On the Use of Ultrafiltration or Microfiltration Polymeric Spiral-Wound Membranes for Cheesemilk Standardization: Impact on Process Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Filtration System
2.3. Operational Modes
2.3.1. Total Recirculation Mode
2.3.2. Concentration/DF of Skim Milk
2.4. Membrane Fouling Characterization
2.5. Chemical Analysis
2.6. Energy Consumption
2.7. Cheesemaking
2.8. Cheesemaking Efficiency
2.9. Economic Assessment
2.10. Statistical Analysis
3. Results
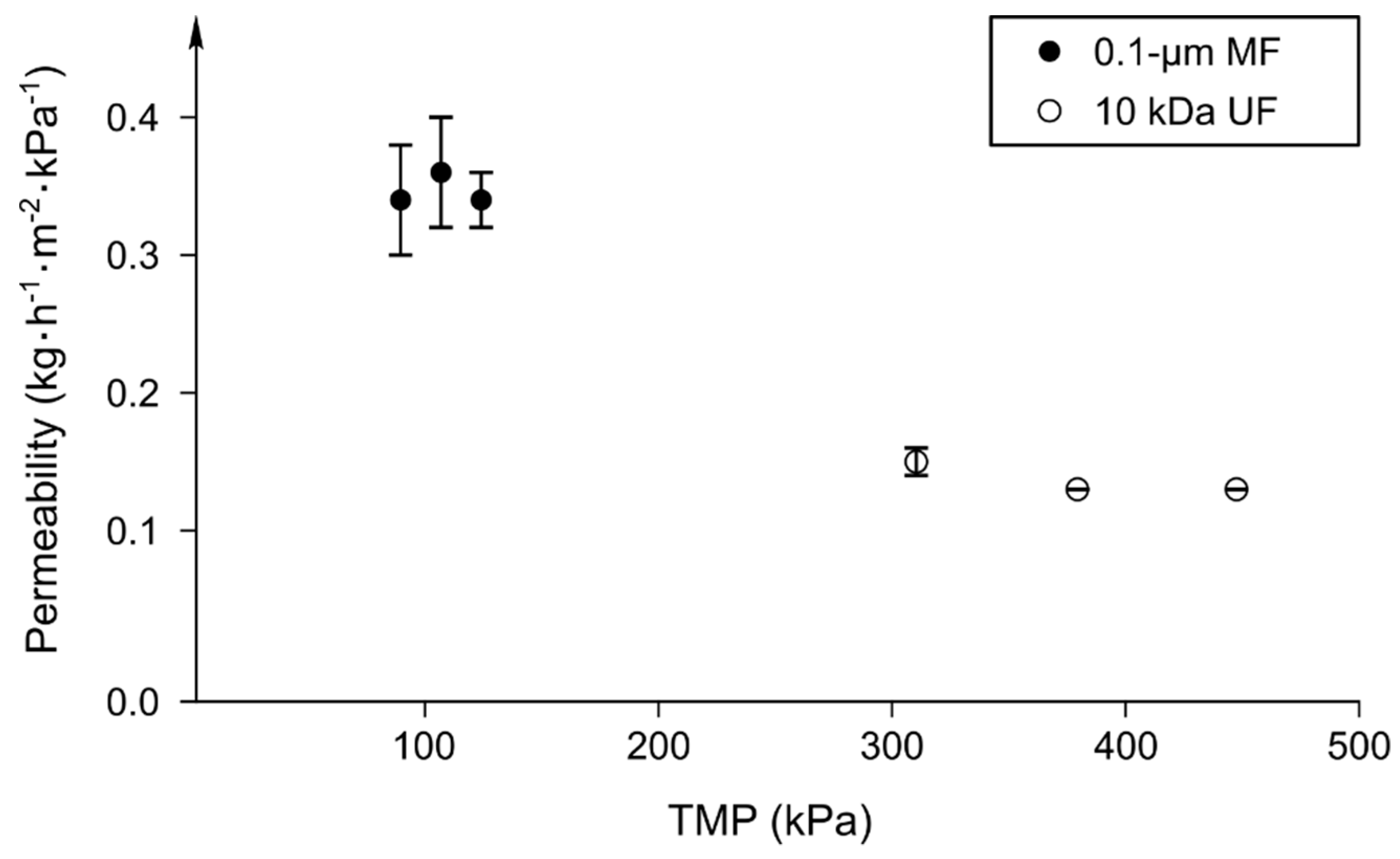
3.1. Effect of TMP on Normalized Permeation Flux in the Total Recirculation Mode
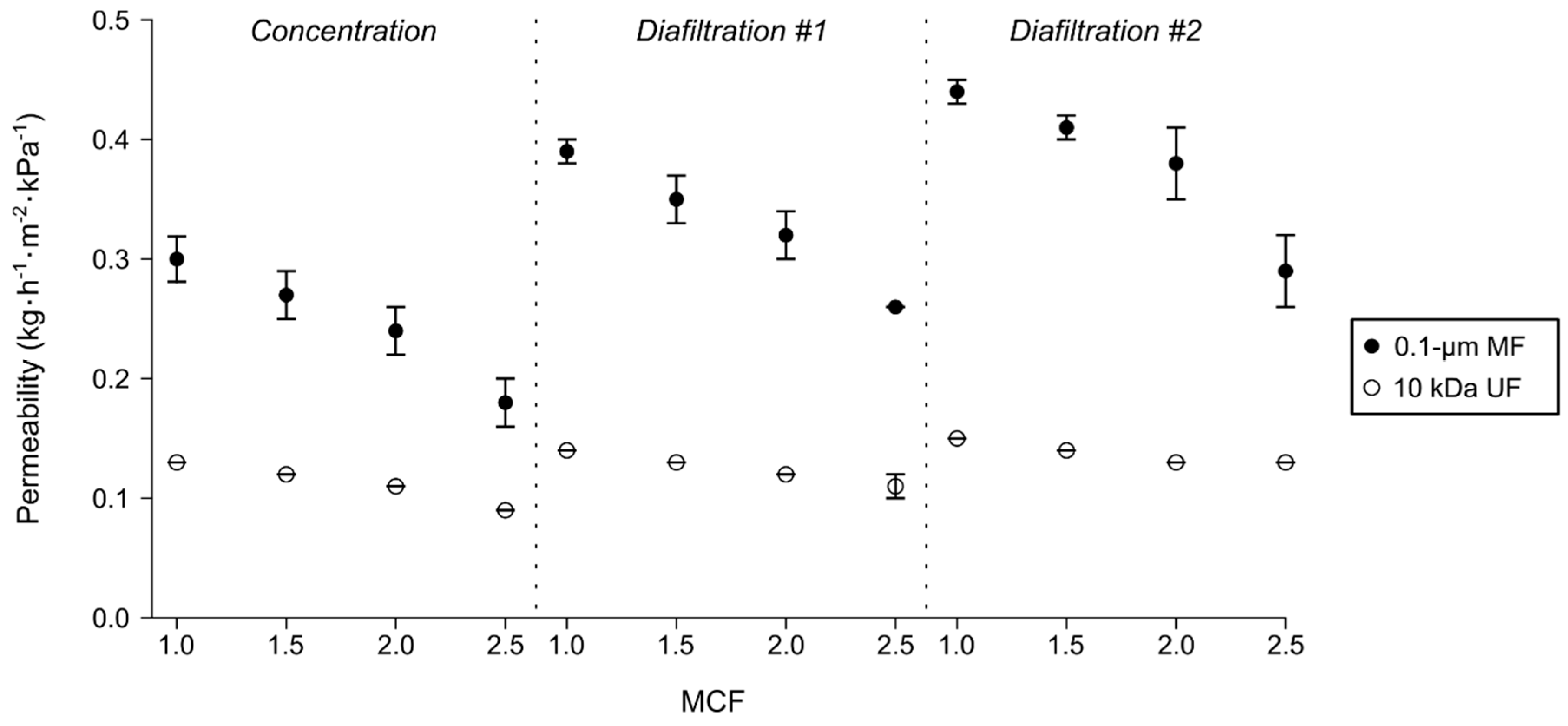
3.2. Effect of Concentration and Diafiltration Modes on Normalized Permeation Flux
3.3. Effect of Concentration and Diafiltration Modes on Retentate Composition
3.4. Effect of Concentration and Diafiltration Modes on Energy Consumption
3.5. Effect of Concentration and Diafiltration Modes on Membrane Fouling
3.6. Cheesemaking Efficiency of Unconcentrated Cheesemilk, and Standardized with MF and UF Retentates
3.7. Economic Assessment of MF and UF Approaches through a Process Simulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guinee, T.P.; O’Kennedy, B.T.; Kelly, P.M. Effect of milk protein standardization using different methods on the composition and yields of Cheddar cheese. J. Dairy Sci. 2006, 89, 468–482. [Google Scholar] [CrossRef]
- Kosikowski, F. New cheese-making procedures utilizing ultrafiltration. Food Technol. 1986, 40, 71–77. [Google Scholar]
- Mistry, V.V.; Maubois, J.-L. Application of membrane separation technology to cheese production. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 677–697. [Google Scholar]
- Cheryan, M. Ultrafiltration and Microfiltration Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Guinee, T.P.; Gorry, C.B.; Callaghan, D.J.O.; Brendan, T.; Kennedy, O.; Brien, N.O.; Fenelon, M.A. The effects of composition and some processing treatments on the rennet coagulation properties of milk. Int. J. Dairy Technol. 1997, 50, 99–106. [Google Scholar] [CrossRef]
