The Effect of In Vitro Digestion on Antioxidant, ACE-Inhibitory and Antimicrobial Potentials of Traditional Serbian White-Brined Cheeses
Abstract
1. Introduction
2. Materials and Methods
2.1. Cheese Samples
2.2. Chemical Characteristics of Cheeses
2.3. Preparation of Water-Soluble and Water-Insoluble Fractions
2.4. In Vitro Simulated Digestion
2.5. Sodium Dodecil Sulfate Polyacrilamide Gel Elecrophoresis (SDS-PAGE)
2.6. Total Free Amino Acid Level
2.7. Total Antioxidant Capacity
2.8. Reducing Power
2.9. Iron (II) Chelating Activity Assay
2.10. ACE-Inhibitor Activity
2.11. Antimicrobial Potential of Digestates Antimicrobial Activity
2.12. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characteristics of Cheeses
3.2. Antioxidant Properties of Cheeses and Their Digestates
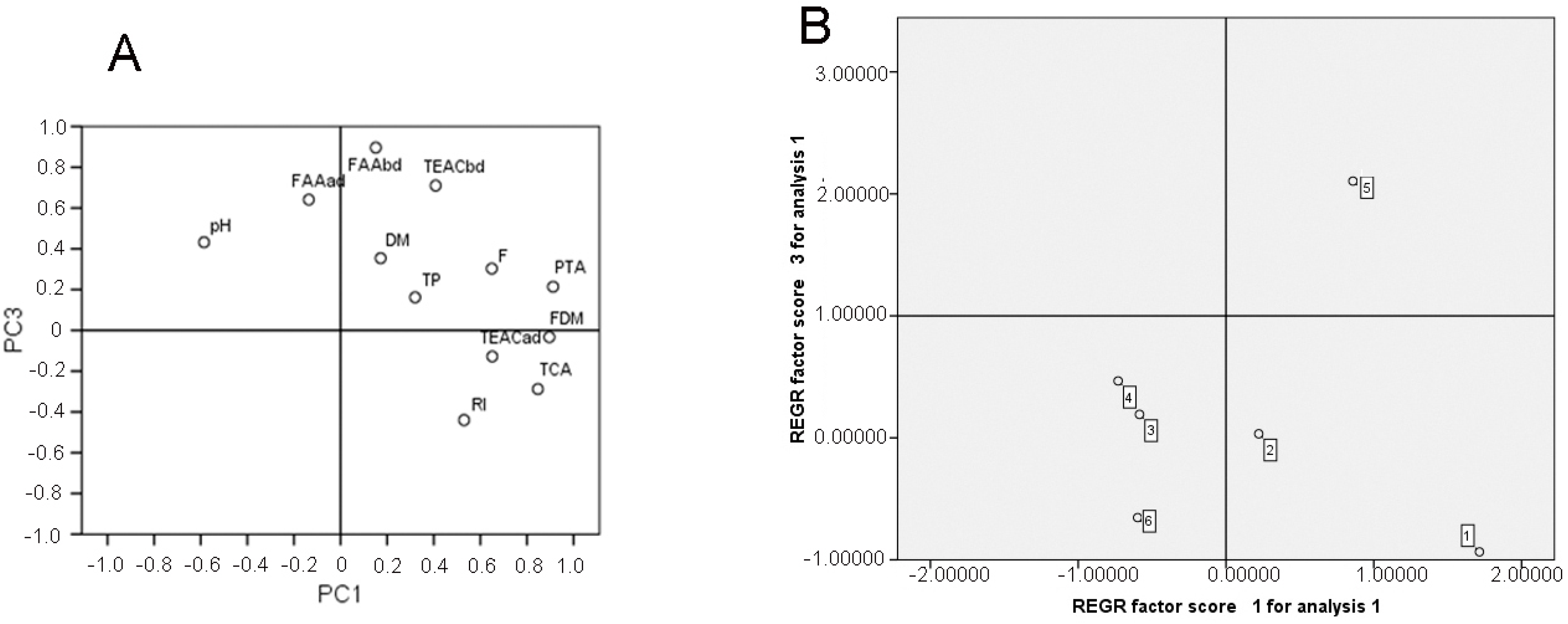
3.3. Principal Component Analysis
3.4. ACE-Inhibiting Potential of Protein Fractions of Traditional Serbian Cheeses
3.5. Antimicrobial Potential of Cheeses
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Vicentini, A.; Liberatore, L.; Mastrocola, D. Functional foods: Trends and development of the global market. Ital. J. Food Sci. 2016, 28, 338–351. [Google Scholar]
- Contor, L. Functional Food Science in Europe. Nutr. Metab. Cardiovasc. Dis. 2001, 11 (Suppl. 4), 20–23. [Google Scholar]
- Pisanu, S.; Pagnozzi, D.; Pes, M.; Pirisi, A.; Roggio, T.; Uzzau, S.; Addis, M.F. Differences in the peptide profile of raw and pasteurised ovine milk cheese and implications for its bioactive potential. Int. Dairy J. 2015, 42, 26–33. [Google Scholar] [CrossRef]
- Mushtaq, M.; Gani, A.; Shetty, P.H.; Masoodi, F.A.; Ahmad, M. Himalayan cheese (Kalari/kradi): Effect of different storage temperatures on its physicochemical, microbiological and antioxidant properties. LWT-Food Sci. Technol. 2015, 63, 837–845. [Google Scholar] [CrossRef]
- Barać, M.; Smiljanić, M.; Žilić, S.; Pesić, M.; Stanojević, S.; Vasić, M.; Vučić, T.; Kostić, A. Protein profiles and total antioxidant capacity of water soluble and insoluble protein fractions of white cow cheese at different stage of ripening. Mljekarstvo 2016, 66, 187–197. [Google Scholar] [CrossRef][Green Version]
- Barac, M.; Smiljanic, M.; Zilic, S.; Pesic, M.; Stanojevic, S.; Vasic, M.; Vucic, T.; Kostic, A. Protein profiles and total antioxidant capacity of water-soluble and water-insoluble fractions of white brined goat cheese at different stages of ripening. Int. J. Food Sci. Technol. 2016, 51, 1140–1149. [Google Scholar] [CrossRef]
- Barbé, F.; Le Feunteun, S.; Rémond, D.; Ménard, O.; Jardin, J.; Henry, G.; Laroche, B.; Dupont, D. Tracking the in vivo release of bioactive peptides in the gut during digestion: Mass spectrometry peptidomic characterization of effluents collected in the gut of dairy matrices fed mini-pigs. Food Res. Int. 2014, 63, 147–156. [Google Scholar] [CrossRef]
