Bridging the Knowledge Gap for the Impact of Non-Thermal Processing on Proteins and Amino Acids
Abstract
1. Introduction
2. Proteins and Amino Acids
3. Food Processing
3.1. Conventional Thermal Processing
3.2. Emerging Non-Thermal Processing
4. Impact of Non-Thermal Processing on Proteins
4.1. Surface Hydrophobicity
4.2. Structural Changes and Aggregation
4.3. Particle Size/Molecular Weight Distribution and Zeta Potential
4.4. Solubility and Gel-Forming/Stability
4.5. Emulsifying Properties
4.6. Reduction in Allergens
5. Impact of Non-Thermal Processing on Amino Acids
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Meade, S.J.; Reid, E.A.; Gerrard, J.A. The impact of processing on the nutritional quality of food proteins. J. AOAC Int. 2005, 88, 904–922. [Google Scholar]
- Korhonen, H.; Pihlanto-Leppäla, A.; Rantamäki, P.; Tupasela, T. Impact of processing on bioactive proteins and peptides. Trends Food Sci. Technol. 1998, 9, 307–319. [Google Scholar] [CrossRef]
- Halász, A.; Baráth, Á.; Simon-Sarkadi, L.; Holzapfel, W. Biogenic amines and their production by microorganisms in food. Trends Food Sci. Technol. 1994, 5, 42–49. [Google Scholar] [CrossRef]
- Bermúdez-Aguirre, D.; Barbosa-Cánovas, G.V. An update on high hydrostatic pressure, from the laboratory to industrial applications. Food Eng. Rev. 2011, 3, 44–61. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77(Pt. 4), 773–798. [Google Scholar] [CrossRef]
- Barba, F.J.; SantAna, A.S.; Orlien, V.; Koubaa, M. Innovative Technologies for Food Preservation: Inactivation of Spoilage and Pathogenic Microorganisms; Academic Press: Cambridge, MA, USA, 2018; pp. 1–315. [Google Scholar]
- Young, V.R.; Pellett, P.L. Plant proteins in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 1994, 59, 1203S–1212S. [Google Scholar] [CrossRef]
- Kent, S.B.H. Total chemical synthesis of proteins. Chem. Soc. Rev. 2009, 38, 338–351. [Google Scholar] [CrossRef]
- Zhang, Y.; Baranov, P.V.; Atkins, J.F.; Gladyshev, V.N. Pyrrolysine and selenocysteine use dissimilar decoding strategies. J. Biol. Chem. 2005, 280, 20740–20751. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Müntz, K. Deposition of storage proteins. In Protein Trafficking in Plant Cells; Springer: Rotterdam, The Netherlands, 1998; pp. 77–99. [Google Scholar]
- Floros, J.D.; Newsome, R.; Fisher, W.; Barbosa-Cánovas, G.V.; Chen, H.; Dunne, C.P.; German, J.B.; Hall, R.L.; Heldman, D.R.; Karwe, M.V.; et al. Feeding the World today and tomorrow: The Importance of food science and technology. Compr. Rev. Food Sci. Food Saf. 2010, 9, 572–599. [Google Scholar] [CrossRef]
- What is a Processed Food? You Might be Surprised! Understanding Our Food Communications Tool Kit; International Food Information Council Foundation: Washington, DC, USA, 2010.
- Van Boekel, M.; Fogliano, V.; Pellegrini, N.; Stanton, C.; Scholz, G.; Lalljie, S.; Somoza, V.; Knorr, D.; Jasti, P.R.; Eisenbrand, G. A review on the beneficial aspects of food processing. Mol. Nutr. Food Res. 2010, 54, 1215–1247. [Google Scholar] [CrossRef]
- Weaver, C.M.; Dwyer, J.; Fulgoni, V.L.; King, J.C.; Leveille, G.A.; MacDonald, R.S.; Ordovas, J.; Schnakenberg, D. Processed foods: Contributions to nutrition. Am. J. Clin. Nutr. 2014, 99, 1525–1542. [Google Scholar] [CrossRef]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci. Technol. 2016, 49, 24–34. [Google Scholar] [CrossRef]
- Mauron, J. Influence of processing on protein quality. J. Nutr. Sci. Vitaminol. 1990, 36 (Suppl. 1), S57–S69. [Google Scholar] [CrossRef]
