Effect of Thermal Processing on Carotenoids and Folate Changes in Six Varieties of Sweet Potato (Ipomoes batata L.)
Abstract
1. Introduction
2. Materials and Methods
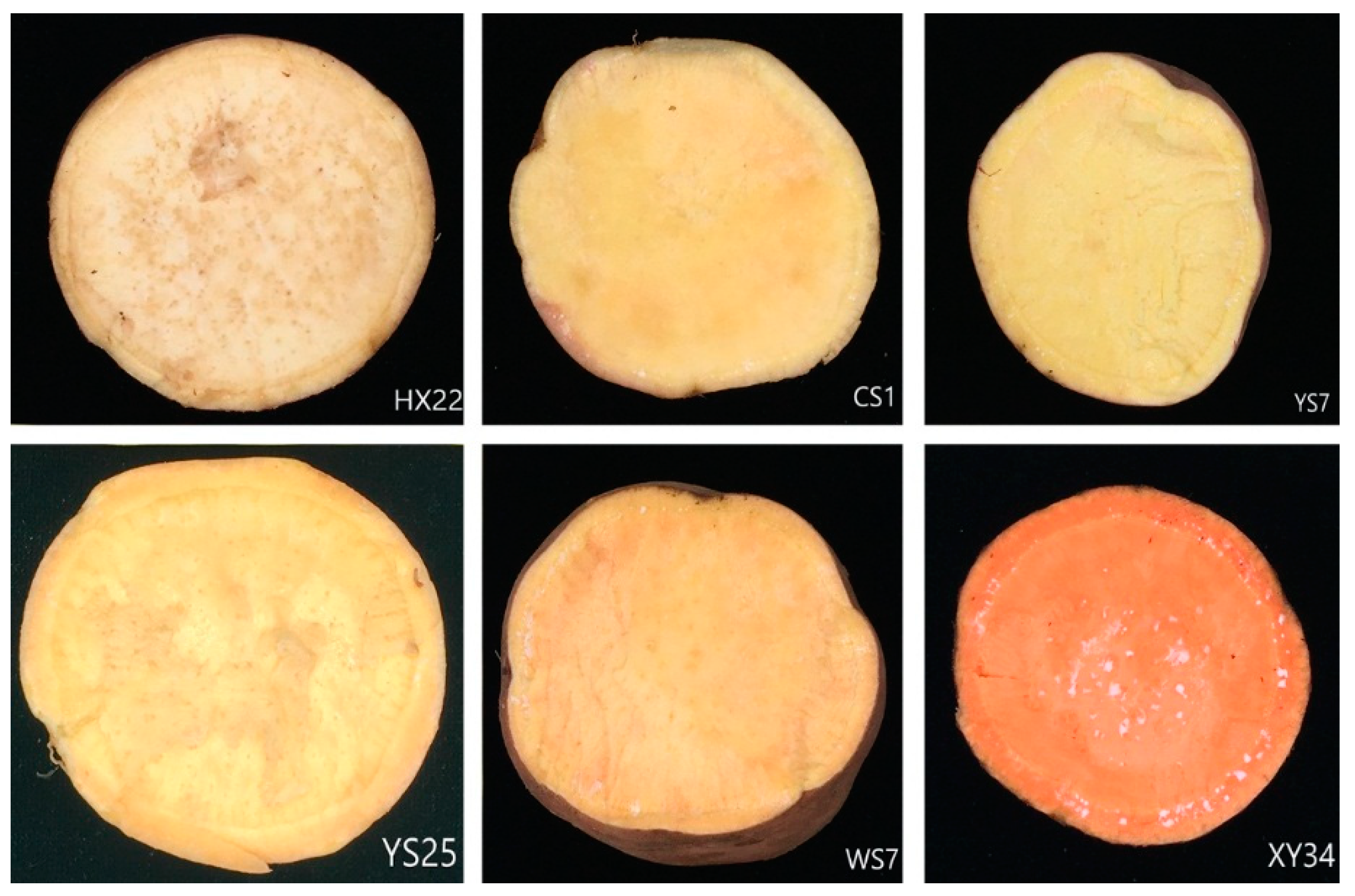
2.1. Samples Preparation
2.2. Carotenoids Extraction and Analysis
2.3. Extraction and Determination of Folate Content
2.4. Statistics Analysis
3. Results
3.1. Thermal Effect on Carotenoids in Roots of Sweet Potatoes
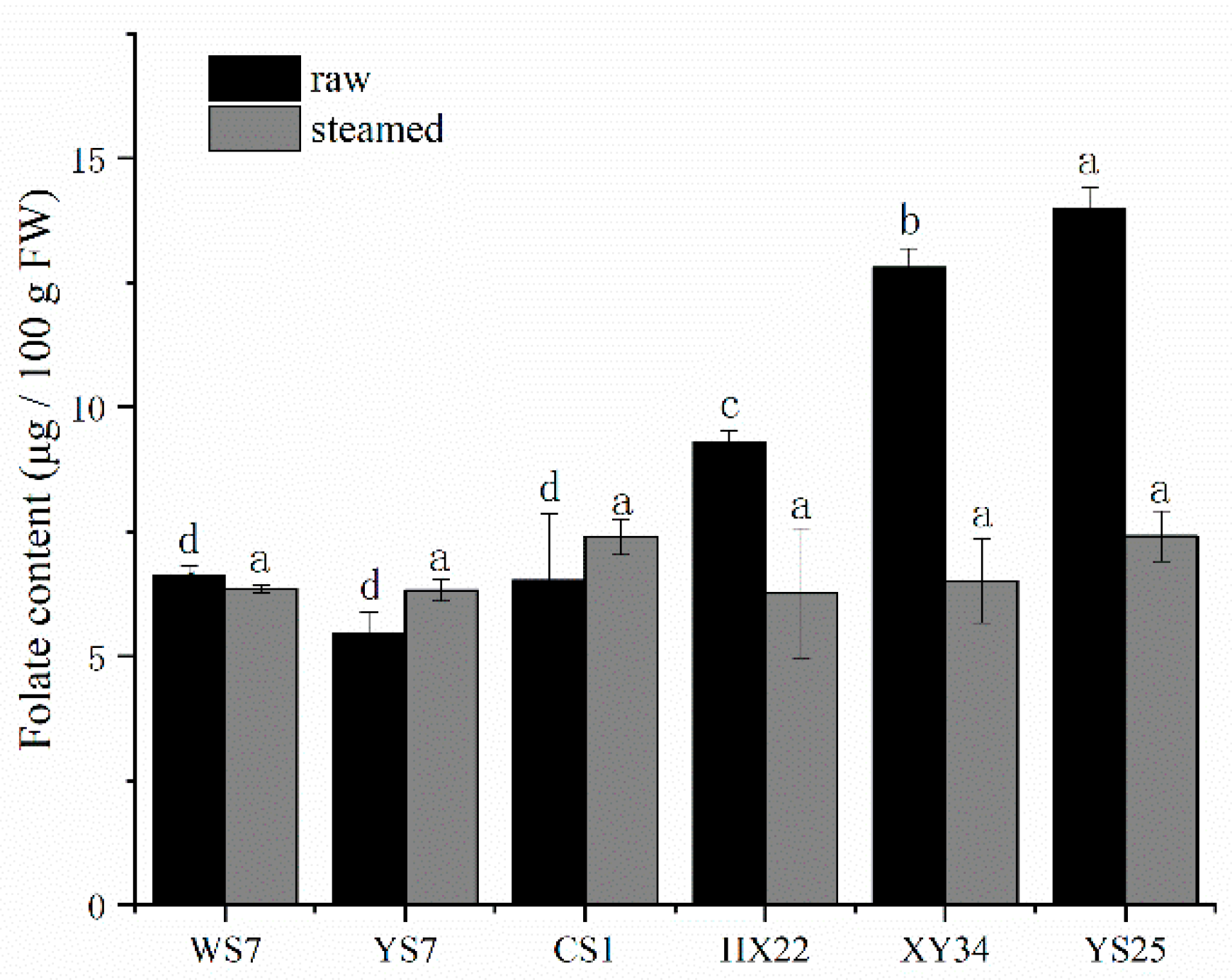
3.2. Thermal Effect on Folate Contents in Roots of Sweet Potatoes
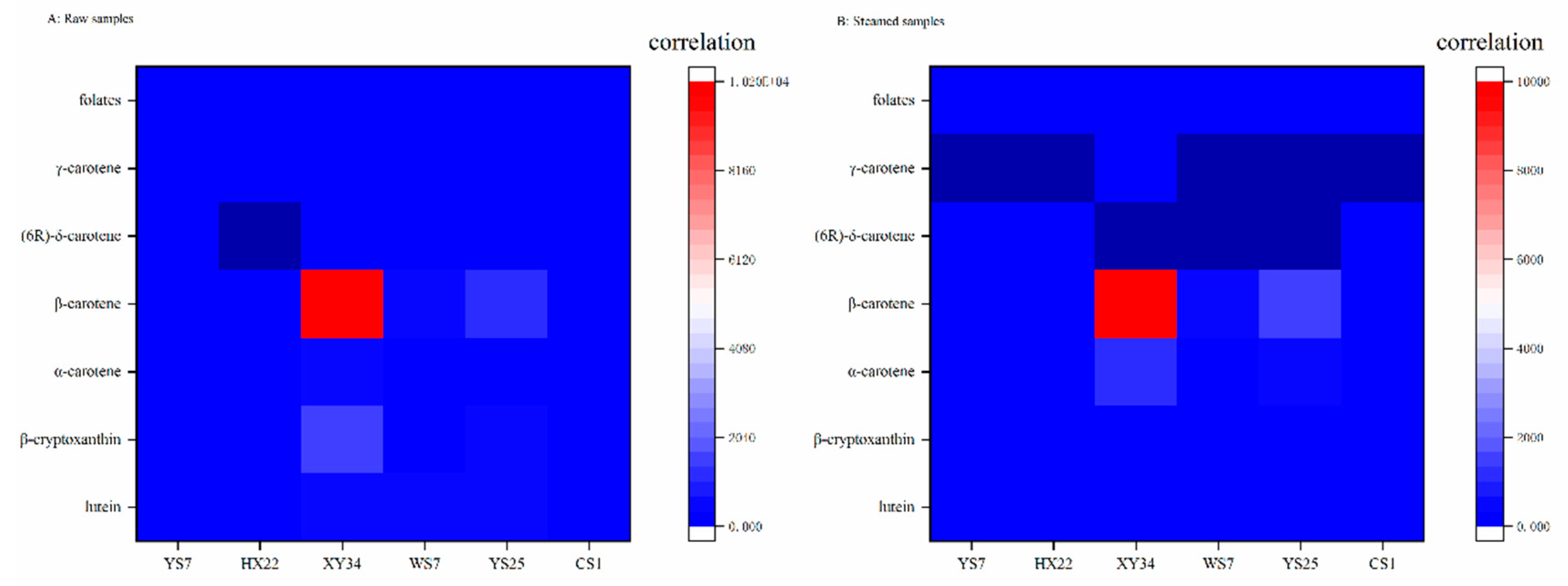
3.3. Correlation Analysis
4. Discussion
4.1. Effect of Thermal Processing On Carotenoids
4.2. Effect of Thermal Processing on Total Folate Content
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Guideline: Fortification of Rice with Vitamins and Minerals as a Public Health Strategy; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Rotondi, M.A.; Khobzi, N. Vitamin A supplementation and neonatal mortality in the developing world: a meta-regression of cluster-randomized trials. Bull. World Health Organ. 2010, 88, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.N.; Nusrat, T.; Begum, P.; Ahsan, M. Carotenoids and β-carotene in orange fleshed sweet potato: A possible solution to vitamin A deficiency. Food Chem. 2016, 199, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed]
