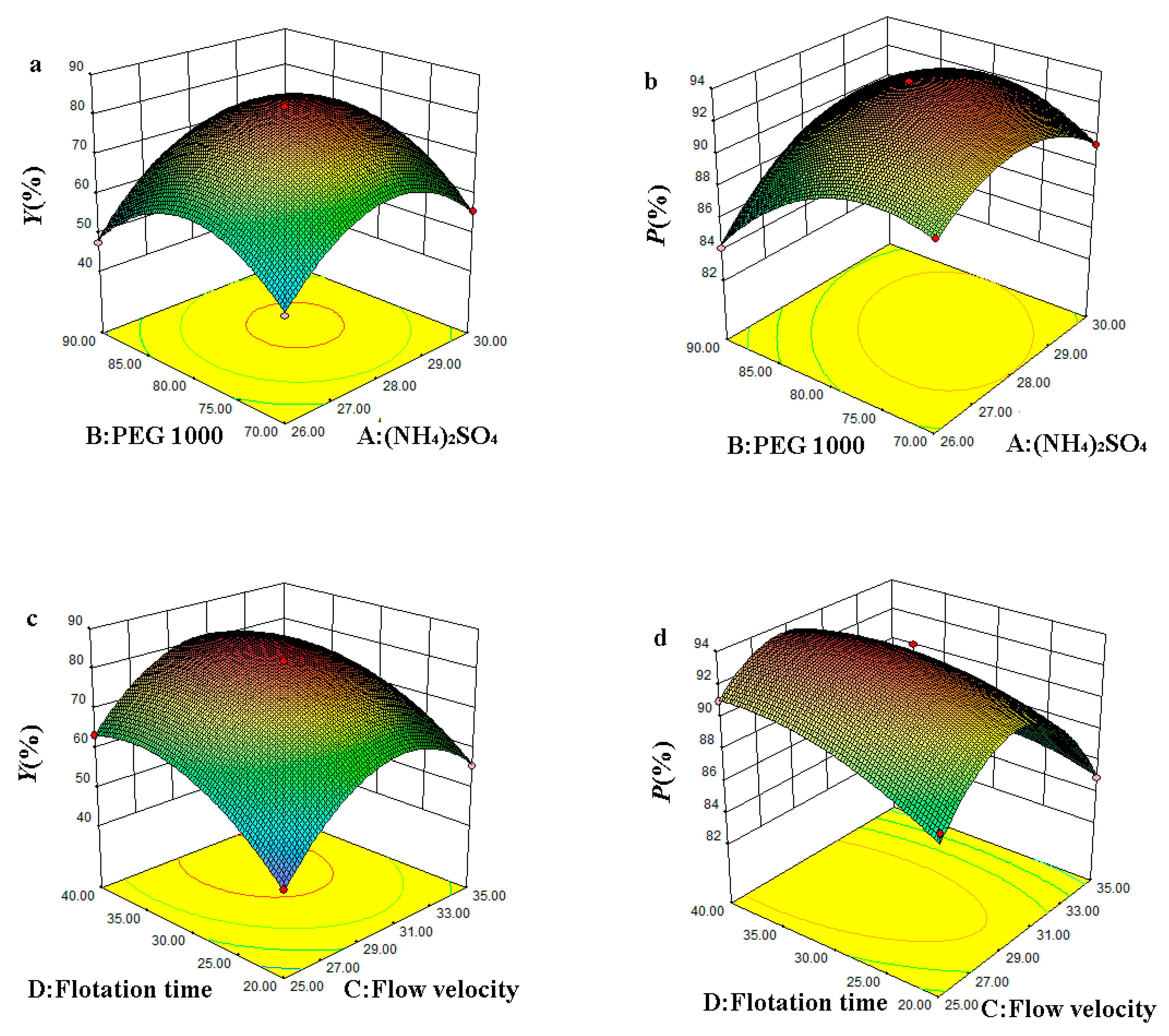
3.2.3. Interactive Analysis
3.2.4. Validation of the Best Extraction Conditions
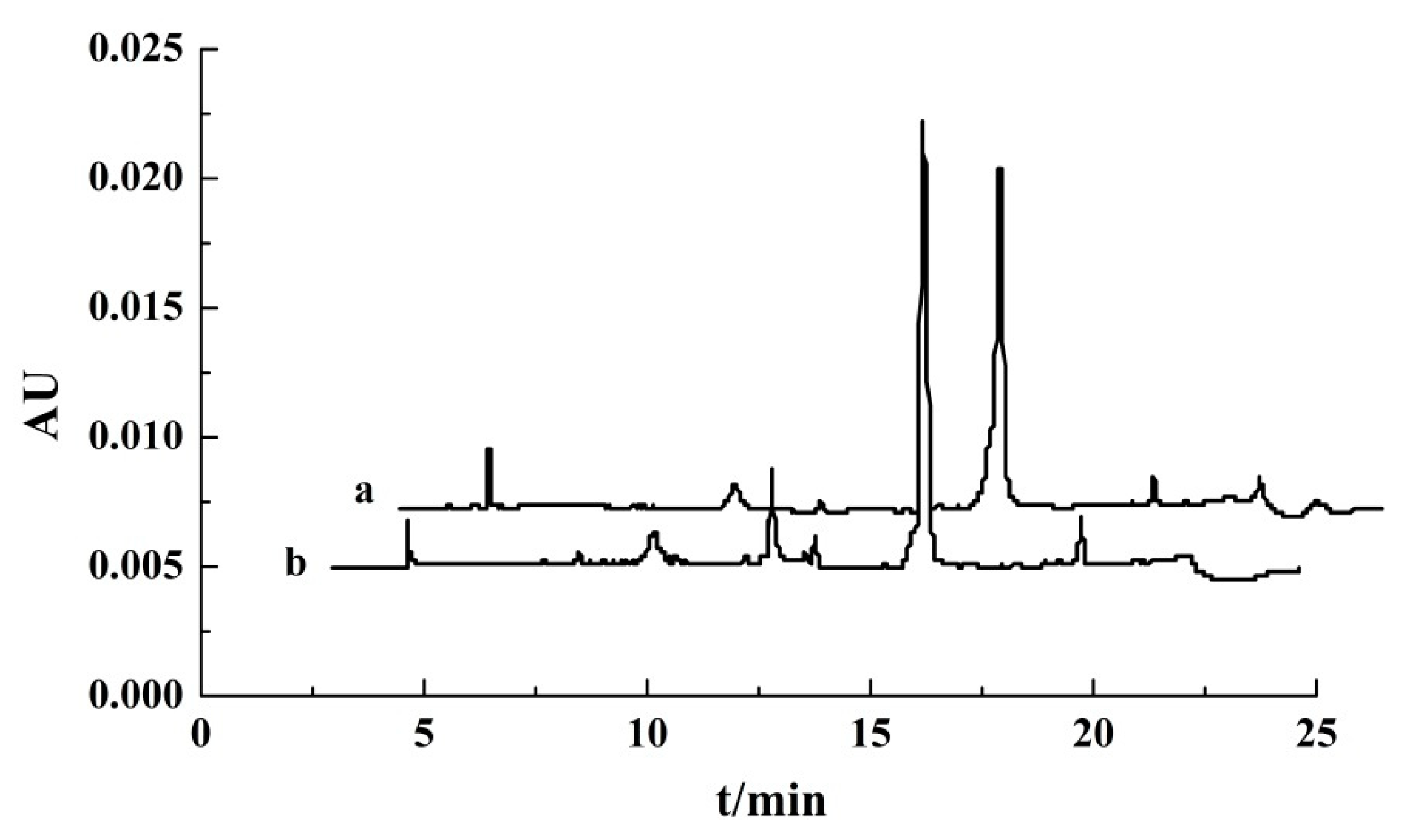
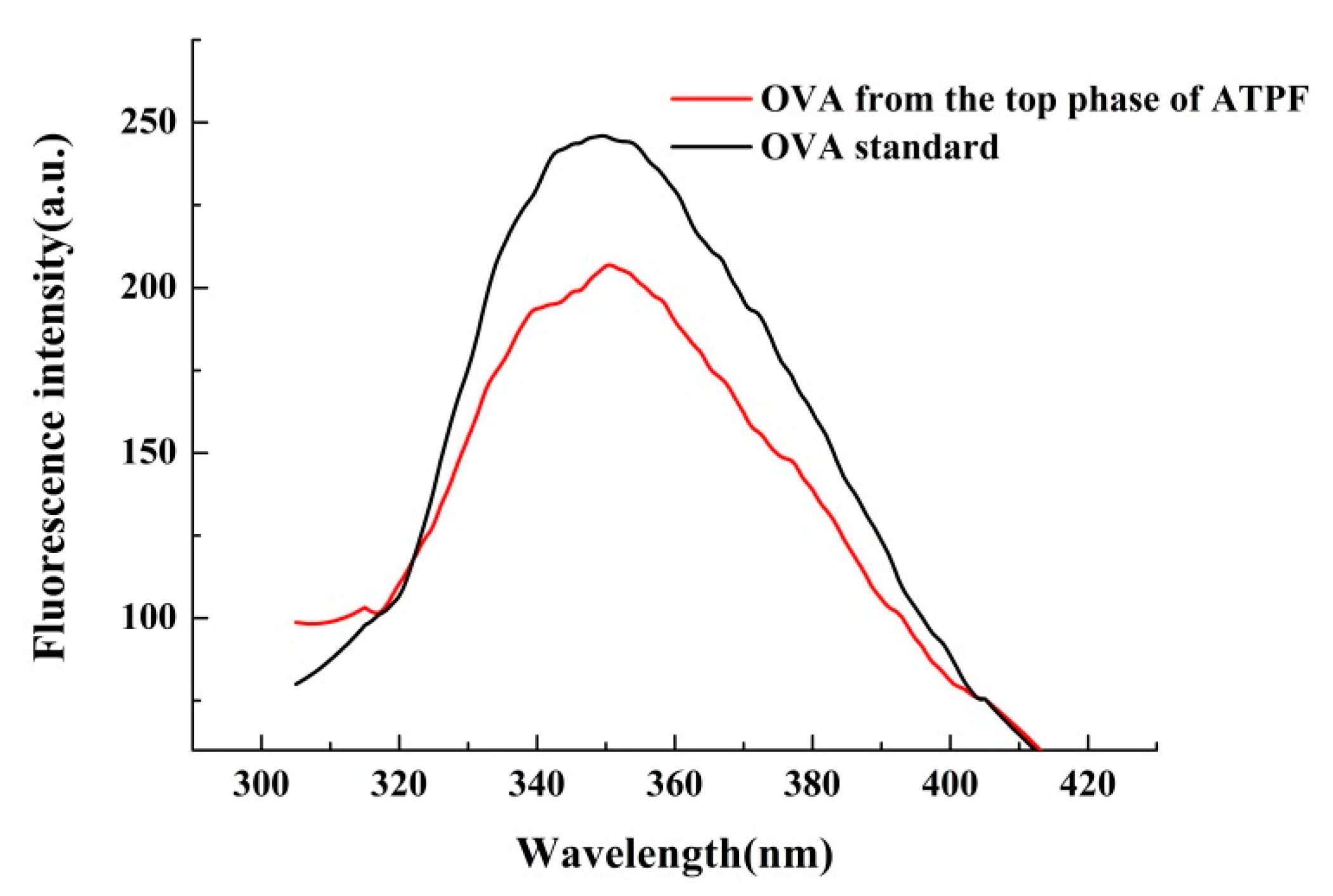
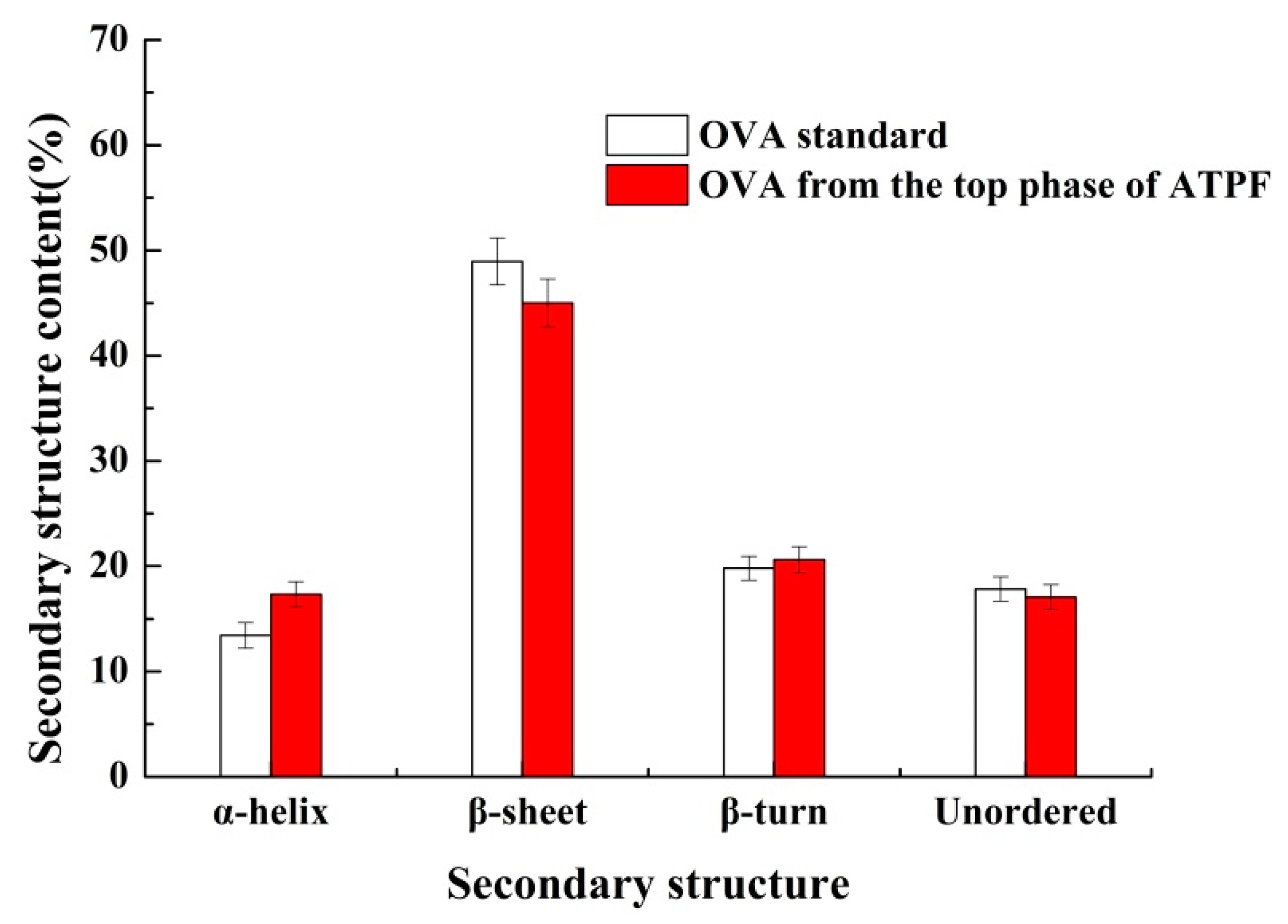
3.3. Characterization of Ovalbumin Extracted Directly from Salt Egg White
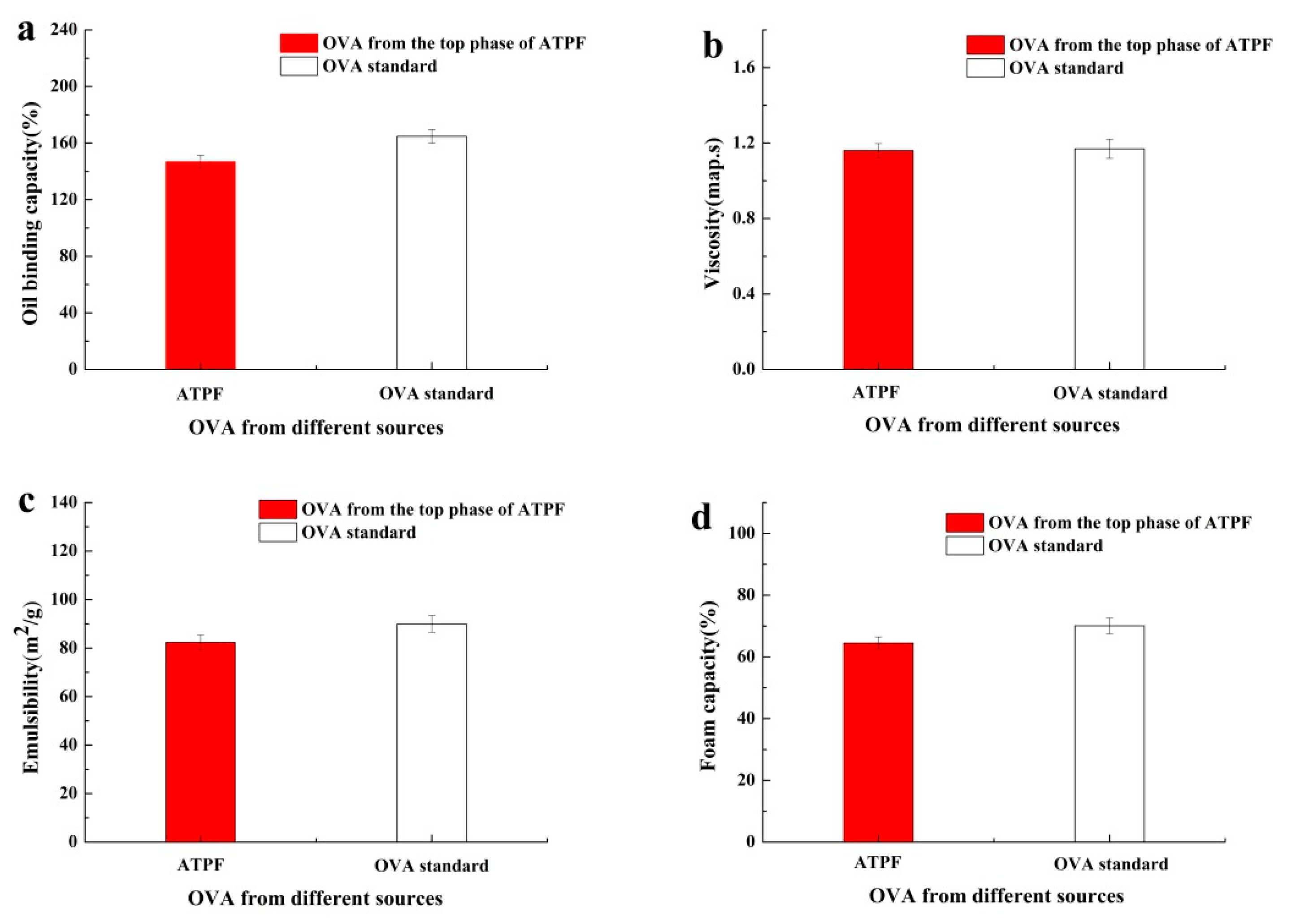
3.4. Determination of Ovalbumin Functional Properties
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, B.; Na, J.X.; Wang, L.L.; Li, D.M.; Liu, C.H.; Feng, Z.B. Eco-innovation in reusing of food by-products: Separation of ovalbumin from salted egg white using aqueous two-phase system of PEG 1000/(NH4)2SO4. Polymers 2019, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, Y.; Xu, M.; Yao, Y.; Wu, N.; Du, H.Y.; Tu, Y.G. Changes in physico-chemical properties, microstructure, protein structures and intermolecular force of egg yolk, plasma and granule gels during salting. Food Chem. 2019, 275, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Raikos, V.; Euston, S.R. Modification of functional properties of egg-white proteins. Mol. Nutr. Food Res. 2003, 47, 369–376. [Google Scholar]
- Pereira, M.M.; Cruz, R.A.P.; Almeida, M.R.; Lima, A.S.; Coutinho, J.A.P.; Freire, M.G. Single-step purification of ovalbumin from egg white using aqueous biphasic systems. Process. Biochem. 2016, 51, 781–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benarafa, C.; Remold-O’Donnell, E. The ovalbumin serpins revisited: Perspective from the chicken genome of clade B serpin evolution in vertebrates. Proc. Natl. Acad. Sci. USA 2005, 102, 11367–11372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs-Nolan, J.; Phillips, M.; Mine, Y. Advances in the Value of Eggs and Egg Components for Human Health. J. Agric. Food Chem. 2005, 53, 8421–8431. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, A.D.; Saundry, R.H.; Moir, A.J.G.; Fothergill, L.A.; Fothergill, J.E. The complete amino-acid sequence of hen ovalbumin. Eur. J. Biochem. 1981, 115, 335–345. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2018, 132, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, F.G. On the separation of a pure albumin from egg-white. J. Physiol. 1900, 25, 306–330. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—A review. Poult. Sci. 2013, 92, 3292–3299. [Google Scholar] [CrossRef]
- Datta, D.; Bhattacharjee, S.; Nath, A. Separation of ovalbumin from chicken egg white using two-stage ultrafiltration technique. Sep. Purif. Technol. 2009, 66, 353–361. [Google Scholar] [CrossRef]
- Geng, F.; Huang, Q.; Wu, X. Co-purification of chicken egg white proteins using polyethylene glycol precipitation and anion-exchange chromatography. Sep. Purif. Technol. 2012, 96, 75–80. [Google Scholar] [CrossRef]
- Zhang, D.D.; Chen, Q.; Hu, L.L.; Chen, X.W.; Wang, J.H. Preparation of a cobalt mono-substituted silicotungstic acid doped with aniline for the selective adsorption of ovalbumin. J. Mater. Chem. B 2015, 3, 4363–4369. [Google Scholar] [CrossRef]
- Liu, J.W.; Wang, M.M.; Zhang, Y.; Han, L.; Chen, X.W.; Wang, J.H. Polymeric ionic liquid modified reduced graphene graphene oxide as adsorbent for highly selective isolation of acidic protein. RSC Adv. 2014, 4, 61936–61943. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, D.H.; Zhang, Y.; Wang, Y.N.; Xu, Z.R. In situ self-assembled reduced graphene oxide aerogel embedded with nickel oxide nanoparticles for the high-efficiency separation of ovalbumin. J. Sep. Sci. 2017, 40, 1765–1772. [Google Scholar] [CrossRef]