- Renzone, G.; Arena, S.; Scaloni, A. Proteomic characterization of intermediate and advanced glycation end-products in commercial milk samples. J. Proteom. 2015, 117, 12–23. [Google Scholar] [CrossRef]
- Martins, S.I.F.; Jongen, W.M.; van Boekel, M.A.J. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Dagostin, J.L.A. Blanching as an acrylamide mitigation technique. In New Perspectives on Food Blanching; Springer: Cham, Switzerland, 2017; pp. 95–122. [Google Scholar]
- Hodge, J.E. Dehydrated foods, chemistry of browning reactions in model systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Barba, F.J.; Koubaa, M.; do Prado-Silva, L.; Orlien, V.; Sant’Ana, A.d.S. Mild processing applied to the inactivation of the main foodborne bacterial pathogens: A review. Trends Food Sci. Technol. 2017, 66, 20–35. [Google Scholar] [CrossRef]
- Knorr, D.; Froehling, A.; Jaeger, H.; Reineke, K.; Schlueter, O.; Schoessler, K. Emerging technologies in food processing. Annu. Rev. Food Sci. Technol. 2011, 2, 203–235. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.-W. Enhancement of food processes by ultrasound: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 570–594. [Google Scholar] [CrossRef]
- Toepfl, S.; Mathys, A.; Heinz, V.; Knorr, D. Review: Potential of high hydrostatic pressure and pulsed electric fields for energy efficient and environmentally friendly food processing. Food Rev. Int. 2006, 22, 405–423. [Google Scholar] [CrossRef]
- Kang, D.; Zou, Y.; Cheng, Y.; Xing, L.; Zhou, G.; Zhang, W. Effects of power ultrasound on oxidation and structure of beef proteins during curing processing. Ultrason. Sonochem. 2016, 33, 47–53. [Google Scholar] [CrossRef]
- Higuera-Barraza, O.A.; Torres-Arreola, W.; Ezquerra-Brauer, J.M.; Cinco-Moroyoqui, F.J.; Rodríguez Figueroa, J.C.; Marquez-Ríos, E. Effect of pulsed ultrasound on the physicochemical characteristics and emulsifying properties of squid (Dosidicus gigas) mantle proteins. Ultrason. Sonochem. 2017, 38, 829–834. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef]
- Surowsky, B.; Fischer, A.; Schlueter, O.; Knorr, D. Cold plasma effects on enzyme activity in a model food system. Innov. Food Sci. Emerg. Technol. 2013, 19, 146–152. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, F.; Sun, D.-W.; Zhao, M. Effect of oxidation on the emulsifying properties of myofibrillar proteins. Food Bioprocess Technol. 2013, 6, 1703–1712. [Google Scholar] [CrossRef]
- Zhang, Z.; Regenstein, J.M.; Zhou, P.; Yang, Y. Effects of high intensity ultrasound modification on physicochemical property and water in myofibrillar protein gel. Ultrason. Sonochem. 2017, 34, 960–967. [Google Scholar] [CrossRef]
- Liu, C.; Xiong, Y.L. Oxidation-initiated myosin subfragment cross-linking and structural instability differences between white and red muscle fiber types. J. Food Sci. 2015, 80, C288–C297. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, J.; Yu, X.; Yagoub, A.E.A.; Zhang, Y.; Ma, H.; Gao, X.; Otu, P.N.Y. Heat and/or ultrasound pretreatments motivated enzymolysis of corn gluten meal: Hydrolysis kinetics and protein structure. LWT 2017, 77, 488–496. [Google Scholar] [CrossRef]