- Gurmu, F.; Hussein, S.; Laing, M. The Potential of Orange-Fleshed Sweet Potato to Prevent Vitamin A Deficiency in Africa. Int. J. Vitam. Nutr. Res. 2014, 84, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Laurie, S.M.; Faber, M.; Claasen, N. Incorporating orange-fleshed sweet potato into the food system as a strategy for improved nutrition: The context of South Africa. Food Res. Int. 2018, 104, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Alpert, J.E.; Fava, M. Nutrition and depression: The role of folate. Nutr. Rev. 1997, 55, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Sung, B.; Kim, N.D. Roles of folate in skeletal muscle cell development and functions. Arch. Pharm. Res. 2019, 42, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Kourouma, V.; Mu, T.-H.; Zhang, M.; Sun, H.-N. Effects of cooking process on carotenoids and antioxidant activity of orange-fleshed sweet potato. LWT Food Sci. Technol. 2019, 104, 134–141. [Google Scholar] [CrossRef]
- Bengtsson, A.; Brackmann, C.; Enejder, A.; Alminger, M.L.; Svanberg, U. Effects of Thermal Processing on the in Vitro Bioaccessibility and Microstructure of beta-Carotene in Orange-Fleshed Sweet Potato. J. Agric. Food Chem. 2010, 58, 11090–11096. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, A.; Narnutebi, A.; Alminger, M.L.; Svanberga, U. Effects of various traditional processing methods on the all-trans-beta-carotene content of orange-fleshed sweet potato. J. Food Compos. Anal. 2008, 21, 134–143. [Google Scholar] [CrossRef]
- Bureau, S.; Mouhoubi, S.; Touloumet, L.; Garcia, C.; Moreau, F.; Bédouet, V.; Renard, C.M.G.C. Are folates, carotenoids and vitamin C affected by cooking? Four domestic procedures are compared on a large diversity of frozen vegetables. LWT Food Sci. Technol. 2015, 64, 735–741. [Google Scholar] [CrossRef]
