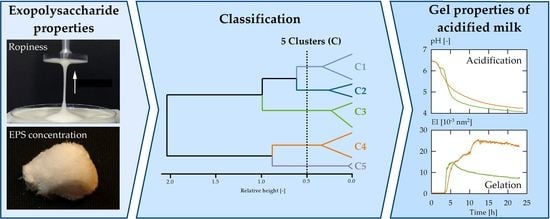
Clustering of Streptococcus thermophilus Strains to Establish a Relation between Exopolysaccharide Characteristics and Gel Properties of Acidified Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Strains
2.2. Production of Acid Gel Suspensions
2.3. Analysis of Acid Gel Suspensions
2.4. Analysis of Gelation Behaviour
2.5. Statistical Data Evaluation
3. Results and Discussion
3.1. Exopolysaccharides in Acid Gel Suspensions
3.2. Strain Classification
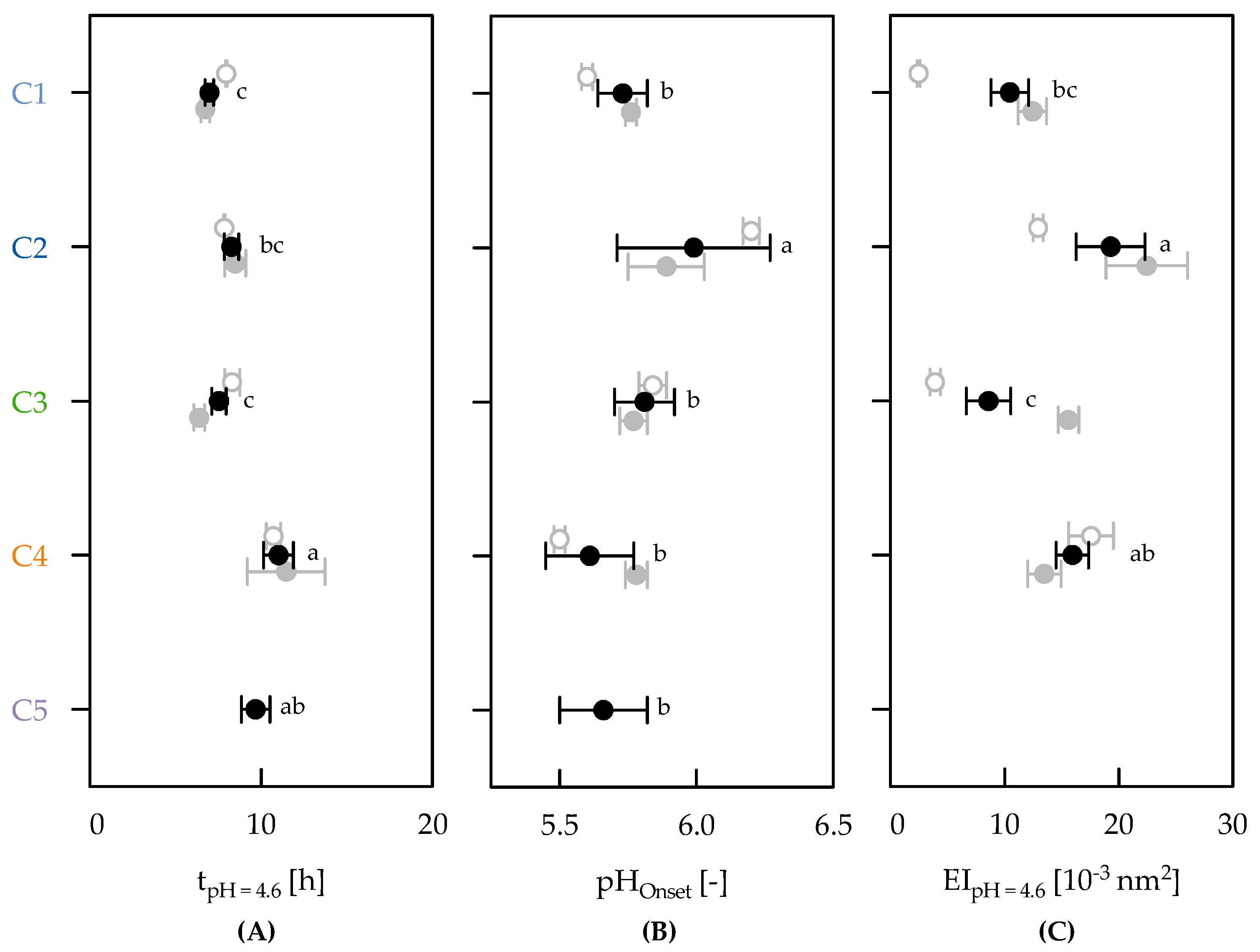
3.3. Effect of Clusters on Gelation Behaviour
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waters, D.M.; Mauch, A.; Coffey, A.; Arendt, E.K.; Zannini, E. Lactic acid bacteria as a cell factory for the delivery of functional biomolecules and ingredients in cereal-based beverages: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 503–520. [Google Scholar] [CrossRef]
- Mende, S.; Rohm, H.; Jaros, D. Influence of exopolysaccharides on the structure, texture, stability and sensory properties of yoghurt and related products. Int. Dairy J. 2016, 52, 57–71. [Google Scholar] [CrossRef]
- Costa, N.E.; Wang, L.; Auty, M.E.; Hannon, J.A.; McSweeney, P.L.H.H.; Beresford, T.P. Rheological, microscopic and primary chemical characterisation of the exopolysaccharide produced by Lactococcus lactis subsp. cremoris DPC6532. Dairy Sci. Technol. 2012, 92, 219–235. [Google Scholar] [CrossRef][Green Version]
- Folkenberg, D.M.; Dejmek, P.; Skriver, A.; Ipsen, R. Interactions between EPS-producing Streptococcus thermophilus strains in mixed yoghurt cultures. J. Dairy Res. 2006, 73, 385–393. [Google Scholar] [CrossRef]
- Hahn, C.; Müller, E.; Wille, S.; Weiss, J.; Atamer, Z.; Hinrichs, J. Control of microgel particle growth in fresh cheese (concentrated fermented milk) with an exopolysaccharide-producing starter culture. Int. Dairy J. 2014, 36, 46–54. [Google Scholar] [CrossRef]