- Govindasamy-Lucey, S.; Jaeggi, J.J.; Martinelli, C.; Johnson, M.E.; Lucey, J.A. Standardization of milk using cold ultrafiltration retentates for the manufacture of Swiss cheese: Effect of altering coagulation conditions on yield and cheese quality. J. Dairy Sci. 2011, 94, 2719–2730. [Google Scholar] [CrossRef] [PubMed]
- Garnot, P. Influence of milk concentration by UF on enzymatic coagulation. Bull. Int. Dairy Fed. 1988, 225, 11–15. [Google Scholar]
- Hu, K.; Dickson, J.M.; Kentish, S.E. Microfiltration for casein and serum protein separation. In Membrane Processing for Dairy Ingredient Separation; Hu, K., Dickson, J.M., Eds.; John Wiley & Sons: Hoboken, NZ, USA, 2015; pp. 1–34. [Google Scholar]
- Fauquant, J.; Maubois, J.L.; Pierre, A. Microfiltration du lait sur membrane minérale. Tech. Laitière Mark. 1988, 1028, 21–23. [Google Scholar]
- Ardisson-Korat, A.V.; Rizvi, S.S.H. Vatless manufacturing of low-moisture part-skim mozzarella cheese from highly concentrated skim milk microfiltration retentates. J. Dairy Sci. 2004, 87, 3601–3613. [Google Scholar] [CrossRef]
- Maubois, J.L. Membrane microfiltration: A tool for a new approach in dairy technology. Aust. J. Dairy Technol. 2002, 57, 92–96. [Google Scholar]
- Gésan-Guiziou, G. Integrated membrane operations in whey processing. In Integrated Membrane Operations in the Food Production; Cassano, A., Drioli, E., Eds.; De Gruyter: Berlin, Germany, 2014; pp. 133–146. [Google Scholar]
- Mercier-Bouchard, D.; Benoit, S.; Doyen, A.; Britten, M.; Pouliot, Y. Process efficiency of casein separation from milk using polymeric spiral-wound microfiltration membranes. J. Dairy Sci. 2017, 100, 8838–8848. [Google Scholar] [CrossRef]
- Zulewska, J.; Newbold, M.; Barbano, D.M. Efficiency of serum protein removal from skim milk with ceramic and polymeric membranes at 50 °C. J. Dairy Sci. 2009, 92, 1361–1377. [Google Scholar] [CrossRef]
- Heino, A. Microfiltration in Cheese and Whey Processing. Master’s Thesis, University of Helsinki, Helsinki, Finland, 2010. [Google Scholar]
- Méthot-Hains, S.; Benoit, S.; Bouchard, C.; Doyen, A.; Bazinet, L.; Pouliot, Y. Effect of transmembrane pressure control on energy efficiency during skim milk concentration by ultrafiltration at 10 and 50 °C. J. Dairy Sci. 2016, 99, 8655–8664. [Google Scholar] [CrossRef] [PubMed]