- Kariluoto, S.; Liukkonen, K.-H.; Myllymäki, O.; Vahteristo, L.; Kaukovirta-Norja, A.; Piironen, V. Effect of Germination and Thermal Treatments on Folates in Rye. J. Agric. Food Chem. 2006, 54, 9522–9528. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Mao, J.H.; Yan, S.J.; Yu, Y.T.; Xie, L.H.; Hu, J.G.; Li, T.; Abbasi, A.M.; Guo, X.B.; Liu, R.H. Evaluation of carotenoid biosynthesis, accumulation and antioxidant activities in sweetcorn (Zea mays L.) during kernel development. Int. J. Foodsci. Technol. 2018, 53, 381–388. [Google Scholar] [CrossRef]
- Okarter, N.; Liu, C.S.; Sorrells, M.E.; Liu, R.H. Phytochemical content and antioxidant activity of six diverse varieties of whole wheat. Food Chem. 2010, 119, 249–257. [Google Scholar] [CrossRef]
- Shan, Q.-J.; Liu, J.-H.; Li, W.; Wang, H.; Hu, X.-D.; Li, T.; Hu, J.-G.; Guo, X.-B.; Liu, R.H. Comprehensive evaluation of biosynthesis, accumulation, regulation of folate and vitamin C in waxy maize (Zea mays L. var. ceratina) with kernel development. J. Cereal Sci. 2019, 87, 215–224. [Google Scholar] [CrossRef]
- Tang, Y.; Cai, W.; Xu, B. Profiles of phenolics, carotenoids and antioxidative capacities of thermal processed white, yellow, orange and purple sweet potatoes grown in Guilin, China. Food Sci. Hum. Wellness 2015, 4, 123–132. [Google Scholar] [CrossRef]
- Tao, N.; Wang, C.; Xu, J.; Cheng, Y. Carotenoid accumulation in postharvest “Cara Cara” navel orange (Citrus sinensis Osbeck) fruits stored at different temperatures was transcriptionally regulated in a tissue-dependent manner. Plant Cell Rep. 2012, 31, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Delchier, N.; Ringling, C.; Maingonnat, J.