Fluidized Bed Drying of Pumpkin (Cucurbita sp.) Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Seeds
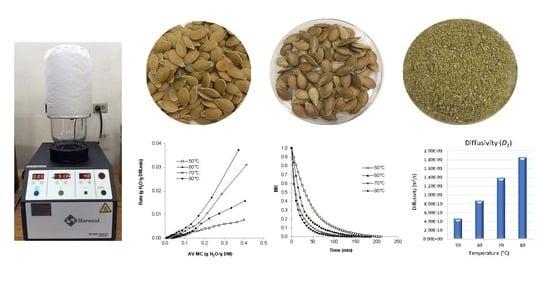
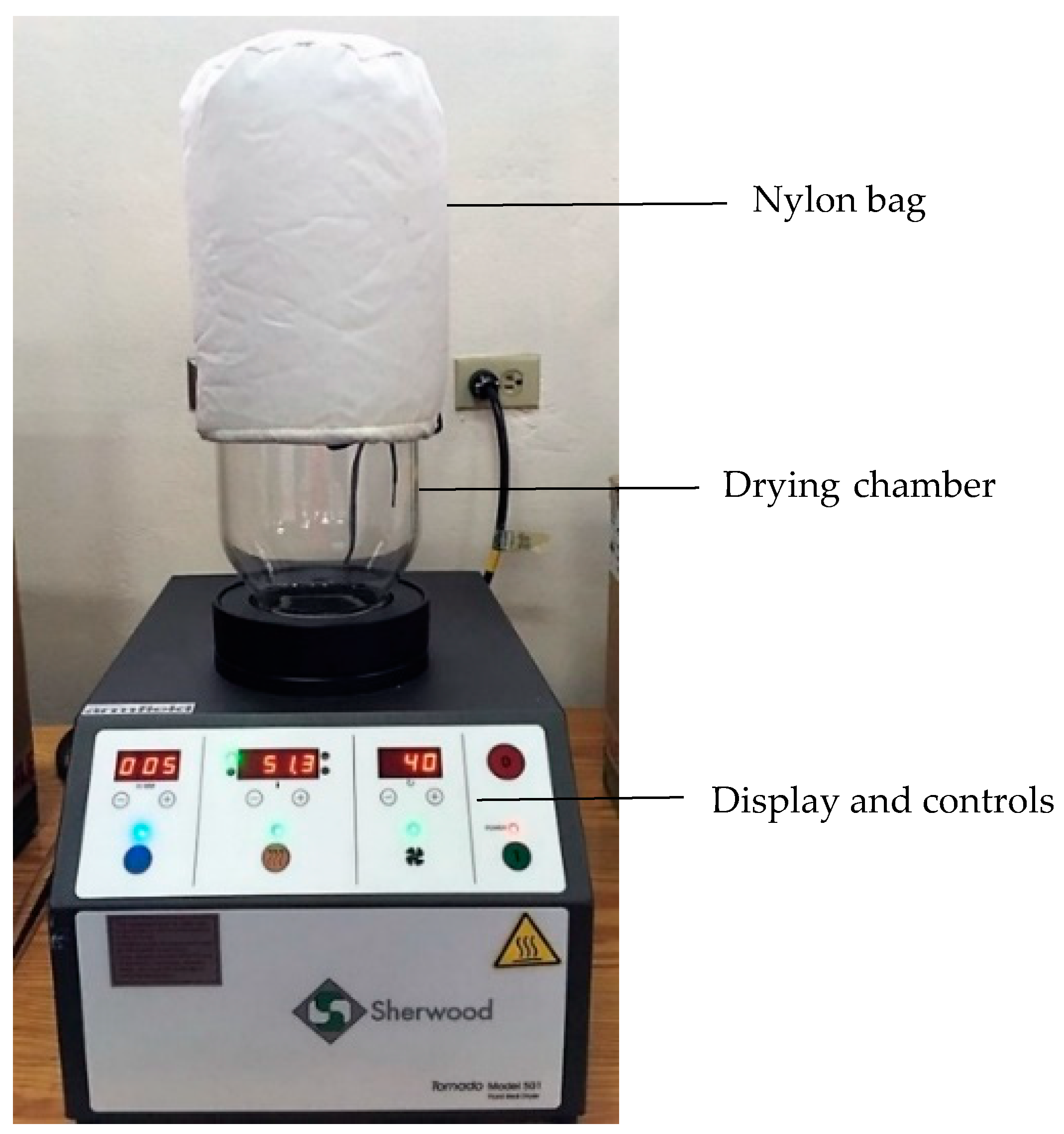
2.2. Drying Equipment
2.3. Drying Procedure
2.4. Drying Variables
2.5. Analytical Methods
2.6. Powder Physical Properties
2.7. Data Analysis
3. Results and Discussion
3.1. Moisture and Water Activity (aw)
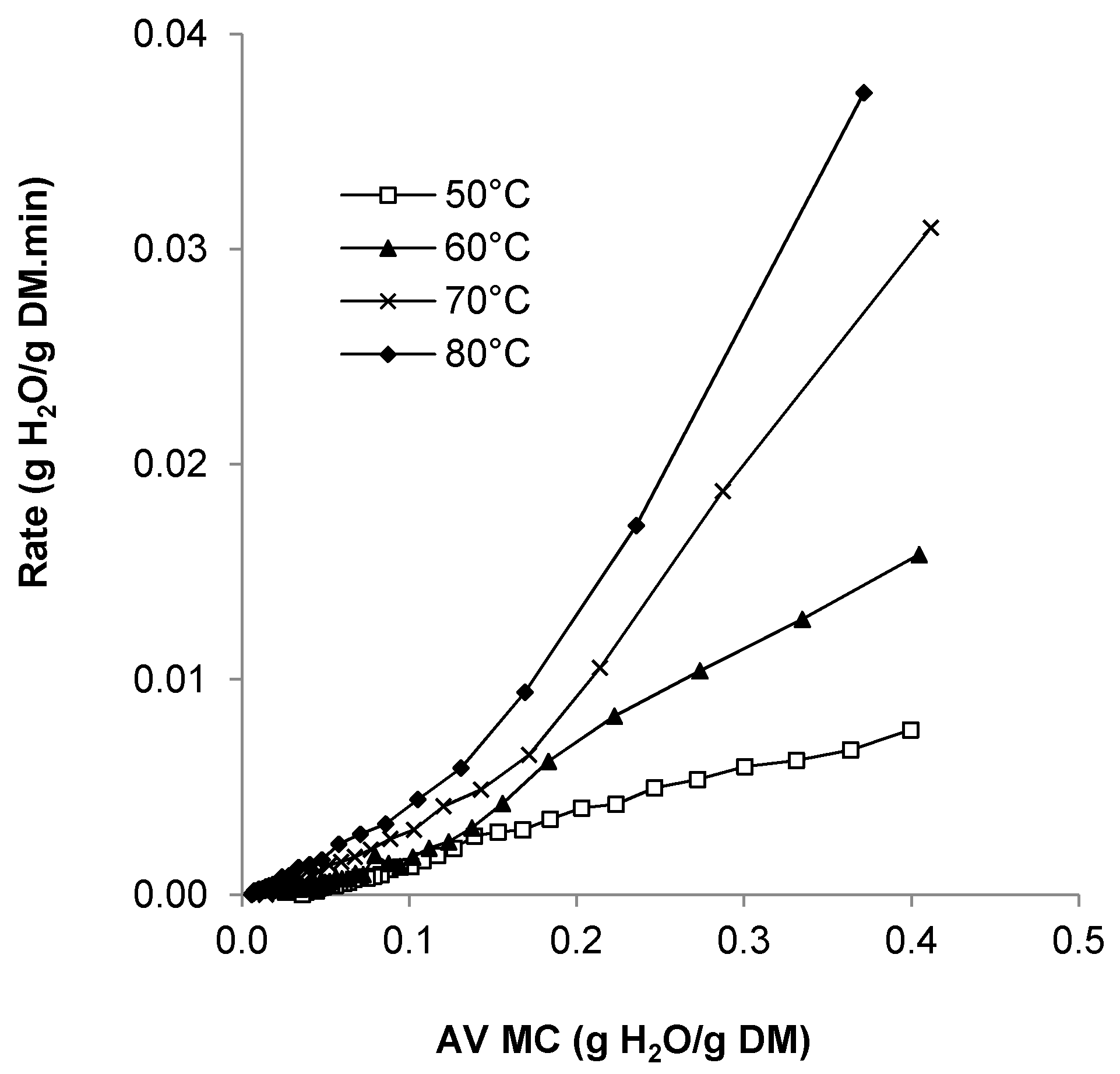
3.2. Drying Rate
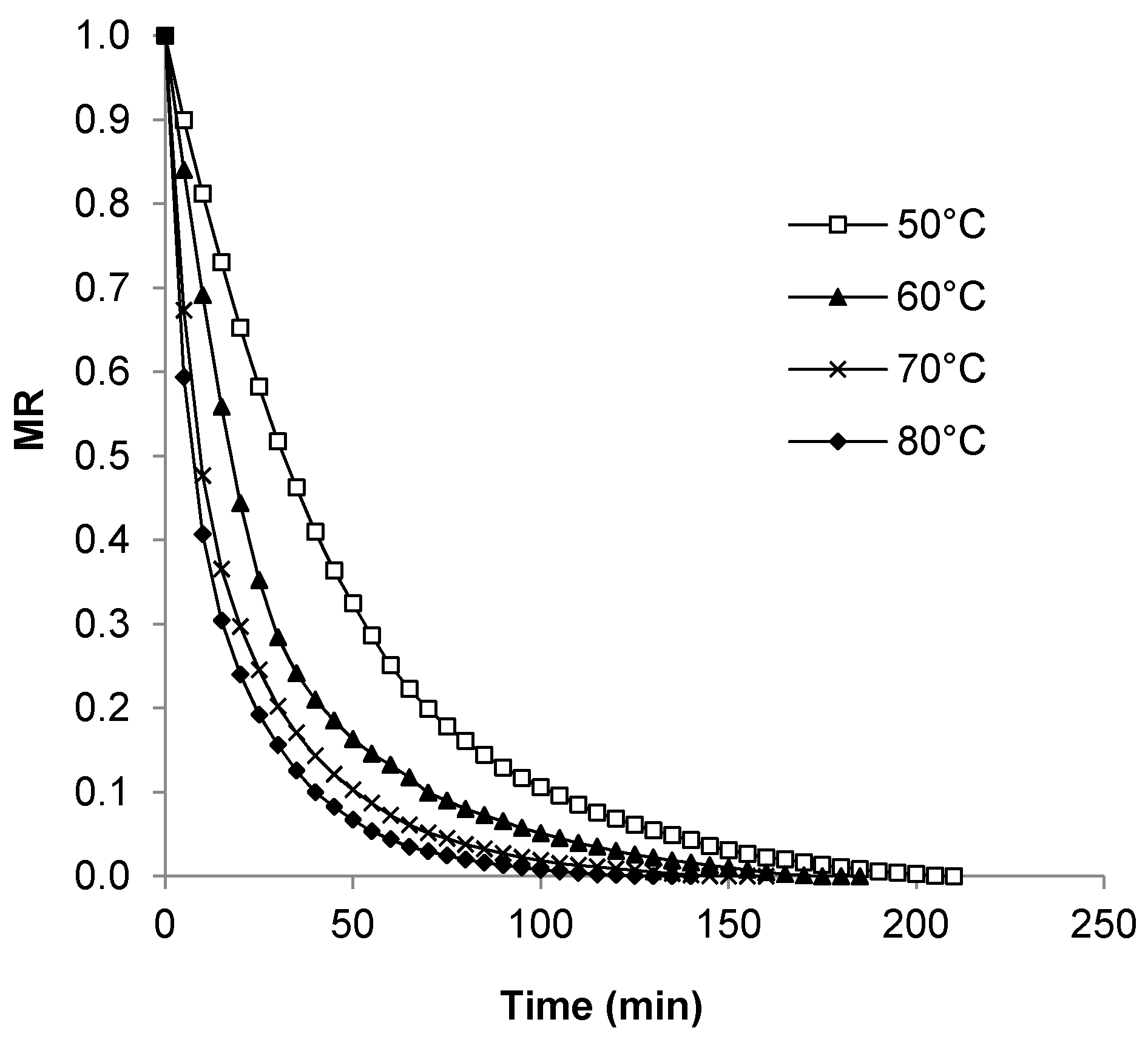
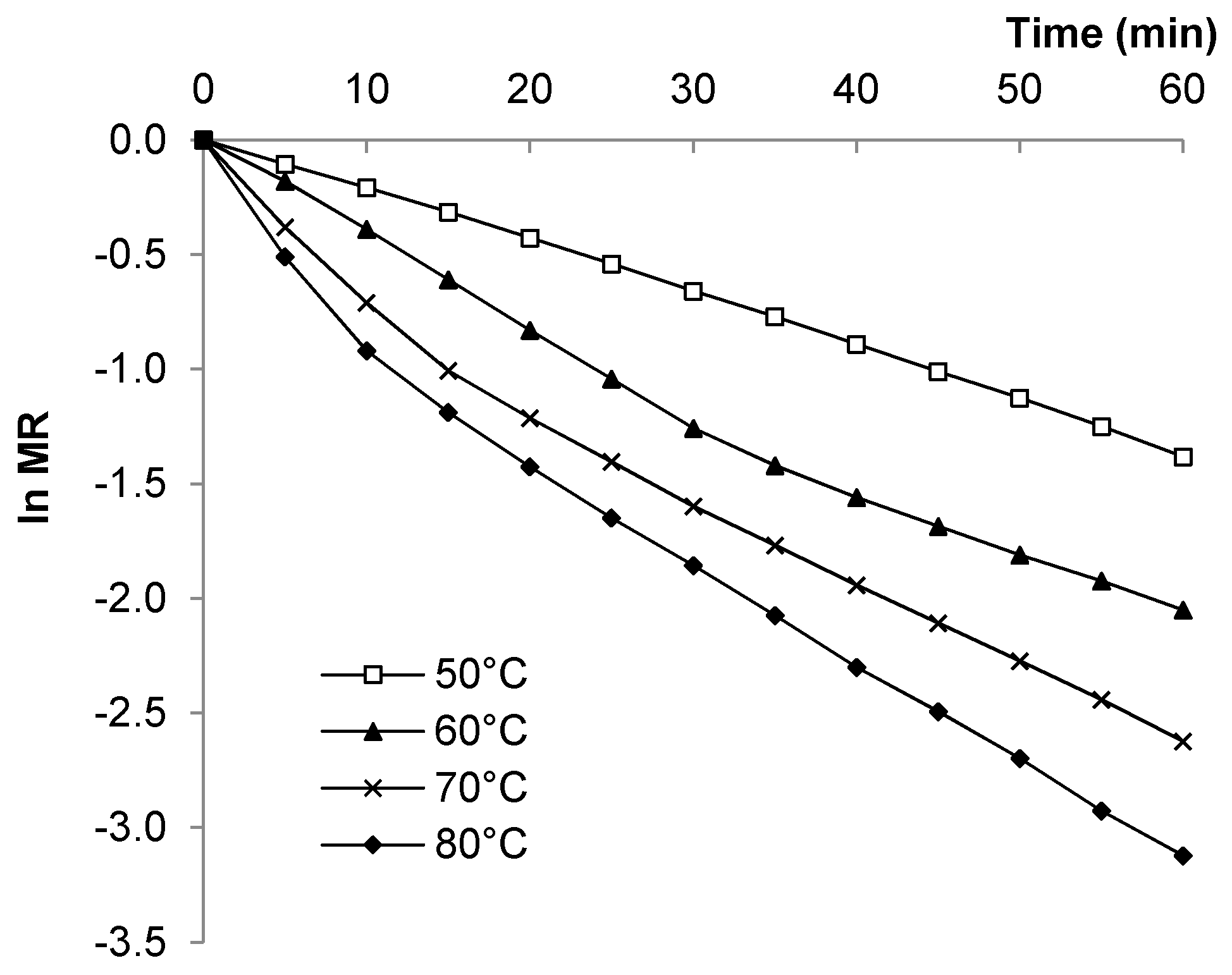
3.3. Moisture Ratio
3.4. Drying Rate Constants (k) and Effective Diffusion Coefficient (Deff)
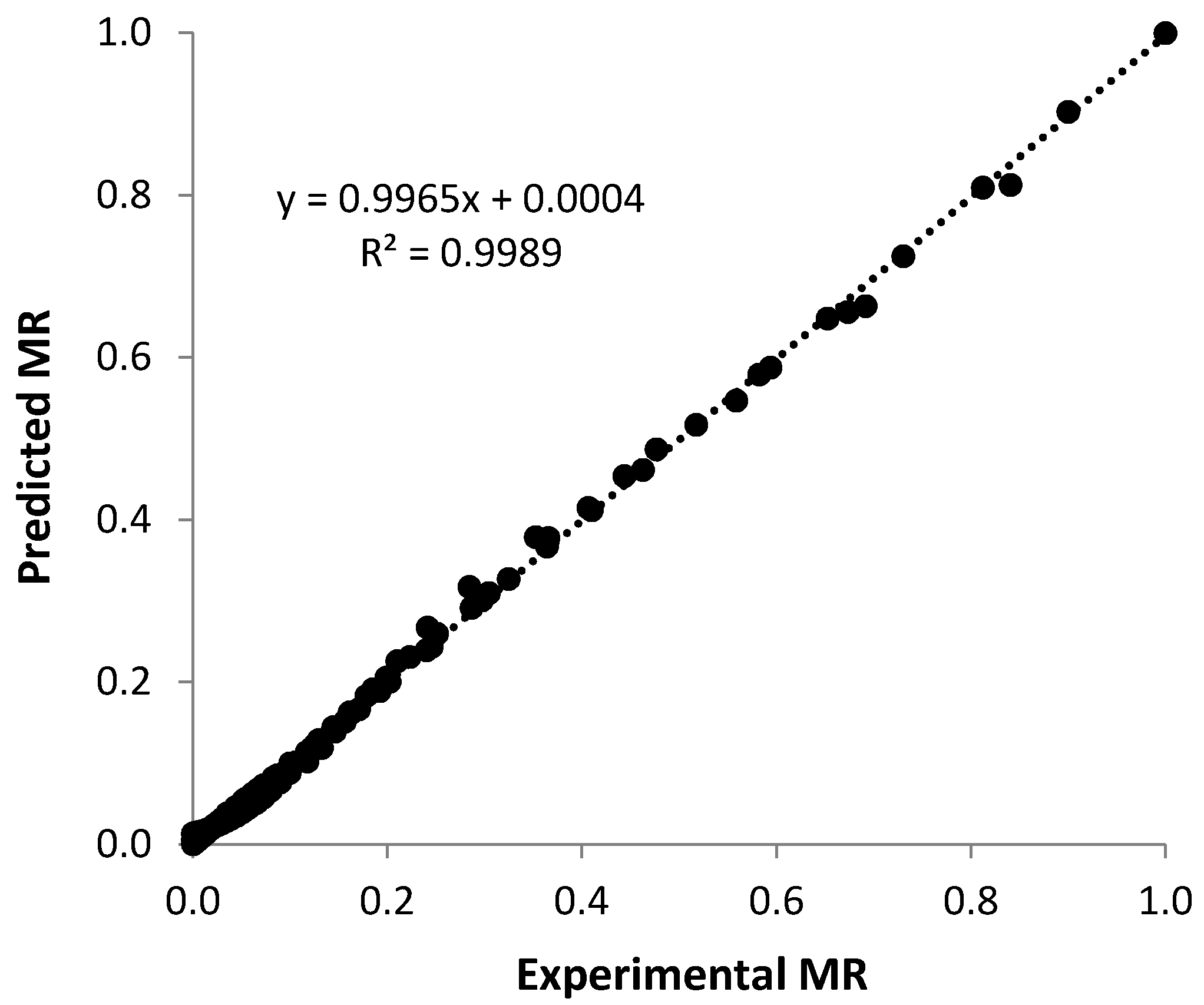
3.5. Thin Layer Models
3.6. Quality Attributes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Ea | Activation energy (KJ/mol) |
| Deff | Effective Diffusion coefficient (m2/s) |
| DM | Dry matter |
| k1, k2 | Drying rate constants (1/min) obtained from slopes of plot of ln MR versus time |
| L | Half-thickness of leaf (m) |
| M | Moisture content (g H2O/g DM) at time = t |