- Lee, S.Y.; Khoiroh, I.; Ling, T.C.; Show, P.L. Aqueous two-phase flotation for the recovery of biomolecules. Sep. Purif. Rev. 2016, 45, 81–92. [Google Scholar] [CrossRef]
- Sankaran, R.; Show, P.L.; Yap, Y.J. Sustainable approach in recycling of phase components of large scale aqueous two-phase flotation for lipase recovery. J. Clean. Prod. 2018, 184, 938–948. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, L.L.; Na, J.X.; Zhang, X.Q.; Yuan, Y.Q.; Liu, C.H.; Feng, Z.B. Environmentally-friendly strategy for separation of α-Lactalbumin from whey by aqueous two phase flotation. Arab. J. Chem. 2018. [Google Scholar] [CrossRef]
- Pakhale, S.V.; Vetal, M.D.; Rathod, V.K. Separation of bromelain by aqueous two phase flotation. Sep. Sci. Technol. 2013, 48, 984–989. [Google Scholar] [CrossRef]
- Jiang, B.; Na, J.X.; Wang, L.L.; Li, D.M.; Liu, C.H.; Feng, Z.B. Separation and Enrichment of Antioxidant Peptides from Whey Protein Isolate Hydrolysate by Aqueous Two-Phase Extraction and Aqueous Two-Phase Flotation. Foods 2019, 8, 34. [Google Scholar] [CrossRef]
- Bi, P.Y.; Chang, L.; Mu, Y.L. Separation and concentration of baicalin from scutellaria baicalensis georgi extract by aqueous two-phase flotation. Sep. Purif. Technol. 2013, 116, 454–457. [Google Scholar] [CrossRef]