- Tremblay-Marchand, D.; Doyen, A.; Britten, M.; Pouliot, Y. A process efficiency assessment of serum protein removal from milk using ceramic graded permeability microfiltration membrane. J. Dairy Sci. 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Guinee, T.P.; Mulholland, E.O.; Kelly, J.; Callaghan, D.J.O. Effect of protein-to-fat ratio of milk on the composition, manufacturing efficiency, and yield of Cheddar cheese. J. Dairy Sci. 2007, 90, 110–123. [Google Scholar] [CrossRef]
- Lauzin, A.; Dussault-Chouinard, I.; Britten, M.; Pouliot, Y. Impact of membrane selectivity on the compositional characteristics and model cheese-making properties of liquid pre-cheese concentrates. Int. Dairy J. 2018, 83, 34–42. [Google Scholar] [CrossRef]
- Fox, P.F.; McSweeney, P.L.H.; Cogan, T.M.T.; Guinee, T.P. Cheese—Chemistry, Physics and Microbiology; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Racine, J.S. RStudio: A platform-independent IDE for R and Sweave. J. Appl. Econom. 2012, 27, 167–172. [Google Scholar] [CrossRef]
- De Mendiburu, F. Agricolae: Statistical procedures for agricultural research. R Packag. Vers. 2014, 1, 1–6. [Google Scholar]
- Beckman, S.L.; Barbano, D.M. Effect of microfiltration concentration factor on serum protein removal from skim milk using spiral-wound polymeric membranes. J. Dairy Sci. 2013, 96, 6199–6212. [Google Scholar] [CrossRef] [PubMed]
- Daviau, C.; Famelart, M.-H.; Pierre, A.; Goudédranche, H.; Maubois, J.-L. Rennet coagulation of skim milk and curd drainage: Effect of pH, casein concentration, ionic strength and heat treatment. Lait 2000, 80, 397–415. [Google Scholar] [CrossRef]
- Pierre, A.; Fauquant, J.; Le Graet, Y.; Piot, M.; Maubois, J.L. Préparation de phosphocaséinate natif par microfiltration sur membrane. Lait 1992, 72, 461–474. [Google Scholar] [CrossRef]
- Caron, A.; St-Gelais, D.; Pouliot, Y. Coagulation of milk enriched with ultrafiltered or diafiltered microfiltered milk retentate powders. Int. Dairy J. 1997, 7, 445–451. [Google Scholar] [CrossRef]
- Oommen, B.S.; Mistry, V.V.; Nair, M.G. Effect of homogenization of cream on composition, yield, and functionality of Cheddar cheese made from milk supplemented with ultrafiltered milk. Lait 2000, 80, 77–91. [Google Scholar] [CrossRef]