- Barać, M.; Pešić, M.; Vučić, T.; Vasić, M.; Smiljanić, M. White cheeses as a potential source of bioactive peptides. Mljekarstvo 2017, 67, 3–16. [Google Scholar] [CrossRef]
- Barać, M.; Kresović, M.; Špirović-Trifunović, B.; Pešić, M.; Vučić, T.; Kostić, A.; Despotović, S. Fatty acid profiles and mineral content of serbian traditional white brined cheeses. Mljekarstvo 2018, 68, 37–45. [Google Scholar] [CrossRef]
- IDF. Determination of the Total Solid Content (Cheese and Processed Cheese); IDF Standard 4A; International Dairy Federation: Brussels, Belgium, 1982. [Google Scholar]
- IDF. Determination of Fat Content (Cheese and Processed Cheese Products); IDF Standard 5B; International Dairy Federation: Brussels, Belgium, 1986; Volume 5B. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 16th ed.; Cunnif, P., Ed.; Association of Official Analytical Chemists International: Arlington, MA, USA, 1998. [Google Scholar]
- IDF. Determination of Chloride Content (Cheese and Processed Cheese); IDF Standard 5B; International Dairy Federation: Brussels, Belgium, 1988. [Google Scholar]
- Ardö, Y.; Polychroniadou, A. Laboratory Manual for Chemical Analysis of Cheese; Office for Official Publications of the European Communities: Luxembourg, 1999; pp. 1–144. [Google Scholar]
- Kuchroo, C.N.; Fox, P.F. Soluble nitrogen in Cheddar cheese: Comparison of extraction procedures. Milchwissenschaft 1982, 37, 331–335. [Google Scholar]
- Petrat-Melin, B.; Andersen, P.; Rasmussen, J.T.; Poulsen, N.A.; Larsen, L.B.; Young, J.F. In vitro digestion of purified β-casein variants A1, A2, B, and I: Effects on antioxidant and angiotensin-converting enzyme inhibitory capacity. J. Dairy Sci. 2015, 98, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Fling, S.P.; Gregerson, D.S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris-buffer system without urea. Anal. Biochem. 1986, 155, 83–88. [Google Scholar] [CrossRef]
- Hayaloglu, A.A. Comparisons of different single-strain starter cultures for their effects on ripening and grading of Beyaz cheese. Int. J. Food Sci. Technol. 2007, 42, 930–938. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Pellegrini, N.; Fogliano, V. Direct measurement of the total antioxidant capacity of cereal products. J. Cereal Sci. 2008, 48, 816–820. [Google Scholar] [CrossRef]
- Meira, S.M.M.; Daroit, D.J.; Helfer, V.E.; Corrêa, A.P.F.; Segalin, J.; Carro, S.; Brandelli, A. Bioactive peptides in water-soluble extracts of ovine cheeses from Southern Brazil and Uruguay. Food Res. Int. 2012, 48, 322–329. [Google Scholar] [CrossRef]
- Lee, B.; Chen, F.; Wang, X.; Baoping, J.; Yonnie, W. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2007, 102, 1135–1143. [Google Scholar]