| M0 | Initial Moisture Content (g H2O/g DM) |
| Me | Equilibrium Moisture Content (g H2O/g DM) |
| MR | Moisture Ratio |
| R | Gas constant (8.314 J/mol.K) |
| R2 | Coefficient of Determination |
| RMSE | Root Mean Square Error |
| t | Time (min) |
| T | Temperature (°C) |
| wb | Wet basis |
| K, a, b, c, g, K1, K2, a0, h | Thin layer model constants obtained by curve fitting |
| χ2 | Chi-Square Statistic |
References
- United States Department of Agriculture (USDA)—Agricultural Research Service; National Nutrient Database for Standard Reference Legacy Release 12014, Seeds, pumpkin and squash seed kernels, dried. Available online: https://ndb.nal.usda.gov/ndb/foods (accessed on 4 March 2019).
- Giami, S.Y.; Isichei, I. Preparation and properties of flours and protein concentrates from raw, fermented and germinated fluted pumpkin (Telfairia occidentalis Hook) seeds. Plant Food Hum. Nutr. 1999, 54, 67–77. [Google Scholar] [CrossRef]
- Hamed, S.Y.; Hassan, N.M.E.; Hassan, A.B.; Eltayeb, M.M.; Babiker, E.E. Nutritional evaluation and physiochemical properties of processed pumpkin (Telfairia occidentalis Hook) seed flour. Pak. J. Nutr. 2008, 7, 330–334. [Google Scholar]
- Nyam, K.L.; Lau, M.; Tan, C.P. Fibre from pumpkin (Cucurbita pepo L.) seeds and rinds: physio-chemical properties, antioxidant capacity and application as bakery product ingredients. Mal. J. Mutr. 2013, 19, 99–109. [Google Scholar]
- Saraswathi, D.; Renu, R.; Maloo, S. Development and quality evaluation of pumpkin seeds and flaxseeds powder incorporated biscuits. Int. J. Food Sci. Nutr. 2018, 3, 78–83. [Google Scholar]
- Dhiman, A.K.; Bavita, K.; Attri, S.; Ramachandran, P. Preparation of pumpkin powder and pumpkin seed kernel powder for supplementation in weaning mix and cookies. Intl. J. Chem. Studies 2018, 6, 167–175. [Google Scholar]
- Bello, F.A.; Udo, I.I.; Mbak, D.L. Proximate composition and functional properties of sprouted sorghum (Sorghum bicolor) and defatted fluted pumpkin seed (Telfairia occidentalis) flour blends. Am. J. Innov. Res. Appl. Sci. 2017, 5, 246–291. [Google Scholar]