- Chang, L.; Shao, Q.; Xi, X.; Chu, Q.; Wei, Y. Separation of four flavonol glycosides from solanum rostratum dunal using aqueous two-phase flotation followed by preparative high-performance liquid chromatography. J. Sep. Sci. 2016, 40, 804. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Liu, J.; Zhang, X.F. Production and Characterization of Ovalbumin and Ovotransferrin from Chicken Egg White. Int. J. Food Eng. 2012, 8, 1–12. [Google Scholar] [CrossRef]
- Nau, F.; Mallard, A.; Pages, J.; Brulé, G.; Nau, F. Reversed-phase liquid chromatography of egg white proteins. Optimization of ovalbumin elution. J. Liq. Chromatogr. 1999, 22, 1129–1147. [Google Scholar]
- Jiang, B.; Feng, Z.B.; Liu, C.H.; Xu, Y.C.; Li, D.M.; Ji, G. Extraction and purification of wheat-esterase using aqueous two-phase systems of ionic liquid and salt. J. Food Sci. Technol. 2015, 52, 2878–2885. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zhang, X. Identification of in vivo protein phosphorylation sites with mass spectrometry. Methods Mol. Biol. 2002, 194, 211–221. [Google Scholar] [PubMed]
- Carbonaro, M.; Nucara, A. Secondary structure of food proteins by Fourier transform spectroscopy in the mid-infrared region. Amino Acids 2010, 38, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhang, M.; Ma, M. Emulsifying properties of ovalbumin: Improvement and mechanism by phosphorylation in the presence of sodium tripolyphosphate. Food Hydrocoll. 2016, 60, 29–37. [Google Scholar] [CrossRef]
- Li, W.Y.; He, Z.Y.; Xiong, Y.L.; Huang, X.L.; Chen, J. Effects of temperature on the foaming properties of soybean protein isolate. Sci. Technol. Food Ind. 2010, 31, 86–88. [Google Scholar]
- Ling, C. Direct recovery of lipase derived from burkholderia cepacia with aqueous two-phase flotation. Sep. Purif. Technol. 2011, 80, 577–584. [Google Scholar]