- Zeidan, A.A.; Poulsen, V.K.; Janzen, T.; Buldo, P.; Derkx, P.M.F.; Øregaard, G.; Neves, A.R. Polysaccharide production by lactic acid bacteria: From genes to industrial applications. FEMS Microbiol. Rev. 2017, 41, S168–S200. [Google Scholar] [CrossRef]
- Paulo, E.M.; Vasconcelos, M.P.; Oliveira, I.S.; de Jesus Affe, H.M.; Nascimento, R.; de Melo, I.S.; de Abreu Roque, M.R.; de Assis, S.A. An alternative method for screening lactic acid bacteria for the production of exopolysaccharides with rapid confirmation. Food Sci. Technol. 2012, 32, 710–714. [Google Scholar] [CrossRef]
- Ricciardi, A.; Parente, E.; Clementi, F. A simple method for the screening of lactic acid bacteria for the production of exopolysaccharides in liquid media. Biotechnol. Tech. 1997, 11, 271–275. [Google Scholar] [CrossRef]
- Ren, W.; Xia, Y.; Wang, G.; Zhang, H.; Zhu, S.; Ai, L. Bioactive exopolysaccharides from a S. thermophilus strain: Screening, purification and characterization. Int. J. Biol. Macromol. 2016, 86, 402–407. [Google Scholar] [CrossRef]
- Manoury, E.; Horvath, P.; Fremaux, C.; Fourcassie, P. Genetic Cluster of Strains of Streptococcus thermophilus having Unique Rheological Properties for Dairy Fermentation. WO2010066907A1, 12 December 2010. [Google Scholar]
- Hess, S.J.; Roberts, R.F.; Ziegler, G.R. Rheological properties of nonfat yogurt stabilized using Lactobacillus delbrueckii ssp. bulgaricus producing exopolysaccharide or using commercial stabilizer systems. J. Dairy Sci. 1997, 80, 252–263. [Google Scholar] [CrossRef]
- Hughes, R.B.; Smith, A.C. Capsule Stain Protocols. Available online: http://www.asmscience.org/content/education/protocol/protocol.3041 (accessed on 29 January 2019).