- Atuonwu, A.; Akobundu, E. Nutritional and sensory quality of cookies supplemented with defatted pumpkin (Cucurbita pepo) seed flour. Pak. J. Nutr. 2010, 9, 672–677. [Google Scholar] [CrossRef]
- Ardabili, A.G.; Farhoosh, R.; Khodaparast, M.H.H. Chemical composition and physicochemical properties of pumpkin seeds (Cucurbita pepo Subsp. pepo Var. Styriaka) grown in Iran. J. Agr. Sci. Tech. 2011, 13, 1053–1063. [Google Scholar]
- Fedha, M.S. Physicochemical Characterization and Food Application Potential of Pumpkin (Cucurbita sp.) Fruit and Seed Kernel Flours. Master’s Thesis, Jomo Kenyatta University of Agriculture and Technology, Juja, Kenya, 2008. [Google Scholar]
- Kaur, M.; Sharma, S. formulation and nutritional evaluation of cookies supplemented with pumpkin seed (Curcubita Moschata) flour. Chem. Sci. Rev. Lett. 2017, 6, 2236–2241. [Google Scholar]
- Sharma, G.S.; Lakhawat, S. Development, Quality Evaluation and Acceptability of Pumpkin Seed Flour Incorporated in Gravy. J. Nutr. Food Sci. 2017, 7, 613. [Google Scholar]