- Jiang, B.; Yuan, Y.Q.; Zhang, X.Q.; Feng, Z.B.; Liu, C.H. Separation and Enrichment of Lectin from Zihua Snap-Bean (Phaseolus vulgaris) Seeds by PEG 600–Ammonium Sulfate Aqueous Two-Phase System. Molecules 2017, 22, 1596. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, Y.; Luo, L.; Kang, W.; Chen, H.; Liu, Y.; Li, Y.; Ni, L. Optimization of separation and determination of chloramphenicol in food using aqueous two-phase flotation coupled with HPLC. J. Iran. Chem. Soc. 2014, 11, 1775–1782. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, X. Solvent Sublation: Theory and Application. Sep. Purif. Method 2007, 30, 157–189. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, B.; Yu, M.; Han, J.; Wang, Y.; Tan, Z.; Yan, Y. Simultaneous separation/enrichment and detection of trace ciprofloxacin and lomefloxacin in food samples using thermosensitive smart polymers aqueous two-phase flotation system combined with HPLC. Food Chem. 2016, 210, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Glyk, A.; Solle, D.; Scheper, T.; Beutel, S. Optimization of PEG salt aqueous two-phase systems by design of experiments. Chemom. Intell. Lab. Syst. 2015, 149, 12–21. [Google Scholar] [CrossRef]
- Rahimpour, F.; Feyzi, F.; Maghsoudi, S.; Hatti, R. Purification of plasmid DNA with polymer-salt aqueous two-phase system: Optimization using response surface methodology. Biotechnol. Bioeng. 2006, 95, 627–637. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, X.J.; Wang, L.L.; Lv, X.M.; Li, D.M.; Liu, C.H.; Feng, Z.B. Two-Step Isolation, Purification, and Characterization of Lectin from Zihua Snap Bean (Phaseolus vulgaris) Seeds. Polymers 2019, 11, 785. [Google Scholar] [CrossRef]