- Mozzi, F.; Vaningelgem, F.; Hébert, E.M.; Van der Meulen, R.; Foulquié Moreno, M.R.; Font de Valdez, G.; De Vuyst, L.; Moreno, M.R.F.; Font de Valdez, G.; De Vuyst, L. Diversity of heteropolysaccharide-producing lactic acid bacterium strains and their biopolymers. Appl. Environ. Microbiol. 2006, 72, 4431–4435. [Google Scholar] [CrossRef]
- Hassan, A.N.N. ADSA Foundation Scholar Award: Possibilities and challenges of exopolysaccharide-producing lactic cultures in dairy foods. J. Dairy Sci. 2008, 91, 1282–1298. [Google Scholar] [CrossRef]
- Robitaille, G.; Moineau, S.; St-Gelais, D.; Vadeboncoeur, C.; Britten, M. Detection and quantification of capsular exopolysaccharides from Streptococcus thermophilus using lectin probes. J. Dairy Sci. 2006, 89, 4156–4162. [Google Scholar] [CrossRef]
- Hilbig, J.; Loeffler, M.; Herrmann, K.; Weiss, J. Application of exopolysaccharide-forming lactic acid bacteria in cooked ham model systems. Food Res. Int. 2019, 119, 761–768. [Google Scholar] [CrossRef]
- Zhang, L.; Folkenberg, D.M.; Qvist, K.B.; Ipsen, R. Further development of a method for visualisation of exopolysaccharides in yoghurt using fluorescent conjugates. Int. Dairy J. 2014, 46, 88–95. [Google Scholar] [CrossRef]
- Rühmann, B.; Schmid, J.; Sieber, V. High throughput exopolysaccharide screening platform: From strain cultivation to monosaccharide composition and carbohydrate fingerprinting in one day. Carbohydr. Polym. 2015, 122, 212–220. [Google Scholar] [CrossRef]
- Van Geel-Schutten, G.H.; Flesch, F.; Ten Brink, B.; Smith, M.R.; Dijkhuizen, L. Screening and characterization of Lactobacillus strains producing large amounts of exopolysaccharides. Appl. Microbiol. Biotechnol. 1998, 50, 697–703. [Google Scholar] [CrossRef]
- Marshall, V.M.; Laws, A.P.; Gu, Y.; Levander, F.; Rådström, P.; De Vuyst, L.; Degeest, B.; Vaningelgem, F.; Dunn, H.; Elvin, M. Exopolysaccharide-producing strains of thermophilic lactic acid bacteria cluster into groups according to their EPS structure. Lett. Appl. Microbiol. 2001, 32, 433–437. [Google Scholar] [CrossRef]
- Pachekrepapol, U.; Lucey, J.A.; Gong, Y.; Naran, R.; Azadi, P. Characterization of the chemical structures and physical properties of exopolysaccharides produced by various Streptococcus thermophilus strains. J. Dairy Sci. 2017, 100, 3424–3435. [Google Scholar] [CrossRef]