- Sacilik, K. Effect of drying methods on thin-layer drying characteristics of hull-less seed pumpkin (Cucurbita pepo L.). J. Fd. Eng. 2007, 79, 23–30. [Google Scholar] [CrossRef]
- Kaur, M. Development and Nutritional Evaluation of Pumpkin Seed (Cucurbita moschata) Supplemented Products. Master’s Thesis, Punjab Agricultural University, Ludhiana, India, 2017. Supervisor: Sonia Sharma. [Google Scholar]
- Jittanit, W. Kinetics and temperature dependent moisture diffusivities of pumpkin seeds during drying. Kasetsart J. Nat. Sci. 2011, 45, 147–158. [Google Scholar]
- Uddin, Z.; Suppakul, P.; Boonsupthip, W. Effect of air temperature and velocity on moisture diffusivity in relation to physical and sensory quality of dried pumpkin seeds. Dry Technol. 2016, 34, 1423–1433. [Google Scholar] [CrossRef]
- Bansal, P.K.; Chung, K.Y. Food Drying Equipment, Chapter 15. In Food Drying Science and Technology Microbiology, Chemistry, Applications; Hui, Y.H., Clary, C., Farid, M.M., Fasina, O.O., Noomhorm, A., Welti-Chanes, J., Eds.; DEStech Publications, Inc.: Lancaster, PA, USA, 2008; pp. 359–402. [Google Scholar]
- Chayjan, R.A.; Salari, K.; Abedi, Q.; Ali Akbar, S. Modeling moisture diffusivity, activation energy and specific energy consumption of squash seeds in a semi fluidized and fluidized bed drying. J. Food Sci. Technol. 2013, 50, 667–677. [Google Scholar] [CrossRef]
- Ramsumair, S. Fluidized Bed Drying of Pumpkin (Cucurbita sp.) Seeds. Master’s Thesis, Food Science and Technology, Department of Chemical Engineering, The University of the West Indies, Saint Augustine, Trinidad and Tobago, Supervisor: S. Mujaffar. 2018, (unpublished). [Google Scholar]
- Mujaffar, S.; Lee Loy, A. Drying kinetics of microwave-dried vegetable amaranth (Amaranthus dubius) leaves. Food Res. Int. 2016, 5, 33–44. [Google Scholar] [CrossRef][Green Version]
- Official Methods of Analysis of AOAC INTERNATIONAL (1999), 16th ed.; AOAC INTERNATIONAL: Gaithersburg, MD, USA, 1999.
- Official Methods of Analysis of AOAC INTERNATIONAL (2000), 17th ed.; AOAC INTERNATIONAL: Gaithersburg, MD, USA, 2000.