-F.; Rychlik, M.; Renard, C.M.G.C. Mechanisms of folate losses during processing: Diffusion vs. heat degradation. Food Chem. 2014, 157, 439–447. [Google Scholar] [CrossRef] [PubMed]
| Carotenoids | HX22 | XY34 | WS7 | YS25 | YS7 | CS1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | Steamed | Raw | Steamed | Raw | Steamed | Raw | Steamed | Raw | Steamed | Raw | Steamed | |
| lut | 128.4 ± 7.4 g | ND | 492.4 ± 6.9 a | 173.5 ± 1.7 e | 377.3 ± 7.3 c | 131.1 ± 2.4 h | 450.1 ± 8.3 b | 218.9 ± 7.1 c | 139.7 ± 1.5 g | 149.2 ± 3.5 f | ND | 132.6 ± 5.7 h |
| β-cry | ND | ND | 1378 ± 60 a | ND | 246.4 ± 31.1 c | ND | 408.5 ± 14.3 b | ND | 127.1 ± 1.4 d | ND | ND | ND |
| α-car | ND | ND | 471.5 ± 16.4 b | 1279 ± 59 a | ND | 175.1 ± 9.4 d | 140.4 ± 0.2 e | 439.2 ± 14.2 c | ND | 72.13 ± 1.35 f | 71.18 ± 0.99 f | 62.64 ± 8.20 g |
| β-car | 133.9 ± 5.6 j | 138.2 ± 12.6 j | 10151 ± 217 a | 9964 ± 517 b | 451.7 ± 48.4 f | 462.6 ± 5.3 e | 1329 ± 216 d | 1383 ± 29 c | 193.1 ± 7.8 h | 155.8 ± 4.5 i | 239.2 ± 7.7 g | 136.2 ± 20.3 j |
| δ-car | ND | ND | ND | ND | ND | ND | ND | ND | 91.44 ± 3.03 a | ND | 89.68 ± 1.56 b | ND |
| γ-car | ND | ND | 158.8±2.5 a | 127.44 ± 1.6 b | ND | ND | ND | ND | ND | ND | ND | ND |
| total | 262.2 ± 13.2 k | 138.2 ± 12.6 l | 12625 ± 303 a | 11544 ± 579 b | 1075 ± 87 e | 768.8 ± 17.1 f | 2328 ± 239 c | 2025 ± 50 d | 551.3 ± 13.7 g | 377.3 ± 9.4 i | 400.1 ± 10.3 h | 331.4 ± 34.2 j |
| Coefficients | raw | steamed | lut | β-cry | α-car | β-car | δ-car | γ-car | TCC | folate |
|---|---|---|---|---|---|---|---|---|---|---|