- Pachekrepapol, U.; Lucey, J.A.; Gong, Y.; Naran, R.; Azadi, P. Corrigendum to “Characterization of the chemical structures and physical properties of exopolysaccharides produced by various Streptococcus thermophilus strains” (J. Dairy Sci. 100:3424–3435). J. Dairy Sci. 2018, 101, 5668. [Google Scholar] [CrossRef]
- Mende, S.; Mentner, C.; Thomas, S.; Rohm, H.; Jaros, D. Exopolysaccharide production by three different strains of Streptococcus thermophilus and its effect on physical properties of acidified milk. Eng. Life Sci. 2012, 12, 466–474. [Google Scholar] [CrossRef]
- Vaningelgem, F.; Zamfir, M.; Mozzi, F.; Adriany, T.; Vancanneyt, M.; Swings, J.; De Vuyst, L. Biodiversity of exopolysaccharides produced by Streptococcus thermophilus strains is reflected in their production and their molecular and functional characteristics. Appl. Environ. Microbiol. 2004, 70, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Buldo, P.; Benfeldt, C.; Folkenberg, D.M.; Jensen, H.B.; Amigo, J.M.; Sieuwerts, S.; Thygesen, K.; van den Berg, F.; Ipsen, R. The role of exopolysaccharide-producing cultures and whey protein ingredients in yoghurt. LWT—Food Sci. Technol. 2016, 72, 189–198. [Google Scholar] [CrossRef]
- Hassan, A.N.N.; Frank, J.F.; Schmidt, K.A.; Shalabi, S.I. Textural properties of yogurt made with encapsulated nonropy lactic cultures. J. Dairy Sci. 1996, 79, 2098–2103. [Google Scholar] [CrossRef]
- Faber, E.J.; Zoon, P.; Kamerling, J.P.; Vliegenthart, J.F.G. The exopolysaccharides produced by Streptococcus thermophilus Rs and Sts have the same repeating unit but differ in viscosity of their milk cultures. Carbohydr. Res. 1998, 310, 269–276. [Google Scholar] [CrossRef]
- Hamet, M.F.; Piermaria, J.A.; Abraham, A.G. Selection of EPS-producing Lactobacillus strains isolated from kefir grains and rheological characterization of the fermented milks. LWT—Food Sci. Technol. 2015, 63, 129–135. [Google Scholar] [CrossRef]
- Poulsen, V.K.; Derkx, P.; Oregaard, G. High-throughput screening for texturing Lactococcus strains. FEMS Microbiol. Lett. 2019, 366, 1–7. [Google Scholar] [CrossRef]