- Roongruangsri, W.; Bronlund, J.E. Effect of air-drying temperature on physico-chemical, powder properties and sorption characteristics of pumpkin powders. Food Res. Int. 2016, 23, 962–972. [Google Scholar]
- Dirim, S.N.; Çalıskan, G.K. Determination of the effect of freeze drying process on the production of pumpkin (Cucurbita moschata) puree powder and the powder properties. J. Food 2012, 37, 203–210. [Google Scholar]
- Mujaffar, S.; Sankat, C.K. The air drying behavior of shark fillets. Can. Biosyst. Eng. 2005, 47, 11–21. [Google Scholar]
- Mujaffar, S.; Sankat, C.K. Modeling the drying behavior of unsalted and salted catfish (Arius sp.) slabs. J. Food Process. Preserv. 2015, 39, 1385–1398. [Google Scholar] [CrossRef]
- Ertekin, C.; Firat, M.Z. A comprehensive review of thin-layer drying models used in agricultural products. Crit. Rev. Food Sci. Nutr. 2017, 57, 701–717. [Google Scholar] [CrossRef] [PubMed]
- Hyams, D.G. Hyams Development—CurveExpert Development. Available online: http://www.curveexpert.net. (accessed on 2 November 2018).
- Assaad, H.I.; Hou, Y.; Zhou, L.; Carroll, R.J.; Wu, G. Rapid publication-ready MS-Word tables for two-way ANOVA. SpringerPlus 2014, 3, 474. [Google Scholar] [CrossRef]
- Sablani, S.S.; Rahman, M.S. Fundamentals of Food Dehydration, Chapter 1. In Food Drying Science and Technology Microbiology, Chemistry, Applications; Hui, Y.H., Clary, C., Farid, M.M., Fasina, O.O., Noomhorm, A., Welti-Chanes, J., Eds.; DEStech Publications, Inc.: Lancaster, PA, USA, 2008; pp. 359–402. [Google Scholar]
- Giami, S.Y.; Bekebain, D.A. proximate composition and functional properties of raw and processed full-fat fluted pumpkin (Telfairia occidentalis) seed flour. J. Sci. Food Agric. 1992, 59, 321–325. [Google Scholar] [CrossRef]
- Buckholz, L.; Daun, H.; Stier, E. Influence of roasting time on sensory attributes of fresh roasted peanuts. J. Food Sci. 1980, 45, 547–554. [Google Scholar] [CrossRef]

| Model Name | Equation |
|---|---|
| Newton | MR = exp (−Kt) |
| Page | MR = exp(−Ktn) |
| Modified Page | MR = exp(−Kt)n |
| Henderson and Pabis | MR = a exp (−Kt) |
| Modified Henderson and Pabis | MR = a exp (−Kt) + b exp (−gt) + c exp (−ht) |
| Logarithmic | MR = a exp (−Kt) + c |
| Two-Term | MR = a exp (−K0 t) + b exp (−K1 t) |
| Two-Term Exponential | MR = a exp (−K t) + (1−a) exp (−K a t) |
| Wang & Singh | MR = 1 + at + bt2 |
| Verma | MR = a exp(−kt)+(1−a) exp(−gt) |
| Hii | MR = a exp(−Ktn) + c exp(−gtn) |
| Midilli | MR = a exp (−Ktn) + b t |
| Weibull distribution | MR = a−b exp (−Ktn) |
| Diffusion approach | MR = a exp(−Kt) + (1−a) exp(−Kbt) |
| Aghbashlo et al. | MR = −K1t/(1 + K2t) |
| Logistic | MR = a0/((1 + a exp (Kt)) |
| Jena and Das | MR = a exp (−Kt + b t1/2) + c |
| Demir et al. | MR = a exp (−Ktn) + c |
| Alibas | MR = a exp (−Ktn + b t) + g |
| Parameter | Drying Temperature | |||
|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | 80 °C | |
| Time to Equilibrium (min) | 207.5 ± 2.5 a | 170.0 ± 5.0 b | 147.5 ± 2.5 c | 122.5 ± 7.5 d |
| Moisture Content (g H2O/g DM) | 0.035 ± 0.0004 a | 0.026 ± 0.0005 b | 0.015 ± 0.0005 c | 0.006 ± 0.001 d |
| Water activity (aw) | 0.418 ± 0.008 a | 0.398 ± 0.008 a | 0.267 ± 0.011 b | 0.267 ± 0.019 b |
| Temperature (°C) | Drying Rate Constant, k (1/min) | Effective Moisture Diffusivity, Deff (m2/s) | ||||