- Preece, K.E.; Hooshyar, N.; Krijgsman, A.; Fryer, P.J.; Zuidam, N.J. Intensified soy protein extraction by ultrasound. Chem. Eng. Process. Process Intensif. 2017, 113, 94–101. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Tang, X.; Ni, W.; Zhou, L. Effects of pulsed ultrasound on rheological and structural properties of chicken myofibrillar protein. Ultrason. Sonochem. 2017, 38, 225–233. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, L.; Li, P.; Cai, P.; Zhang, M.; Sun, Z.; Sun, C.; Geng, Z.; Xu, W.; Xu, X.; et al. Effects of ultrasound assisted extraction on the physiochemical, structural and functional characteristics of duck liver protein isolate. Process Biochem. 2017, 52, 174–182. [Google Scholar] [CrossRef]
- Stanic-Vucinic, D.; Stojadinovic, M.; Atanaskovic-Markovic, M.; Ognjenovic, J.; Grönlund, H.; van Hage, M.; Lantto, R.; Sancho, A.I.; Velickovic, T.C. Structural changes and allergenic properties of β-lactoglobulin upon exposure to high-intensity ultrasound. Mol. Nutr. Food Res. 2012, 56, 1894–1905. [Google Scholar] [CrossRef]
- Li, Z.; Lin, H.; Cao, L.; Jameel, K. Effect of high intensity ultrasound on the allergenicity of shrimp. J. Zhejiang Univ. Sci. B 2006, 7, 251–256. [Google Scholar] [CrossRef]
- Tian, Y.; Huang, J.; Xie, T.; Huang, L.; Zhuang, W.; Zheng, Y.; Zheng, B. Oenological characteristics, amino acids and volatile profiles of Hongqu rice wines during pottery storage: Effects of high hydrostatic pressure processing. Food Chem. 2016, 203, 456–464. [Google Scholar] [CrossRef]
- Xia, Q.; Green, B.D.; Zhu, Z.; Li, Y.; Gharibzahedi, S.M.T.; Roohinejad, S.; Barba, F.J. Innovative processing techniques for altering the physicochemical properties of wholegrain brown rice (Oryza sativa L.)—opportunities for enhancing food quality and health attributes. Crit. Rev. Food Sci. Nutr. 2018, 1–22. [Google Scholar] [CrossRef]
- Jin, Y.; Deng, Y.; Qian, B.; Zhang, Y.; Liu, Z.; Zhao, Y. Allergenic response to squid (Todarodes pacificus) tropomyosin Tod p1 structure modifications induced by high hydrostatic pressure. Food Chem. Toxicol. 2014, 76, 86–93. [Google Scholar] [CrossRef]
- Peñas, E.; Préstamo, G.; Polo, F.; Gomez, R. Enzymatic proteolysis, under high pressure of soybean whey: Analysis of peptides and the allergen Gly m 1 in the hydrolysates. Food Chem. 2006, 99, 569–573. [Google Scholar] [CrossRef]
- Barba, F.J.; Poojary, M.M.; Wang, J.; Olsen, K.; Orlien, V. Effect of high pressure processing and storage on the free amino acids in seedlings of Brussels sprouts. Innov. Food Sci. Emerg. Technol. 2017, 41, 188–192. [Google Scholar] [CrossRef]
- Segat, A.; Misra, N.N.; Cullen, P.J.; Innocente, N. Atmospheric pressure cold plasma (ACP) treatment of whey protein isolate model solution. Innov. Food Sci. Emerg. Technol. 2015, 29, 247–254. [Google Scholar] [CrossRef]
- Pal, P.; Kaur, P.; Singh, N.; Kaur, A.; Misra, N.N.; Tiwari, B.K.; Cullen, P.J.; Virdi, A.S. Effect of nonthermal plasma on physico-chemical, amino acid composition, pasting and protein characteristics of short and long grain rice flour. Food Res. Int. 2016, 81, 50–57. [Google Scholar] [CrossRef]
- Meinlschmidt, P.; Ueberham, E.; Lehmann, J.; Reineke, K.; Schlüter, O.; Schweiggert-Weisz, U.; Eisner, P. The effects of pulsed ultraviolet light, cold atmospheric pressure plasma, and gamma-irradiation on the immunoreactivity of soy protein isolate. Innov. Food Sci. Emerg. Technol. 2016, 38, 374–383. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Arias-Gil, M.; Marsellés-Fontanet, A.R.; Ancín-Azpilicueta, C.; Martín-Belloso, O. Effects of thermal and non-thermal processing treatments on fatty acids and free amino acids of grape juice. Food Control 2007, 18, 473–479. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, J.H.; Yook, H.S.; Kang, K.O.; Lee, S.Y.; Hwang, H.J.; Byun, M.W. Effects of gamma radiation on the allergenic and antigenic properties of milk proteins. J. Food Prot. 2001, 64, 272–276. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, A.D.; Karn, J. Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature 1982, 299, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Gülseren, İ.; Güzey, D.; Bruce, B.D.; Weiss, J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason. Sonochem. 2007, 14, 173–183. [Google Scholar] [CrossRef]
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O.E.; Pilosof, A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Serpone, N.; Colarusso, P.; Sonochemistry, I. Effects of ultrasounds on heterogeneous chemical reactions—A useful tool to generate radicals and to examine reaction mechanisms. Res. Chem. Intermed. 1994, 20, 635–679. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, M.; Zhao, H.; Sun, W.; Cui, C. Effects of oxidative modification on gel properties of isolated porcine myofibrillar protein by peroxyl radicals. Meat Sci. 2014, 96, 1432–1439. [Google Scholar] [CrossRef]
- Saleem, R.; Ahmad, R. Effect of low frequency ultrasonication on biochemical and structural properties of chicken actomyosin. Food Chem. 2016, 205, 43–51. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Herrero, A.M. Raman spectroscopy a promising technique for quality assessment of meat and fish: A review. Food Chem. 2008, 107, 1642–1651. [Google Scholar] [CrossRef]
- Li, S.-Q.; Bomser, J.A.; Zhang, Q.H. Effects of pulsed electric fields and heat treatment on stability and secondary structure of bovine immunoglobulin G. J. Agric. Food Chem. 2005, 53, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Mo, H. Effects of pulsed electric fields on physicochemical properties of soybean protein isolates. LWT 2007, 40, 1167–1175. [Google Scholar] [CrossRef]
- Yeom, H.W.; Zhang, Q.H.; Dunne, C.P. Inactivation of papain by pulsed electric fields in a continuous system. Food Chem. 1999, 67, 53–59. [Google Scholar] [CrossRef]
- Yang, R.; Li, S.-Q.; Zhang, Q.H. Effects of pulsed electric fields on the activity and structure of pepsin. J. Agric. Food Chem. 2004, 52, 7400–7406. [Google Scholar] [CrossRef] [PubMed]
- Desobry-Banon, S.; Richard, F.; Hardy, J. Study of acid and rennet coagulation of high pressurized milk. J. Dairy Sci. 1994, 77, 3267–3274. [Google Scholar] [CrossRef]
- Needs, E.C.; Stenning, R.A.; Gill, A.L.; Ferragut, V.; Rich, G.T. High-pressure treatment of milk: Effects on casein micelle structure and on enzymic coagulation. J. Dairy Res. 2000, 67, 31–42. [Google Scholar] [CrossRef]
- Galazka, V.B.; Dickinson, E.; Ledward, D.A. Influence of high pressure processing on protein solutions and emulsions. Curr. Opin. Colloid Interface Sci. 2000, 5, 182–187. [Google Scholar] [CrossRef]
- Johnston, D.E.; Austin, B.A.; Murphy, R.J. Properties of acid-set gels prepared from high pressure treated skim milk. Milchwissenschaft 1993, 48, 206–209. [Google Scholar]
- Johnston, D.E.; Austin, B.A.; Murphy, R.J. Effects of high hydrostatic pressure on milk. Milchwissenschaft 1992, 47, 760–763. [Google Scholar]
- Puppo, C.; Chapleau, N.; Speroni, F.; Lamballerie-Anton, M.; Michel, F.; Añón, C.; Anton, M. Physicochemical modifications of high-pressure-treated soybean protein isolates. J. Agric. Food Chem. 2004, 52, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Balny, C.; Masson, P. Effects of high pressure on proteins. Food Rev. Int. 1993, 9, 611–628. [Google Scholar] [CrossRef]