- Heino, A.; Uusi-Rauva, J.; Outinen, M. Pre-treatment methods of Edam cheese milk. Effect on cheese yield and quality. LWT-Food Sci. Technol. 2010, 43, 640–646. [Google Scholar] [CrossRef]
- Neocleous, M.; Barbano, D.M.; Rudan, M.A. Impact of low concentration factor microfiltration on milk component recovery and Cheddar cheese yield. J. Dairy Sci. 2002, 85, 2415–2424. [Google Scholar] [CrossRef]
- Papadatos, A.; Neocleous, M.; Berger, A.M.; Barbano, D.M. Economic feasibility evaluation of microfiltration of milk prior to cheesemaking. J. Dairy Sci. 2003, 86, 1564–1577. [Google Scholar] [CrossRef]
| Sample | Membrane Type | Filtration Step | MCF | TP (% w/w) | NPN (%w/w) | TP Rejection Coefficient | Total Solids (%w/w) |
|---|---|---|---|---|---|---|---|
| Retentate | 0.1-µm (MF) | Concentration | 1.0× | 3.13 ± 0.12 cd | 0.15 ± 0.01 a | 97.07 ± 0.75 d | 8.77 ± 0.15 de |
| 2.5× | 7.08 ± 1.62 b | 0.09 ± 0.03 abcd | 14.97 ± 0.65 a | ||||
| DF #1 | 1.0× | 3.05 ± 0.22 cd | 0.07 ± 0.01 bcd | 97.11 ± 0.52 d | 5.25 ± 0.17 g | ||
| 2.5× | 8.94 ± 0.45 a | 0.13 ± 0.06 abc | 11.48 ± 0.42 b | ||||
| DF #2 | 1.0× | 2.92 ± 0.11 d | 0.05 ± 0.01 d | 98.92 ± 0.21 bc | 4.56 ± 0.87 g | ||
| 2.5× | 8.35 ± 0.08 ab | 0.14 ±0.11 ab | 9.88 ± 0.02 cd | ||||
| 10 kDa (UF) | Concentration | 1.0× | 3.53 ± 0.56 cd | 0.13 ± 0.02 abc | 99.36 ± 0.06 ab | 8.48 ± 0.11 ef | |
| 2.5× | 8.91 ± 0.82 a | 0.14 ± 0.02 ab | 16.12 ± 0.05 a | ||||
| DF #1 | 1.0× | 4.40 ± 1.22 c | 0.07 ± 0.01 bcd | 98.38 ± 0.13 ab | 7.25 ± 1.01 f | ||
| 2.5× | 9.23 ± 0.27 a | 0.09 ± 0.03 abcd | 11.96 ± 0.74 b | ||||
| DF #2 | 1.0× | 3.67 ± 0.48 cd | 0.06 ± 0.04 cd | 99.82 ± 0.04 a | 4.92 ± 0.57 g | ||
| 2.5× | 9.15 ± 1.50 a | 0.11 ± 0.07 abcd | 10.09 ± 1.77 c | ||||
| Permeate | 0.1-µm (MF) | Concentration | 2.5× | 0.22 ± 0.11 a | 0.09 ± 0.06 ab | - | 5.93 ± 0.22 a |
| DF #1 | 2.5× | 0.26 ± 0.04 a | 0.15 ± 0.08 a | - | 2.06 ± 0.03 b | ||
| DF #2 | 2.5× | 0.09 ± 0.02 b | 0.06 ± 0.02 bc | - | 0.76 ± 0.01 c | ||
| 10 kDa (UF) | Concentration | 2.5× | N.D. | 0.15 ± 0.01 a | - | 5.50 ± 0.06 a | |
| DF #1 | 2.5× | N.D. | 0.05 ± 0.01 bc | - | 1.41 ± 0.77 bc | ||
| DF #2 | 2.5× | N.D. | 0.02 ± 0.00 c | - | 0.92 ± 0.56 c |