- Qi, B.K.; Ding, J.; Wang, Z.J.; Li, Y.; Ma, C.G.; Chen, F.S.; Sui, X.N.; Jiang, L.Z. Deciphering the characteristics of soybean oleosome-associated protein in maintaining the stability of oleosomes as affected by pH. Food Res. Int. 2017, 100, 551–557. [Google Scholar] [CrossRef]


| Variables | Coded Variable Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1 PEG1000 (w/w)% | 70 | 80 | 90 |
| X2(NH4)2SO4 (w/w)% | 26 | 28 | 30 |
| X3 flow rate of nitrogen (mL/min) | 25 | 30 | 35 |
| X4 flotation time (min) | 20 | 30 | 40 |
| Number | A:PEG 1000 (w/w)% | B:(NH4)2SO4 (w/w)% | C:Flow Velocity (mL/min) | D:Flotatiom Time (min) | Y (%) | P (%) |
|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 0 | -1 | 50.82 | 89.82 |
| 2 | −1 | 1 | 0 | 0 | 47.44 | 84.12 |
| 3 | 0 | 0 | 0 | 0 | 82.56 | 93.03 |
| 4 | 0 | 0 | 0 | 0 | 81.15 | 93.06 |
| 5 | 0 | −1 | 0 | −1 | 53.43 | 90.61 |
| 6 | 0 | 0 | 0 | 0 | 81.97 | 93.08 |
| 7 | −1 | 0 | −1 | 0 | 48.11 | 86.53 |
| 8 | 0 | 1 | 0 | −1 | 50.38 | 86.23 |
| 9 | −1 | 0 | 1 | 0 | 49.26 | 83.13 |
| 10 | 0 | 1 | 1 | 0 | 60.27 | 83.56 |
| 11 | 1 | 1 | 0 | 0 | 61.97 | 88.88 |
| 12 | 0 | 1 | −1 | 0 | 47.67 | 85.19 |
| 13 | 0 | 1 | 0 | 1 | 72.32 | 90.28 |
| 14 | 1 | 0 | 0 | 1 | 74.31 | 91.52 |
| 15 | −1 | −1 | 0 | 0 | 49.09 | 89.73 |
| 16 | 1 | −1 | 0 | 0 | 55.64 | 89.45 |
| 17 | −1 | 0 | 0 | 1 | 59.04 | 89.64 |
| 18 | 0 | −1 | 1 | 0 | 55.31 | 83.56 |
| 19 | 0 | 0 | −1 | 1 | 63.42 | 90.98 |
| 20 | 0 | −1 | −1 | 0 | 48.29 | 89.91 |
| 21 | 0 | 0 | 0 | 0 | 81.78 | 93.09 |