- Bolumar, T.; Middendorf, D.; Toepfl, S.; Heinz, V. Structural Changes in Foods Caused by High-Pressure Processing; Springer: New York, NY, USA, 2016; pp. 509–537. [Google Scholar]
- Doi, E.; Shimizu, A.; Oe, H.; Kitabatake, N. Melting of heat-induced ovalbumin gel by pressure. Food Hydrocoll. 1991, 5, 409–425. [Google Scholar] [CrossRef]
- Okamoto, M.; Kawamura, Y.; Hayashi, R. Application of high pressure to food processing: Textural comparison of pressure- and heat-induced gels of food proteins. Agric. Biol. Chem. 1990, 54, 183–189. [Google Scholar]
- Apichartsrangkoon, A.; Ledward, D.A.; Bell, A.E.; Brennan, J.G. Physicochemical properties of high pressure treated wheat gluten. Food Chem. 1998, 63, 215–220. [Google Scholar] [CrossRef]
- Marcos, B.; Kerry, J.P.; Mullen, A.M. High pressure induced changes on sarcoplasmic protein fraction and quality indicators. Meat Sci. 2010, 85, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Laakkonen, E.; Sherbon, J.W.; Wellington, G.H. Low-temperature, longtime heating of bovine muscle 2. Changes in electrophoretic pattern. J. Food Sci. 1970, 35, 178–180. [Google Scholar] [CrossRef]
- Fischer, C.; Hamm, R.; Honikel, K.O. Changes in solubility and enzymic activity of muscle glycogen phosphorylase in PSE-muscles. Meat Sci. 1979, 3, 11–19. [Google Scholar] [CrossRef]
- Lopez-Bote, C.; Warriss, P.D.; Brown, S.N. The use of muscle protein solubility measurements to assess pig lean meat quality. Meat Sci. 1989, 26, 167–175. [Google Scholar] [CrossRef]
- Joo, S.; Kauffman, R.; Kim, B.; Park, G. The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water-holding capacity in porcine longissimus muscle. Meat Sci. 1999, 52, 291–297. [Google Scholar] [CrossRef]
- Miyaguchi, Y.; Nagayama, K.; Tsutsumi, M. Thermal and functional properties of porcine sarcoplasmic proteins: A comparison with some water-soluble animal proteins. Nihon Chikusan Gakkaiho 2000, 71, 416–424. [Google Scholar] [CrossRef][Green Version]
- Sun, X.D.; Holley, R.A. High hydrostatic pressure effects on the texture of meat and meat products. J. Food Sci. 2010, 75, R17–R23. [Google Scholar] [CrossRef] [PubMed]
- Cheftel, J.C.; Culioli, J. Effects of high pressure on meat: A review. Meat Sci. 1997, 46, 211–236. [Google Scholar] [CrossRef]
- Kennick, W.H.; Elgasim, E.A.; Holmes, Z.A.; Meyer, P.F. The effect of pressurisation of pre-rigor muscle on post-rigor meat characteristics. Meat Sci. 1980, 4, 33–40. [Google Scholar] [CrossRef]
- Riffero, L.M.; Holmes, Z.A. Characteristics of pre-rigor pressurized versus conventionally processed beef cooked by microwaves and by broiling. J. Food Sci. 1983, 48, 346–350. [Google Scholar] [CrossRef]
- Jung, S.; De Lamballerie-Anton, M.; Ghoul, M. Textural changes in bovine meat treated with high pressure. Int. J. High Press. Res. 2000, 19, 69–74. [Google Scholar] [CrossRef]
- Ma, H.-J.; Ledward, D. High pressure/thermal treatment effects on the texture of beef muscle. Meat Sci. 2004, 68, 347–355. [Google Scholar] [CrossRef]
- Lee, E.-J.; Kim, Y.-J.; Lee, N.-H.; Hong, S.-I.; Yamamoto, K. Differences in properties of myofibrillar proteins from bovinesemitendinosus muscle after hydrostatic pressure or heat treatment. J. Sci. Food Agric. 2007, 87, 40–46. [Google Scholar] [CrossRef]
- Macfarlane, J.J. Pre-rigor pressurization of muscle: Effects on pH, shear value and taste panel assessment. J. Food Sci. 1973, 38, 294–298. [Google Scholar] [CrossRef]