|---|---|---|---|---|---|---|
| k1 | R2 | k2 | R2 | D1 | D2 | |
| 50 | 0.0226 a | 0.9994 | - | - | 4.68 × 10−10 | - |
| 60 | 0.0423 b | 0.9985 | 0.0241 a | 0.9963 | 8.76 × 10−10 | 1.25 × 10−10 |
| 70 | 0.0673 c | 0.9969 | 0.0349 b | 0.9993 | 13.94 × 10−10 | 1.81 × 10−10 |
| 80 | 0.0900 d | 0.9961 | 0.0426 c | 0.9996 | 18.63 × 10−10 | 2.21 × 10−10 |
| a) 50 °C, 2.87 m/s | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | Model Constants obtained by curve fitting | R2 | RMSE | χ2 | |||||||||
| k | n | a | b | c | g | k1 | k2 | a0 | h | ||||
| Alibas | 1.2780 | 1.0005 | 1.0047 | 1.2581 | - | −0.0022 | - | - | - | - | 0.9999 | 0.004237 | 0.000020 |
| Logistic | 0.0240 | - | 8.2151 | - | - | - | - | - | 9.2575 | - | 0.9999 | 0.004534 | 0.000022 |
| Aghbashlo et al. | - | - | - | - | - | - | 0.0217 | −0.0005 | - | - | 0.9998 | 0.005065 | 0.000027 |
| b) 60 °C, 2.87 m/s | |||||||||||||
| Model | Model Constants | R2 | RMSE | χ2 | |||||||||
| k | n | a | b | c | g | k1 | k2 | a0 | h | ||||
| Aghbashlo et al. | - | - | - | - | - | - | 0.0429 | 0.0037 | - | - | 0.9986 | 0.012437 | 0.000163 |
| Alibas | 2.8099 | 0.9987 | 1.0116 | 2.7577 | - | 0.0102 | - | - | - | - | 0.9982 | 0.014473 | 0.000241 |
| c) 70 °C, 2.87 m/s | |||||||||||||
| Model | Model Constants | R2 | RMSE | χ2 | |||||||||
| k | n | a | b | c | g | k1 | k2 | a0 | h | ||||
| Alibas | 0.5837 | 0.9668 | 1.0439 | 0.4725 | - | −0.0405 | - | - | - | - | 0.9998 | 0.004460 | 0.000023 |
| Aghbashlo et al. | - | - | - | - | - | 0.0756 | 0.0112 | - | - | 0.9980 | 0.013693 | 0.000206 | |
| d) 80 °C, 2.87 m/s | |||||||||||||
| Model | Model Constants | R2 | RMSE | χ2 | |||||||||
| k | n | a | b | c | g | k1 | k2 | a0 | h | ||||
| Modified Henderson & Pabis | 0.2153 | - | 0.4646 | 0.1883 | 0.3472 | 0.0416 | - | - | - | 0.0416 | 1.0000 | 0.001141 | 0.000002 |
| Alibas | 0.3455 | 0.9090 | 1.0688 | 0.2007 | - | −0.0681 | - | - | - | 0.9999 | 0.002719 | 0.000009 | |
| Aghbashlo et al. | - | - | - | - | - | - | 0.0987 | 0.0154 | - | - | 0.9978 | 0.014857 | 0.000238 |
| Quality Attribute | Temperature (°C) | |||
|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | 80 °C | |
| L* | 64.21 ± 0.12 a | 63.13 ± 0.32 b | 57.43 ± 0.51 c | 54.94 ± 0.15 d |
| a* | 3.04 ± 0.01 a | 2.90 ± 0.05 a | 2.58 ± 0.50 a | 2.83 ± 0.02 b |
| b* | 18.98 ± 0.04 a | 18.40 ± 0.53 a | 18.07 ± 0.13 a | 17.93 ± 0.16 b |
| Hue (°) | 80.89 ± 0.04 | 81.04 ± 0.40 | 81.85 ± 1.76 | 81.02 ± 0.12 |
| Fat (g/g DM) | 0.35 ± 0.004 a | 0.34 ± 0.010 a | 0.32 ± 0.002 b | 0.32 ± 0.004 b |
| Crude Fiber (g/g DM) | 0.22 ± 0.006 | 0.24 ± 0.010 | 0.23 ± 0.020 | 0.22 ± 0.004 |
| Protein g/g DM | 0.27 ± 0.004 a | 0.29 ± 0.010 ab | 0.29 ± 0.013 ab | 0.31 ± 0.003 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujaffar, S.; Ramsumair, S. Fluidized Bed Drying of Pumpkin (Cucurbita sp.) Seeds. Foods 2019, 8, 147. https://doi.org/10.3390/foods8050147
Mujaffar S, Ramsumair S. Fluidized Bed Drying of Pumpkin (Cucurbita sp.) Seeds. Foods. 2019; 8(5):147. https://doi.org/10.3390/foods8050147
Chicago/Turabian StyleMujaffar, Saheeda, and Sheena Ramsumair. 2019. "Fluidized Bed Drying of Pumpkin (Cucurbita sp.) Seeds" Foods 8, no. 5: 147. https://doi.org/10.3390/foods8050147
APA StyleMujaffar, S., & Ramsumair, S. (2019). Fluidized Bed Drying of Pumpkin (Cucurbita sp.) Seeds. Foods, 8(5), 147. https://doi.org/10.3390/foods8050147