| 22 | 1 | 0 | 1 | 0 | 66.01 | 85.52 |
| 23 | 0 | 0 | 0 | 0 | 81.58 | 93.88 |
| 24 | 0 | −1 | 0 | 1 | 64.13 | 91.41 |
| 25 | 1 | 0 | −1 | 0 | 49.43 | 89.22 |
| 26 | 0 | 0 | −1 | −1 | 44.64 | 87.89 |
| 27 | 0 | 0 | 1 | −1 | 55.67 | 84.93 |
| 28 | 0 | 0 | 1 | 1 | 68.76 | 84.27 |
| 29 | −1 | 0 | 0 | −1 | 49.31 | 87.28 |
| Source | Sum of Squares | df | Mean Square | F | p1 |
|---|---|---|---|---|---|
| Model | 4430.17 | 14 | 316.44 | 457.18 | <0.0001 |
| Residual | 9.69 | 14 | 0.69 | -- | -- |
| Lack of fit | 8.61 | 10 | 0.86 | 3.20 | 0.1369 |
| Pure error | 1.08 | 4 | 0.27 | -- | -- |
| CV% | -- | -- | 1.38 | -- | -- |
| R12 | -- | -- | 0.9978 | -- | -- |
| Source | Sum of Squares | df | Mean Square | F | p2 |
|---|---|---|---|---|---|
| Model | 306.56 | 14 | 21.90 | 101.07 | <0.0001 |
| Residual | 3.03 | 14 | 0.22 | -- | -- |
| Lack of fit | 2.50 | 10 | 0.25 | 1.87 | 0.2856 |
| Pure error | 0.53 | 4 | 0.13 | -- | -- |
| CV% | -- | -- | 0.53 | -- | -- |
| R12 | -- | -- | 0.9902 | -- | -- |
| Hits | Protein Mass | No. of Peptide | Protein | UniprotKB Databases | Relative Abundance | Probability |
|---|---|---|---|---|---|---|
| 1 | 43,195.66 | 17 | OVA of chick | P01012 | 99.4% | 99.0% |
| 2 | 22,535.07 | 3 | Alpha-1-acid glycoprotein of chick | Q8JIG5 | 0.6% | 99.0% |
| Scan No. | Peptide Mass | Peptide Sequence of Protein from Chick | Peptide Probability |
|---|---|---|---|
| 6633 | 1772.89 | ISQAVHAAHAEINEAGR | 96.2% |
| 6896 | 887.56 | IKVYLPR | 87.6% |
| 6955 | 1554.71 | AFKDEDTQAMPFR | 96.6% |
| 7045 | 1580.71 | LTEWTSSNVMEER | 93.2% |
| 7037 | 943.53 | DILNQITK | 89.5% |
| 7155 | 1354.65 | PNDVYSFSLASR | 93.9% |