- Otte, J.; Shalaby, S.; Zakora, M.; Nielsen, M.S. Fractionation and identification of ACE-inhibitory peptides from α-lactalbumin and β-casein produced by thermolysin-catalysed hydrolysis. Int. Dairy J. 2007, 17, 1460–1472. [Google Scholar] [CrossRef]
- Vermeirssen, V.; van Camp, J.; Verstraete, W. Bioavailability of angiotensin I-converting enzyme inhibitory peptides. Br. J. Nutr. 2004, 92, 357–366. [Google Scholar] [CrossRef]
- Soković, D.M. Antifungal Activities of Essential Oil of Selected Aromatic and Medicinal Plants In Vitro and In Vivo. Ph.D. Thesis, Faculty of Biology, University of Belgrade, Yugoslavia, Serbia, 2001. [Google Scholar]
- Tsukatani, T.; Suenaga, H.; Noguchi, K.; Ishiyama, M.; Matsumoto, K.; Ezoe, T. Comparison of the WST-8 colorimetric method and the CLSI broth microdilution method for susceptibility testing against drug-resistant bacteria. J. Microbiol. Methods 2012, 90, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius. Codex Group Standard for Cheeses in Brine (Codex Standard 208-1999); Joint FAO/WHO Food Standards Program; FAO: Rome, Italy, 1999. [Google Scholar]
- Hayaloglu, A.A.; Ozer, B.H.; Fox, P.F. Cheeses of Turkey: 2. Varieties ripened under brine. Dairy Sci. Technol. 2008, 88, 225–244. [Google Scholar] [CrossRef]
- Hayaloglu, A.A.; Guven, M.; Fox, P.F.; Hannon, J.A.; McSweeney, P.L.H. Proteolysis in Turkish White-brined cheese made with defined strains of Lactococcus. Int. Dairy J. 2004, 14, 599–610. [Google Scholar] [CrossRef]
- Goncu, A.; Alpkent, Z. Sensory and chemical properties of white pickled cheese produced using kefir, yoghurt or a commercial cheese culture as a starter. Int. Dairy J. 2005, 15, 771–776. [Google Scholar] [CrossRef]
- Žilić, S.; Akıllıoğlu, G.; Serpen, A.; Barać, M.; Gökmen, V. Effects of isolation, enzymatic hydrolysis, heating, hydratation and Maillard reaction on the antioxidant capacity of cereal and legume proteins. Food Res. Int. 2012, 49, 1–6. [Google Scholar] [CrossRef]
- Di Benedetto, N.A.; Alfarano, M.; Laus, M.N.; Caporizzi, R.; Soccio, M.; Robbe, C.; Flagella, Z.; Pastore, D. Antioxidant capacity of durum wheat large flour particles may be evaluated by QUENCHER ABTS assay by adopting a proper calculation mode. Cereal Res. Commun. 2015, 43, 682–689. [Google Scholar] [CrossRef]
- Apak, R.; Ozyurek, M.; Guclu, K.; Capanoglu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Cömert, E.D.; Gökmen, V. Antioxidants Bound to an Insoluble Food Matrix: Their Analysis, Regeneration Behavior, and Physiological Importance. Compr. Rev. Food Sci. Food Saf. 2017, 16, 382–399. [Google Scholar] [CrossRef]
- Silva, R.A.; Lima, M.S.F.; Viana, J.B.M.; Bezerra, V.S.; Pimentel, M.C.B.; Porto, A.L.F.; Cavalcanti, M.T.H.; Lima Filho, J.L. Can artisanal ‘‘Coalho’’ cheese from Northeastern Brazil be used as a functional food? Food Chem. 2012, 135, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Pattom, S.; Hongsprabhas, P. Effect of coagulants on antioxidant capacity of milk protein curds and their tryptic hydrolysates. J. Food Biochem. 2013, 37, 203–211. [Google Scholar]