- Van der Meulen, R.; Grosu-Tudor, S.; Mozzi, F.; Vaningelgem, F.; Zamfir, M.; Font de Valdez, G.; De Vuyst, L. Screening of lactic acid bacteria isolates from dairy and cereal products for exopolysaccharide production and genes involved. Int. J. Food Microbiol. 2007, 118, 250–258. [Google Scholar] [CrossRef]
- Hassan, A.N.N.; Corredig, M.; Frank, J.F. Capsule formation by nonropy starter cultures affects the viscoelastic properties of yogurt during structure formation. J. Dairy Sci. 2002, 85, 716–720. [Google Scholar] [CrossRef]
- Purwandari, U.; Shah, N.P.; Vasiljevic, T. Effects of exopolysaccharide-producing strains of Streptococcus thermophilus on technological and rheological properties of set-type yoghurt. Int. Dairy J. 2007, 17, 1344–1352. [Google Scholar] [CrossRef]
- Bullard, J.; St-Gelais, D.; Turgeon, S.L. Production of set yoghurts using thermophilic starters composed of two strains with different growth biocompatibilities and producing different exopolysaccharides. Int. Dairy J. 2018, 79, 33–42. [Google Scholar] [CrossRef]
- Mårtensson, O.; Öste, R.; Holst, O. Lactic acid bacteria in an oat-based non-dairy milk substitute: Fermentation characteristics and exopolysaccharide formation. LWT—Food Sci. Technol. 2000, 33, 525–530. [Google Scholar] [CrossRef]
- Jaros, D.; Heidig, C.; Rohm, H. Enzymatic modification through microbial transglutaminase enhances the viscosity of stirred yogurt. J. Texture Stud. 2007, 38, 179–198. [Google Scholar] [CrossRef]
- Jaros, D.; Haque, A.; Kneifel, W.; Rohm, H. Influence of the starter culture on the relationship between dry matter content and physical properties of stirred yogurt. Milchwiss. Milk Sci. Int. 2002, 57, 447–450. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Mende, S.; Dong, T.; Rathemacher, A.; Rohm, H.; Jaros, D. Physicochemical characterisation of the exopolysaccharides of Streptococcus thermophilus ST-143. Int. J. Food Sci. Technol. 2014, 49, 1254–1263. [Google Scholar] [CrossRef]
- Amatayakul, T.; Sherkat, F.; Shah, N.P. Physical characteristics of set yoghurt made with altered casein to whey protein ratios and EPS-producing starter cultures at 9 and 14% total solids. Food Hydrocoll. 2006, 20, 314–324. [Google Scholar] [CrossRef]
- van Marle, M.E.; van den Ende, D.; de Kruif, C.G.; Mellema, J. Steady-shear viscosity of stirred yogurts with varying ropiness. J. Rheol. 1999, 43, 1643–1662. [Google Scholar] [CrossRef]