- Ivanov, I.; Bert, Y.; Lebedeva, N. Changes in some properties of myosin, actomyosin, and actin under the influence of high pressure. Biochem.-Mosc. 1960, 25, 386–389. [Google Scholar]
- Ohshima, T.; Ushio, H.; Koizumi, C. High-pressure processing of fish and fish products. Trends Food Sci. Technol. 1993, 4, 370–375. [Google Scholar] [CrossRef]
- Cheah, P.B.; Ledward, D.A. High pressure effects on lipid oxidation in minced pork. Meat Sci. 1996, 43, 123–134. [Google Scholar] [CrossRef]
- Molina, E.; Papadopoulou, A.; Ledward, D.A. Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins. Food Hydrocoll. 2001, 15, 263–269. [Google Scholar] [CrossRef]
- Grinberg, V.Y.; Haertlé, T. Reducer driven baric denaturation and oligomerisation of whey proteins. J. Biotechnol. 2000, 79, 205–209. [Google Scholar] [CrossRef]
- Dumay, E.M.; Kalichevsky, M.T.; Cheftel, J.C. Characteristics of pressure-induced gels of β-lactoglobulin at various times after pressure release. LWT Food Sci. Technol. 1998, 31, 10–19. [Google Scholar] [CrossRef]
- Zasypkin, D.V.; Dumay, E.; Cheftel, J.C. Pressure- and heat-induced gelation of mixed β-lactoglobulin/xanthan solutions. Food Hydrocoll. 1996, 10, 203–211. [Google Scholar] [CrossRef]
- Galazka, V.B.; Dickinson, E.; Ledward, D.A. Emulsifying properties of ovalbumin in mixtures with sulphated polysaccharides: Effects of pH, ionic strength, heat and high-pressure treatment. J. Sci. Food Agric. 2000, 80, 1219–1229. [Google Scholar] [CrossRef]
- Dickinson, E.; James, J.D. Effect of high pressure processing on properties of emulsions made. In High Pressure Food Science, Bioscience and Chemistry; Isaacs, N.S., Ed.; Woodhead Publishing: Cambridge, UK, 1998; pp. 152–159. [Google Scholar]
- Shriver, S.K. Effect of Selected Nonthermal Processing Methods on the Allergen Reactivity of Atlantic White Shrimp (Litopenaeus setiferus). Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2011. [Google Scholar]
- Nooji, J.K. Reduction of Wheat Allergen Potency by Pulsed Ultraviolet Light, High Hydrostatic Pressure, and Non-Thermal Plasma. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2011. [Google Scholar]
- Tammineedi, C.V.R.K.; Choudhary, R.; Perez-Alvarado, G.C.; Watson, D.G. Determining the effect of UV-C, high intensity ultrasound and nonthermal atmospheric plasma treatments on reducing the allergenicity of α-casein and whey proteins. LWT Food Sci. Technol. 2013, 54, 35–41. [Google Scholar] [CrossRef]
- Wan, A.; Yu, W. Effect of wool fiber modified by ecologically acceptable ozone-assisted treatment on the pilling of knit fabrics. Text. Res. J. 2012, 82, 27–36. [Google Scholar] [CrossRef]
- Zhenxing, L.; Hong, L.; Limin, C.; Jamil, K. The influence of gamma irradiation on the allergenicity of shrimp (Penaeus vannamei). J. Food Eng. 2007, 79, 945–949. [Google Scholar] [CrossRef]
- Byun, M.W.; Lee, K.H.; Kim, D.H.; Kim, J.H.; Yook, H.S.; Ahn, H.J. Effects of gamma radiation on sensory qualities, microbiological and chemical properties of salted and fermented squid. J. Food Prot. 2000, 63, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-H.; Kim, J.-H.; Lee, J.-W.; Yoo, Y.-C.; Kim, M.R.; Park, K.-S.; Byun, M.-W. Ovalbumin modified by gamma irradiation alters its immunological functions and allergic responses. Int. Immunopharmacol. 2007, 7, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Song, K. Bin Effect of gamma-irradiation on the molecular properties of myoglobin. J. Biochem. Mol. Biol. 2002, 35, 590–594. [Google Scholar] [PubMed]