| 7195 | 1686.83 | GGLEPINFQTAADQAR | 94.9% |
| 7241 | 2007.94 | EVVGSAEAGVDAASVSEEFR | 95.7% |
| 7277 | 1246.62 | ADHPFLFCIK | 83.1% |
| 7295 | 1344.73 | HIATNAVLFFGR | 95.7% |
| 7490 | 1521.79 | YPILPEYLQCVK | 90.7% |
| 7618 | 2280.17 | DILNQITKPNDVYSFSLASR | 91.1% |
| 7661 | 1481.75 | PVQMMYQIGLFR | 92.3% |
| 7735 | 2283.14 | VTEQESKPVQMMYQIGLFR | 91.0% |
| 8705 | 2459.31 | NVLQPSSVDSQTAMVLVNAIVFK | 77.0% |
| 10304 | 1857.96 | ELINSWVESQTNGIIR | 92.4% |
| 9088 | 3032.51 | VHHANENIFYCPIAIMSALAMVYLGAK | 77.6% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Na, J.; Wang, L.; Li, D.; Liu, C.; Feng, Z. Reutilization of Food Waste: One-Step Extration, Purification and Characterization of Ovalbumin from Salted Egg White by Aqueous Two-Phase Flotation. Foods 2019, 8, 286. https://doi.org/10.3390/foods8080286
Jiang B, Na J, Wang L, Li D, Liu C, Feng Z. Reutilization of Food Waste: One-Step Extration, Purification and Characterization of Ovalbumin from Salted Egg White by Aqueous Two-Phase Flotation. Foods. 2019; 8(8):286. https://doi.org/10.3390/foods8080286
Chicago/Turabian StyleJiang, Bin, Jiaxin Na, Lele Wang, Dongmei Li, Chunhong Liu, and Zhibiao Feng. 2019. "Reutilization of Food Waste: One-Step Extration, Purification and Characterization of Ovalbumin from Salted Egg White by Aqueous Two-Phase Flotation" Foods 8, no. 8: 286. https://doi.org/10.3390/foods8080286
APA StyleJiang, B., Na, J., Wang, L., Li, D., Liu, C., & Feng, Z. (2019). Reutilization of Food Waste: One-Step Extration, Purification and Characterization of Ovalbumin from Salted Egg White by Aqueous Two-Phase Flotation. Foods, 8(8), 286. https://doi.org/10.3390/foods8080286