- Hassan, A.N.N.; Ipsen, R.; Janzen, T.; Qvist, K.B. Microstructure and rheology of yogurt made with cultures differing only in their ability to produce exopolysaccharides. J. Dairy Sci. 2003, 86, 1632–1638. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; Alting, A.C.; Zoon, P. Effect of exopolysaccharides and proteolytic activity of Lactococcus lactis subsp. cremoris strains on the viscosity and structure of fermented milks. Int. Dairy J. 2005, 15, 155–164. [Google Scholar] [CrossRef]
- Marshall, V.M.; Rawson, H.L. Effects of exopolysaccharide-producing strains of thermophilic lactic acid bacteria on the texture of stirred yoghurt. Int. J. Food Sci. Technol. 1999, 34, 137–143. [Google Scholar] [CrossRef]
- Petry, S.; Furlan, S.; Waghorne, E.; Saulnier, L.; Cerning, J.; Maguin, E. Comparison of the thickening properties of four Lactobacillus delbrueckii subsp. bulgaricus strains and physicochemical characterization of their exopolysaccharides. FEMS Microbiol. Lett. 2003, 221, 285–291. [Google Scholar] [CrossRef]
- Mende, S.; Peter, M.; Bartels, K.; Rohm, H.; Jaros, D. Addition of purified exopolysaccharide isolates from S. thermophilus to milk and their impact on the rheology of acid gels. Food Hydrocoll. 2013, 32, 178–185. [Google Scholar] [CrossRef]
- Mozzi, F.; Torino, M.I.; de Valdez, G.F. Identification of exopolysaccharide-producing: Lactic acid bacteria a method for the isolation of polysaccharides in milk cultures. In Food Microbiology Protocols; Spencer, J.F.T., de Ragout Spencer, A.L., Eds.; Humana Press: New York City, NY, USA, 2001; Volume 14, pp. 183–190. [Google Scholar]
- Rohart, A.; Michon, C.; Confiac, J.; Bosc, V. Evaluation of ready-to-use SMLS and DWS devices to study acid-induced milk gel changes and syneresis. Dairy Sci. Technol. 2016, 96, 459–475. [Google Scholar] [CrossRef]
- Han, Y.; Mei, Y.; Li, K.; Xu, Y.; Wang, F. Effect of transglutaminase on rennet-induced gelation of skim milk and soymilk mixtures. J. Sci. Food Agric. 2019, 99, 1820–1827. [Google Scholar] [CrossRef]