| Filtration Step | Specific Energy Consumption 1 (Wh per kg of Permeate Removed) | |
|---|---|---|
| MF | UF | |
| Concentration | 10.81 ± 0.77 bA | 15.60 ± 0.28 aA |
| Diafiltration #1 | 8.05 ± 0.40 bB | 12.89 ± 0.33 aB |
| Diafiltration #2 | 6.92 ± 0.51 bB | 11.47 ± 0.10 aC |
| Whole process | 8.59 ± 0.50 b | 13.31 ± 0.23 a |
| Membrane Type | Resistance Type (10−13 m−1) | |||
|---|---|---|---|---|
| Rm | Rrev | Rirrev | Rtot | |
| MF (0.1-μm) | 0.47 ± 0.01 b | 1.87 ± 0.92 a | 0.81 ± 0.13 b | 3.15 ± 0.96 b |
| UF (10 kDa) | 0.72 ± 0.05 a | 3.38 ± 0.30 a | 3.00 ± 0.39 a | 7.09 ± 0.23 a |
| Indicator | Unconcentrated Cheesemilk | 0.1-μm MF Retentate | 10 kDa UF Retentate |
|---|---|---|---|
| TS (%) | 51.11 ± 0.23 b | 53.30 ± 1.29 a | 52.09 ± 0.50 ab |
| MNFS (%) | 67.69 ± 0.61 a | 67.85 ± 1.53 a | 67.75 ± 0.67 a |
| FDM (%) | 54.34 ± 0.92 c | 58.50 ± 0.93 a | 56.23 ± 0.87 b |
| YVat (%) | 14.13 ± 0.25 b | 28.46 ± 0.53 a | 28.21 ± 0.47 a |
| YMAVat (%) | 14.13 ± 0.30 c | 29.67 ± 0.51 a | 28.75 ± 0.42 b |
| YFVat (%) | 89.46 ± 0.41 c | 96.91 ± 0.26 a | 96.29 ± 0.12 b |
| YPVat (%) | 76.15 ± 0.26 c | 90.73 ± 0.13 a | 86.17 ± 0.20 b |
| Indicator | Scenarios | ||
|---|---|---|---|
| Unconcentrated Milk | MF-Standardized Cheesemilk | UF-Standardized Cheesemilk | |
| Inputs (kg) | |||
| Whole milk | 1,000,000 | 1,000,000 | 1,000,000 |
| Cream | 24,070 | 23,178 | 30,012 |
| Calcium chloride | 143 | 73 | 78 |
| Starter culture 1 | 20 | 10 | 11 |
| Coagulant enzyme | 232 | 119 | 127 |
| Outputs (kg) | |||
| Cheese 2 | 145,049 | 147,531 | 154,592 |
| Cheese whey | 879,995 | 376,863 | 405,647 |
| Permeate | - | 602,466 | 628,049 |
| Diafiltrate | - | 1,204,932 | 1,256,099 |
| Predicted mass yield 3 (%) | |||
| YVat | 14.16 | 28.13 | 27.59 |
| YInp | 14.16 | 14.42 | 15.01 |
| YPInp | 76.15 | 84.57 | 85.73 |
| Indicator | Price | Scenarios | ||
|---|---|---|---|---|
| Unconcentrated Milk | MF-Standardized Cheesemilk | UF-Standardized Cheesemilk | ||
| Expenditures | ||||
| Inputs | ||||
| Whole milk | 84.56 Can$ 100 kg−1 | 845,600 | 845,600 | 845,600 |
| Cream | 3.95 Can$ kg−1 | 95,077 | 91,553 | 118,549 |
| Calcium chloride | 2.23 Can$ kg−1 | 320 | 164 | 175 |
| Starter culture 1 | 600 Can$ kg−1 | 12,289 | 6293 | 6723 |