| raw | 1 | −1.000 ** | 0.426 | 0.471 | −0.297 | 0.006 | 0.447 | 0.049 | 0.040 | 0.458 |
| steamed | 1 | −0.426 | −0.471 | 0.297 | −0.006 | −0.447 | −0.049 | −0.040 | −0.458 | |
| lut | 1 | 0.772 ** | 0.181 | 0.455 | −0.377 | 0.438 | 0.507 | 0.753 ** | ||
| β-cry | 1 | 0.142 | 0.618 * | −0.135 | 0.679 * | 0.659 * | 0.714 ** | |||
| α-car | 1 | 0.842 ** | −0.231 | 0.760 ** | 0.822 ** | 0.067 | ||||
| β-car | 1 | −0.229 | 0.987 ** | 0.998 ** | 0.359 | |||||
| δ-car | 1 | −0.199 | −0.234 | −0.330 | ||||||
| γ-car | 1 | 0.986 ** | 0.354 | |||||||
| TCC | 1 | 0.401 | ||||||||
| folate | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Sun, Y.; Zhang, F.; Guo, X.; Liao, Z. Effect of Thermal Processing on Carotenoids and Folate Changes in Six Varieties of Sweet Potato (Ipomoes batata L.). Foods 2019, 8, 215. https://doi.org/10.3390/foods8060215
Pan Z, Sun Y, Zhang F, Guo X, Liao Z. Effect of Thermal Processing on Carotenoids and Folate Changes in Six Varieties of Sweet Potato (Ipomoes batata L.). Foods. 2019; 8(6):215. https://doi.org/10.3390/foods8060215
Chicago/Turabian StylePan, Zhijun, Yiming Sun, Fangyuan Zhang, Xinbo Guo, and Zhihua Liao. 2019. "Effect of Thermal Processing on Carotenoids and Folate Changes in Six Varieties of Sweet Potato (Ipomoes batata L.)" Foods 8, no. 6: 215. https://doi.org/10.3390/foods8060215
APA StylePan, Z., Sun, Y., Zhang, F., Guo, X., & Liao, Z. (2019). Effect of Thermal Processing on Carotenoids and Folate Changes in Six Varieties of Sweet Potato (Ipomoes batata L.). Foods, 8(6), 215. https://doi.org/10.3390/foods8060215