- Raak, N.; Leidner, R.; Rohm, H.; Jaros, D. Potential of thromboelastometry and multispeckle diffusing wave difference during polymer melt extrusion flows sprectroscopy for monitoring acid-induced casein gelation. In Annual Transactions of the Nordic Rheology Society; Kádár, R., Ed.; Nordic Rheology Society: Helsinki, Finland, 2016; Volume 24, pp. 81–85. [Google Scholar]
- Dickinson, E. Colloid science of mixed ingredients. Soft. Matter 2006, 2, 642–652. [Google Scholar] [CrossRef]
- Khanal, S.N.; Lucey, J.A. Evaluation of the yield, molar mass of exopolysaccharides, and rheological properties of gels formed during fermentation of milk by Streptococcus thermophilus strains St-143 and ST-10255y. J. Dairy Sci. 2017, 100, 6906–6917. [Google Scholar] [CrossRef]
- Kristo, E.; Miao, Z.; Corredig, M. The role of exopolysaccharide produced by Lactococcus lactis subsp. cremoris in structure formation and recovery of acid milk gels. Int. Dairy J. 2011, 21, 656–662. [Google Scholar] [CrossRef]
- Lucey, J.A. Formation, structural properties and rheology of acid-coagulated milk gels. In Cheese: Chemistry, Physics and Microbiology; Fox, P.F., McSweeney, P.L.H., Cogan, T.M., Guinee, T.P., Eds.; Elsevier: Heidelberg, Germany, 2004; Volume 1, pp. 105–122. [Google Scholar]
- Folkenberg, D.M.; Dejmek, P.; Skriver, A.; Skov Guldager, H.; Ipsen, R. Sensory and rheological screening of exopolysaccharide producing strains of bacterial yoghurt cultures. Int. Dairy J. 2006, 16, 111–118. [Google Scholar] [CrossRef]
- Jaros, D.; Rohm, H.; Haque, A.; Bonaparte, C.; Kneifel, W. Influence of the starter culture on the relationship between dry matter content and physical properties of set-style yogurt. Milchwiss. Milk Sci. Int. 2002, 57, 325–328. [Google Scholar]
- Zisu, B.; Shah, N.P. Effects of pH, temperature, supplementation with whey protein concentrate, and adjunct cultures on the production of exopolysaccharides by Streptococcus thermophilus 1275. J. Dairy Sci. 2003, 86, 3405–3415. [Google Scholar] [CrossRef]
- Ayala-Hernández, I.; Hassan, A.N.N.; Goff, H.D.; Corredig, M. Effect of protein supplementation on the rheological characteristics of milk permeates fermented with exopolysaccharide-producing Lactococcus lactis subsp. cremoris. Food Hydrocoll. 2009, 23, 1299–1304. [Google Scholar]