- Vaz, A.F.M.; Souza, M.P.; Medeiros, P.L.; Melo, A.M.M.A.; Silva-Lucca, R.A.; Santana, L.A.; Oliva, M.L.V.; Perez, K.R.; Cuccovia, I.M.; Correia, M.T.S. Low-dose gamma irradiation of food protein increases its allergenicity in a chronic oral challenge. Food Chem. Toxicol. 2013, 51, 46–52. [Google Scholar] [CrossRef]
- Vaz, A.F.M.; Souza, M.P.; Carneiro-da-Cunha, M.G.; Medeiros, P.L.; Melo, A.M.M.A.; Aguiar, J.S.; Silva, T.G.; Silva-Lucca, R.A.; Oliva, M.L.V.; Correia, M.T.S. Molecular fragmentation of wheat-germ agglutinin induced by food irradiation reduces its allergenicity in sensitised mice. Food Chem. 2012, 132, 1033–1039. [Google Scholar] [CrossRef]
- Krishnamurthy, K.; Irudayaraj, J.; Jun, S.; Demirci, A.; Yang, W. UV pasteurization of food materials. In Food Processing Operations and Modeling: Design and Analysis; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Yang, W.W.; Chung, S.-Y.; Ajayi, O.; Krishnamurthy, K.; Konan, K.; Goodrich-Schneider, R. Use of pulsed ultraviolet light to reduce the allergenic potency of soybean extracts. Int. J. Food Eng. 2010, 6. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, J.; Wang, Y.; Peng, B.; Zhu, J.; Yang, L.; Wang, Y. Elevated tropospheric ozone increased grain protein and amino acid content of a hybrid rice without manipulation by planting density. J. Sci. Food Agric. 2015, 95, 72–78. [Google Scholar] [CrossRef]
- Kotiaho, T.; Eberlin, M.N.; Vainiotalo, P.; Kostiainen, R. Electrospray mass and tandem mass spectrometry identification of ozone oxidation products of amino acids and small peptides. J. Am. Soc. Mass Spectrom. 2000, 11, 526–535. [Google Scholar] [CrossRef]
- Shigematsu, T.; Hayashi, M.; Nakajima, K.; Uno, Y.; Sakano, A.; Murakami, M.; Narahara, Y.; Ueno, S.; Fujii, T. Effects of high hydrostatic pressure on distribution dynamics of free amino acids in water soaked brown rice grain. J. Phys. Conf. Ser. 2010, 215, 12171. [Google Scholar] [CrossRef]
- Ueno, S.; Shigematsu, T.; Watanabe, T.; Nakajima, K.; Murakami, M.; Hayashi, M.; Fujii, T. Generation of free amino acids and γ-aminobutyric acid in water-soaked soybean by high-hydrostatic pressure processing. J. Agric. Food Chem. 2010, 58, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Patil, G.R.; Singh, A.K. High hydrostatic pressure technology in dairy processing: A review. J. Food Sci. Technol. 2011, 48, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Peñas, E.; Préstamo, G.; Gomez, R. High pressure and the enzymatic hydrolysis of soybean whey proteins. Food Chem. 2004, 85, 641–648. [Google Scholar] [CrossRef]
- Eisenmenger, M.J.; Reyes-De-Corcuera, J.I. High pressure enhancement of enzymes: A review. Enzyme Microb. Technol. 2009, 45, 331–347. [Google Scholar] [CrossRef]
- Takai, E.; Kitamura, T.; Kuwabara, J.; Ikawa, S.; Yoshizawa, S.; Shiraki, K.; Kawasaki, H.; Arakawa, R.; Kitano, K. Chemical modification of amino acids by atmospheric-pressure cold plasma in aqueous solution. J. Phys. D Appl. Phys. 2014, 47, 285403. [Google Scholar] [CrossRef]
- Khoroshilova, E.V.; Repeyev, Y.A.; Nikogosyan, D.N. UV protolysis of aromatic amino acids and related dipeptides and tripeptides. J. Photochem. Photobiol. B Biol. 1990, 7, 159–172. [Google Scholar] [CrossRef]
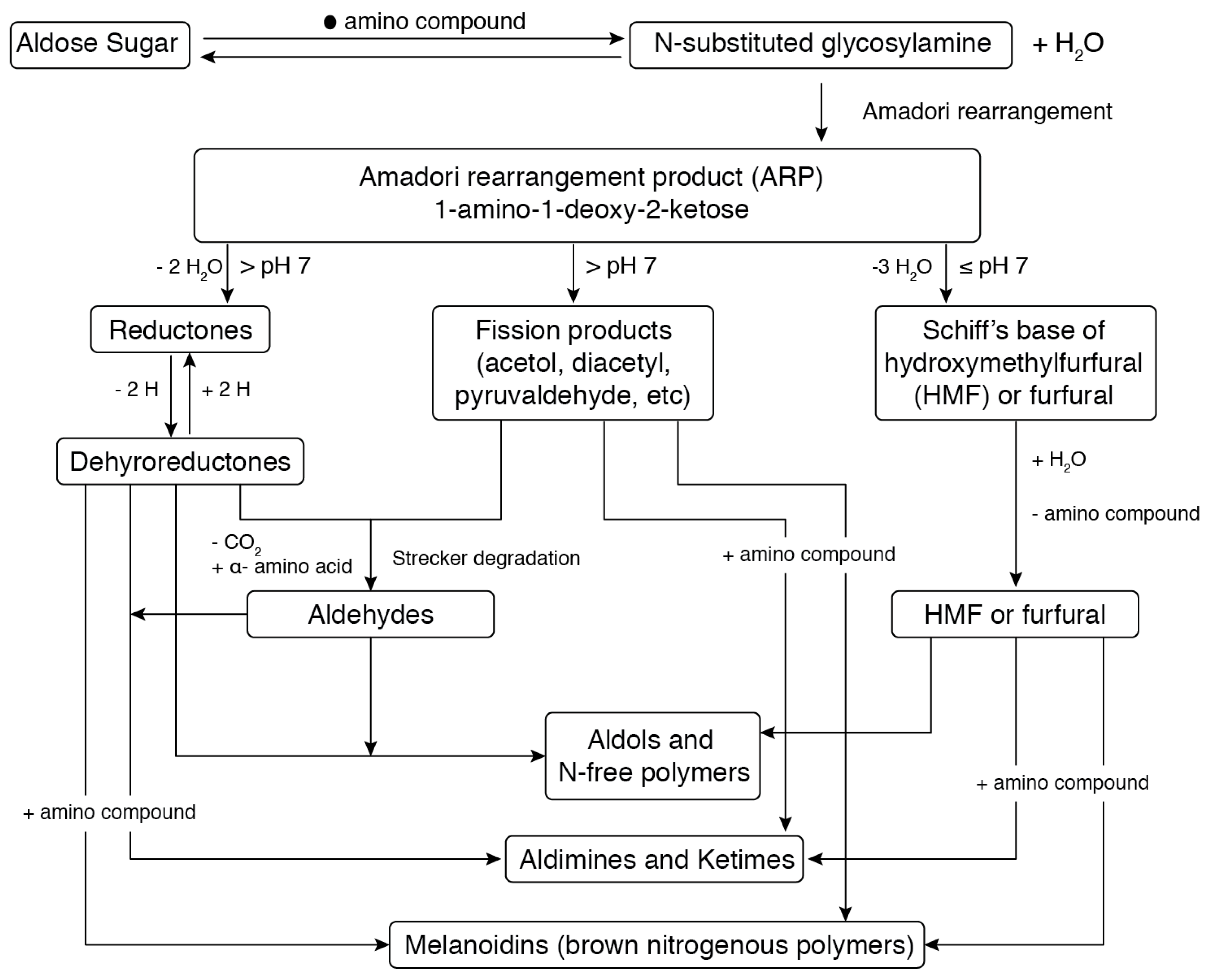

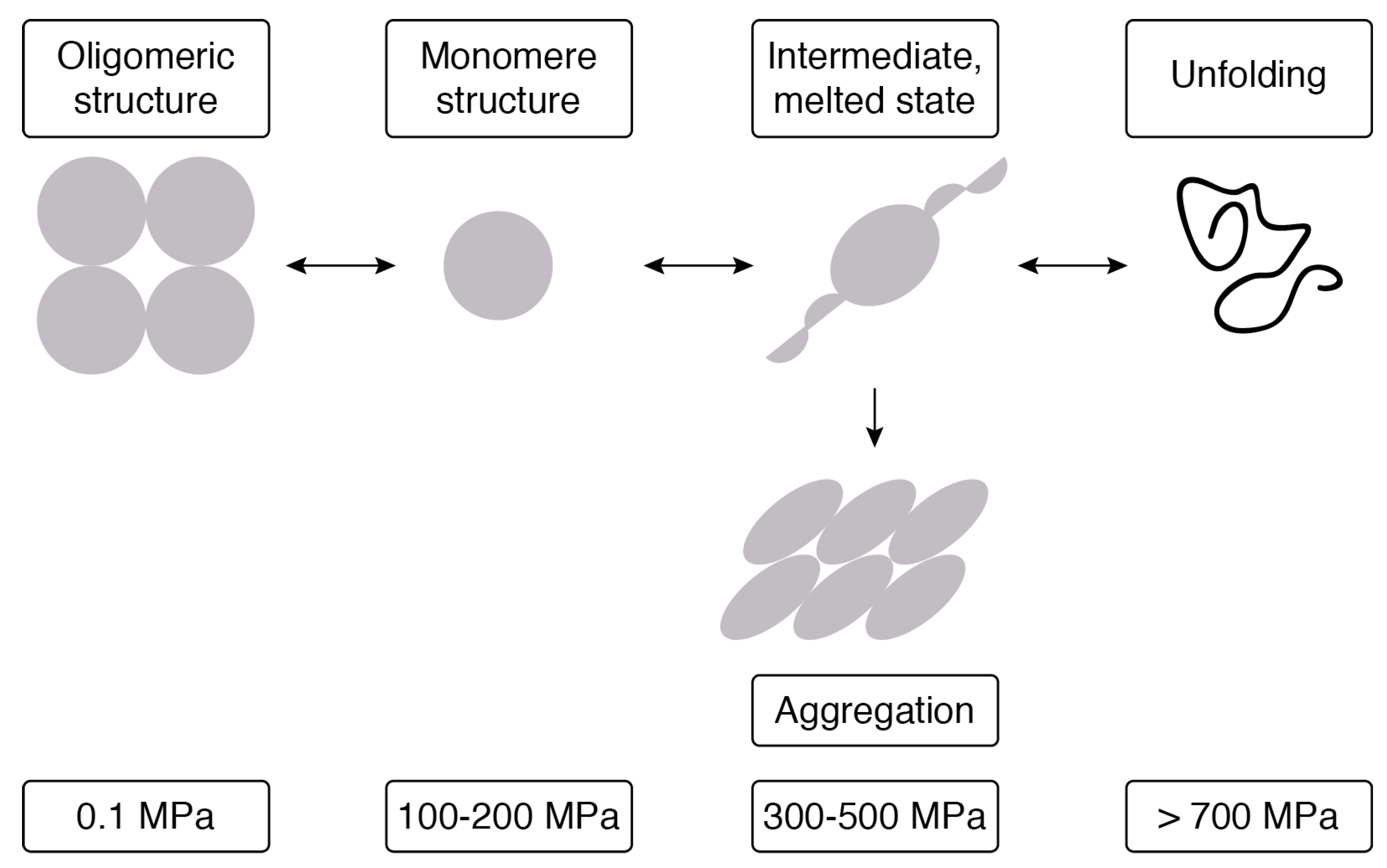
| Treatment | Substrate | Condition | Results | Reference |
|---|---|---|---|---|