| Coagulant enzyme | 28.32 Can$ kg−1 | 6583 | 3371 | 3602 |
| Subtotal | Can$ | 959,869 | 946,981 | 974,649 |
| Filtration | ||||
| Membrane replacement 2 | 0.23 Can$ m−2 day−1 | 0 | 1,169 | 714 |
| Electricity | 0.0327 Can$ kWh−1 | 0 | 508 | 821 |
| Power | 0.42 Can$ kW−1 | 0 | 821 | 1327 |
| Subtotal | Can$ | 0 | 2498 | 2862 |
| Operating costs | Can$ | 959,869 | 949,479 | 977,511 |
| Can$ kg of cheese−1 | 6.62 | 6.44 | 6.32 | |
| Indicator | Price | Scenarios | ||
|---|---|---|---|---|
| Unconcentrated Milk | MF-Standardized Cheesemilk | UF-Standardized Cheesemilk | ||
| Expenditures | ||||
| Inputs | Can$ | 879,859 | 891,849 | 895,681 |
| Filtration | Can$ | 0 | 2431 | 2815 |
| Operating costs | Can$ | 879,859 | 894,280 | 898,496 |
| Can$ kg of cheese−1 | 6.87 | 6.55 | 6.52 | |
| YInp | % | 12.85 | 13.53 | 13.65 |
| YPInp | % | 76.15 | 85.10 | 86.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamberland, J.; Mercier-Bouchard, D.; Dussault-Chouinard, I.; Benoit, S.; Doyen, A.; Britten, M.; Pouliot, Y. On the Use of Ultrafiltration or Microfiltration Polymeric Spiral-Wound Membranes for Cheesemilk Standardization: Impact on Process Efficiency. Foods 2019, 8, 198. https://doi.org/10.3390/foods8060198
Chamberland J, Mercier-Bouchard D, Dussault-Chouinard I, Benoit S, Doyen A, Britten M, Pouliot Y. On the Use of Ultrafiltration or Microfiltration Polymeric Spiral-Wound Membranes for Cheesemilk Standardization: Impact on Process Efficiency. Foods. 2019; 8(6):198. https://doi.org/10.3390/foods8060198
Chicago/Turabian StyleChamberland, Julien, Dany Mercier-Bouchard, Iris Dussault-Chouinard, Scott Benoit, Alain Doyen, Michel Britten, and Yves Pouliot. 2019. "On the Use of Ultrafiltration or Microfiltration Polymeric Spiral-Wound Membranes for Cheesemilk Standardization: Impact on Process Efficiency" Foods 8, no. 6: 198. https://doi.org/10.3390/foods8060198
APA StyleChamberland, J., Mercier-Bouchard, D., Dussault-Chouinard, I., Benoit, S., Doyen, A., Britten, M., & Pouliot, Y. (2019). On the Use of Ultrafiltration or Microfiltration Polymeric Spiral-Wound Membranes for Cheesemilk Standardization: Impact on Process Efficiency. Foods, 8(6), 198. https://doi.org/10.3390/foods8060198