- Gentès, M.C.; St-Gelais, D.; Turgeon, S.L. Gel formation and rheological properties of fermented milk with in situ exopolysaccharide production by lactic acid bacteria. Dairy Sci. Technol. 2011, 91, 645–661. [Google Scholar] [CrossRef]
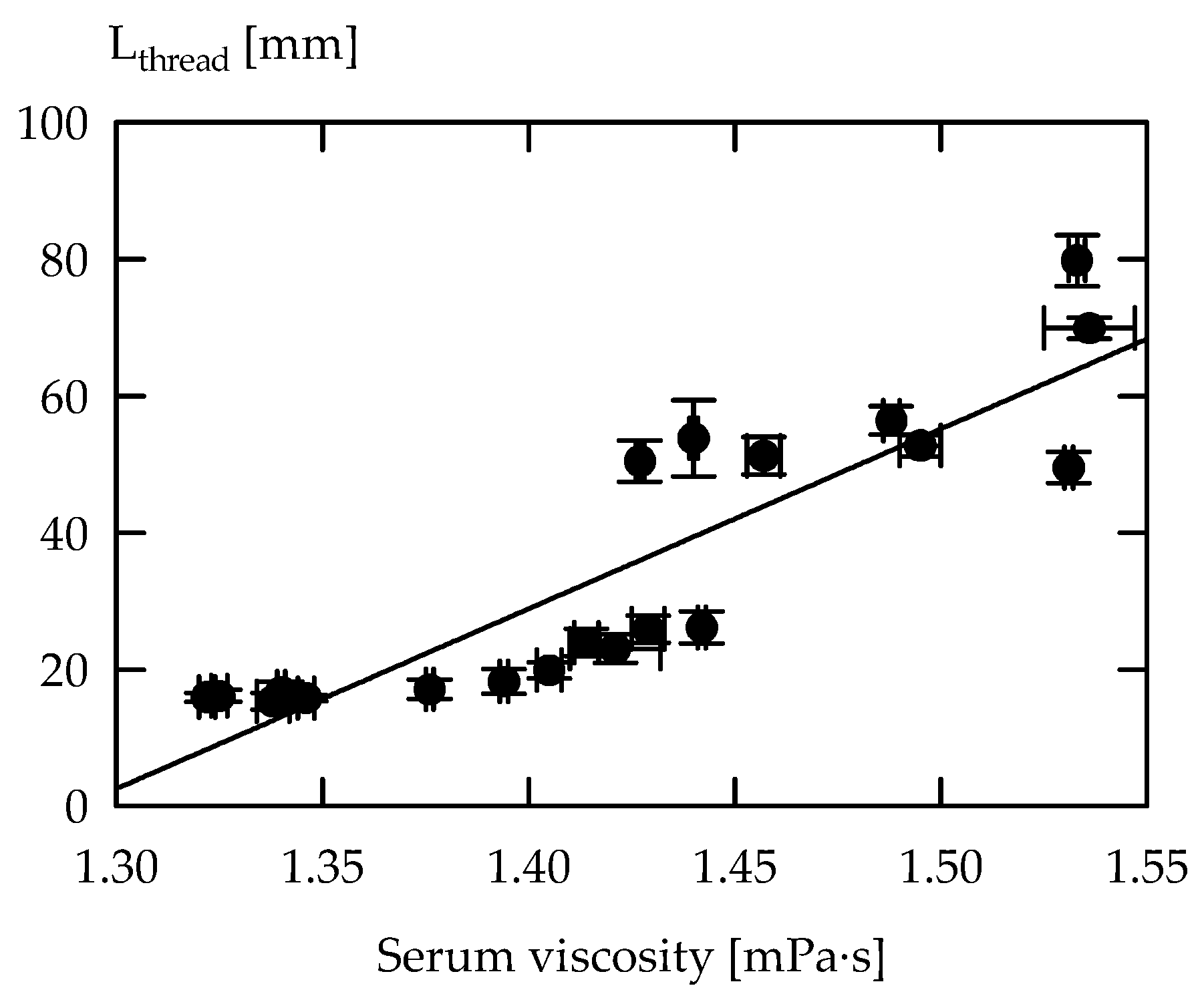
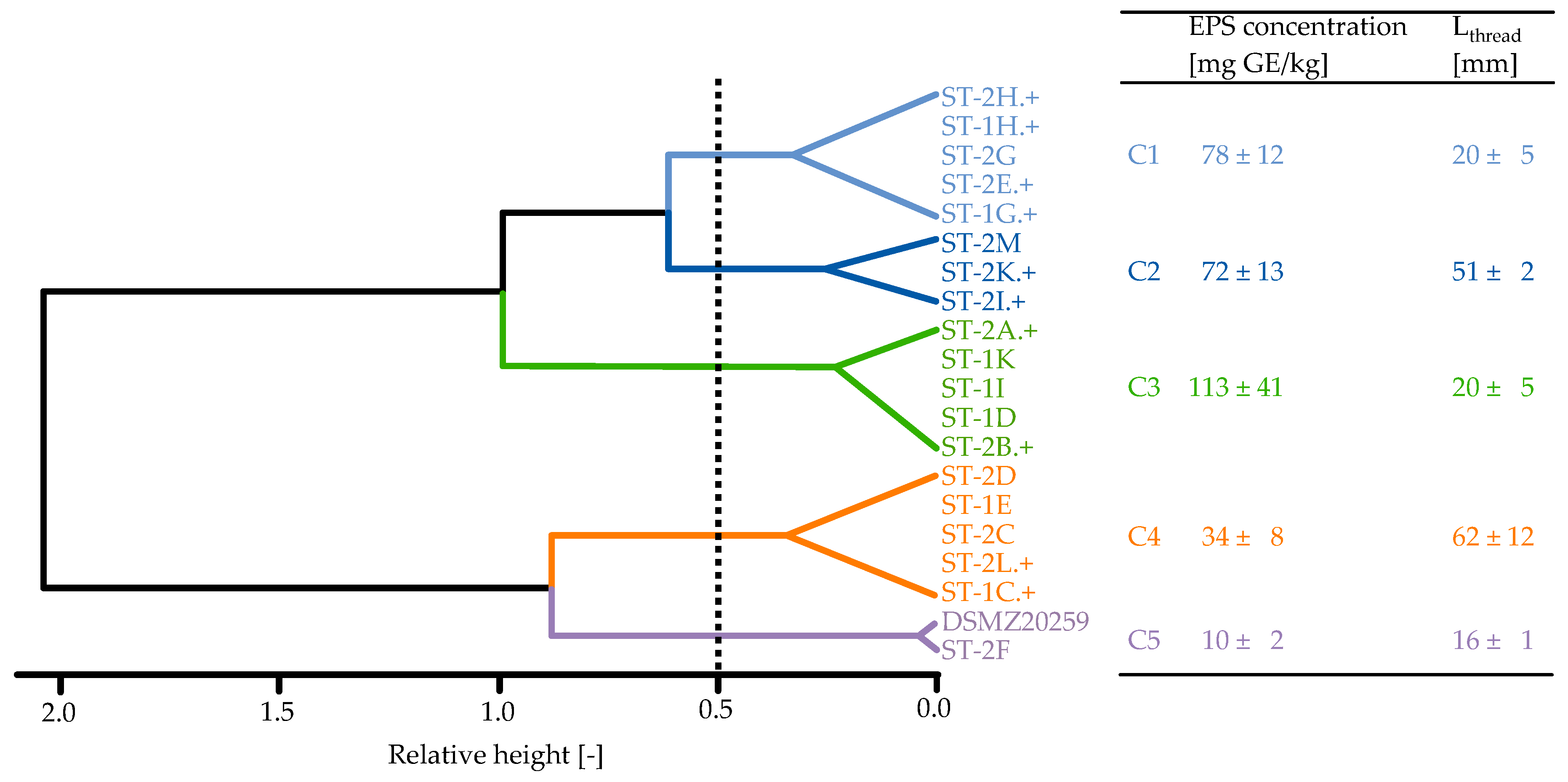

| Strain 1 | EPS Concentration (mg GE/kg) 2 | Serum Viscosity (mPa·s) |
|---|---|---|
| ST-1C.+ | 25 ± 4.9 fg | 1.34 ± 0.004 f |
| ST-1D | 106 ± 36.3 b | 1.30 ± 0.006 h |
| ST-1E | 25 ± 1.1 fg | 1.42 ± 0.020 d |
| ST-1G.+ | 80 ± 18.2 c | 1.40 ± 0.005 e |
| ST-1H.+ | 59 ± 6.6 d | 1.32 ± 0.004 g |
| ST-1I | 110 ± 7.7 b | 1.35 ± 0.001 f |
| ST-1K | 125 ± 16.7 a | 1.31 ± 0.008 gh |
| ST-2A.+ | 118 ± 17.4 ab | 1.43 ± 0.008 cd |
| ST-2B.+ | 106 ± 9.9 b | 1.45 ± 0.006 c |
| ST-2C | 33 ± 2.3 ef | 1.42 ± 0.005 d |
| ST-2D | 44 ± 8.8 e | 1.49 ± 0.006 b |
| ST-2E.+ | 87 ± 13.0 c | 1.44 ± 0.002 cd |
| ST-2F | 8 ± 2.4 h | 1.30 ± 0.001 h |
| ST-2G | 89 ± 7.6 c | 1.30 ± 0.003 h |
| ST-2H.+ | 71 ± 7.9 cd | 1.40 ± 0.001 e |
| ST-2I.+ | 57 ± 3.1 d | 1.44 ± 0.001 cd |
| ST-2K.+ | 82 ± 14.7 c | 1.51 ± 0.027 a |
| ST-2L.+ | 40 ± 3.4 ef | 1.39 ± 0.004 e |
| ST-2M | 75 ± 3.3 c | 1.43 ± 0.004 cd |
| DSMZ20259 | 11 ± 2.4 gh | 1.31 ± 0.008 gh |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surber, G.; Mende, S.; Jaros, D.; Rohm, H. Clustering of Streptococcus thermophilus Strains to Establish a Relation between Exopolysaccharide Characteristics and Gel Properties of Acidified Milk. Foods 2019, 8, 146. https://doi.org/10.3390/foods8050146
Surber G, Mende S, Jaros D, Rohm H. Clustering of Streptococcus thermophilus Strains to Establish a Relation between Exopolysaccharide Characteristics and Gel Properties of Acidified Milk. Foods. 2019; 8(5):146. https://doi.org/10.3390/foods8050146
Chicago/Turabian StyleSurber, Georg, Susann Mende, Doris Jaros, and Harald Rohm. 2019. "Clustering of Streptococcus thermophilus Strains to Establish a Relation between Exopolysaccharide Characteristics and Gel Properties of Acidified Milk" Foods 8, no. 5: 146. https://doi.org/10.3390/foods8050146
APA StyleSurber, G., Mende, S., Jaros, D., & Rohm, H. (2019). Clustering of Streptococcus thermophilus Strains to Establish a Relation between Exopolysaccharide Characteristics and Gel Properties of Acidified Milk. Foods, 8(5), 146. https://doi.org/10.3390/foods8050146