| Ultrasound | Corn gluten meal | 40 kHz, pulsed on- 10 s and off 3 s, 40 min and 20 °C. |
| [33] |
| Soy protein | 20 kHz, power 65 W, 0.5, 1, 5 & 15 min | Protein extraction yield enhanced due to increasing in the solubility | [34] | |
| Beef proteins | 2.39, 6.23, 11.32 and 20.96 Wcm−2, 30, 60, 90 and 120 min |
| [26] | |
| Myofibrillar proteins | 200, 400, 600, 800 and 1000 W, 88, 117, 150, 173 and 193 Wcm−2 | Increase in S0, decrease in particle size | [31] | |
| Squid (Dosidicus gigas) mantle proteins | 20 kHz, 0, 20, and 40%), 0, 30, 60, and 90 s |
| [27] | |
| Chicken myofibrillar protein | 240 w, 0, 3, 6, 9, 12 and 15 min) |
| [35] | |
| Duck liver protein isolate | 24 kHz, 266 W by a pulsed on-time of 2 s and off-time of 3 s for 42 min |
| [36] | |
| Β-Lg In Cow Milk | 9.5 W, 135 W/cm2 | No significant alteration in allergenicity | [37] | |
| Tropomyosin from shrimp | 30 Hz, 800 W for 30–180 min | Allergenicity was reduced | [38] | |
| High pressure processing | Hongqu Rice wines | 200 and 550 MPa, 25 °C, 30 min | Free amino acids content was decreased after 6 months storage | [39] |
| Brown rice | 0.1–500 MPa,10 min | Free amino acids especially essential ones were increased | [40] | |
| Tropomyosin from shrimp | 200, 400 and 600 MPa at 20 °C for 20 min |
| [41] | |
| Soy allergen (Glycinin) | 100, 200 and 300 MPa for 15 min |
| [42] | |
| Brussels sprouts | 200 and 800 MPa for 3 min, 5 °C |
| [43] | |
| Cold plasma | Whey protein isolate | 70 kV, 1, 5, 10, 15, 30, and 60 min |
| [44] |
| Grain rice flour | - |
| [45] | |
| Pulsed ultraviolet light | Soy protein isolate (SPI) | 1, 2, 4 and 6 min Three pulses per second with a width of 300 μs | Vanishing Gly m5 & Gly m6 bands after few minutes and decrease in allergenicity | [46] |
| Cold atmospheric pressure plasma | 1, 2.5, 5, 7.5 and 10 min without stirring | Reduction in immunoreactivity of SPI | ||
| Gamma-irradiation | Target doses were 3, 5, 10, 25, 50, and 100 kGy | Decrease in SPI allergenicity (Gly m5 & Gly m6) was dependent on the irradiation dose | ||
| Pulsed electric field | Grape juice | 4 µs width and with a field strength of 35 kV/cm, 1000 Hz and the total time 1 ms |
| [47] |
| Radiation | Β-Lg in cow milk | 3, 5, and 10 kGy | Protein aggregation and alteration of IgE binding epitopes | [48] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteghlal, S.; Gahruie, H.H.; Niakousari, M.; Barba, F.J.; Bekhit, A.E.-D.; Mallikarjunan, K.; Roohinejad, S. Bridging the Knowledge Gap for the Impact of Non-Thermal Processing on Proteins and Amino Acids. Foods 2019, 8, 262. https://doi.org/10.3390/foods8070262
Esteghlal S, Gahruie HH, Niakousari M, Barba FJ, Bekhit AE-D, Mallikarjunan K, Roohinejad S. Bridging the Knowledge Gap for the Impact of Non-Thermal Processing on Proteins and Amino Acids. Foods. 2019; 8(7):262. https://doi.org/10.3390/foods8070262
Chicago/Turabian StyleEsteghlal, Sara, Hadi Hashemi Gahruie, Mehrdad Niakousari, Francisco J. Barba, Alaa El-Din Bekhit, Kumar Mallikarjunan, and Shahin Roohinejad. 2019. "Bridging the Knowledge Gap for the Impact of Non-Thermal Processing on Proteins and Amino Acids" Foods 8, no. 7: 262. https://doi.org/10.3390/foods8070262
APA StyleEsteghlal, S., Gahruie, H. H., Niakousari, M., Barba, F. J., Bekhit, A. E.-D., Mallikarjunan, K., & Roohinejad, S. (2019). Bridging the Knowledge Gap for the Impact of Non-Thermal Processing on Proteins and Amino Acids. Foods, 8(7), 262. https://doi.org/10.3390/foods8070262