- Power, O.; Jakeman, P.; FitzGerald, R.J. Antioxidative peptides: Enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids 2013, 44, 797–820. [Google Scholar] [CrossRef] [PubMed]
- Pates, G.O.; Guler, L.; Nash, J.J.; Kenttämaa, H.I. Reactivity and selectivity of charged phenyl radicals toward amino acids in a fourier transform ion cyclotron resonance mass spectrometer. J. Am. Chem. Soc. 2011, 133, 9331–9342. [Google Scholar] [CrossRef]
- Yates, Z.; Gunasekaran, K.; Zhou, H.; Hu, Z.; Liu, Z.; Ketchem, R.R. Histidine residue mediates radical-induced hinge cleavage of human IgG1. J. Biol. Chem. 2010, 285, 18662–18671. [Google Scholar] [CrossRef]
- Medina-Navarro, R.; Durán-Reyes, G.; Díaz-Flores, M.; Vilar-Rojas, C. Protein Antioxidant Response to the Stress and the Relationship between Molecular Structure and Antioxidant Function. PLoS ONE 2010, 5, e8971. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, A.; Maurizi, A.; Frigola, A.; Esteve, M.J.; Coli, R.; Burini, G. Antioxidant capacity of cow milk, whey and deproteinized milk. Int. Dairy J. 2009, 19, 380–385. [Google Scholar] [CrossRef]
- Hilario, M.C.; Puga, C.D.; Ocaña, A.N.; Romo, F.P. Antioxidant activity, bioactive polyphenols in Mexican goats’ milk cheeses on summer grazing. J. Dairy Res. 2010, 77, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Mattera, M.; Durazzo, A.; Nicoli, S.; Di Costanzo, M.G.; Manzi, P. Chemical, nutritional, physical and antioxidant properties of “PECORINO D’ABRUZZO” cheese. Ital. J. Food Sci. 2016, 28, 579–597. [Google Scholar]
- Timón, M.L.; Parra, V.; Otte, J.; Broncano, J.M.; Petrón, M.J. Identification of radical scavenging peptides (<3 kDa) from Burgos-type cheese. LWT-Food Sci. Technol. 2014, 57, 359–365. [Google Scholar]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Sun-Waterhouse, D.; Everett, D.W. Total phenolic content and antioxidant properties of hard low-fat cheese fortified with catechin as affected by in vitro gastrointestinal digestion. LWT-Food Sci. Technol. 2015, 62, 393–399. [Google Scholar] [CrossRef]
- Lucas, A.; Rock, E.; Chamba, J.F.; Verdier-Metz, I.; Brachet, P.; Coulon, J.B. Respective effects of milk composition and the cheese-making process on cheese compositional variability in components of nutritional interest. Lait 2006, 86, 21–41. [Google Scholar] [CrossRef]
- Revilla, I.; González-Martín, M.I.; Vivar-Quintana, A.M.; Blanco-López, M.A.; Lobos-Ortega, I.A.; Hernández-Hierro, J.M. Antioxidant capacity of different cheeses: Affecting factors and prediction by near infrared spectroscopy. J. Dairy Sci. 2016, 99, 5074–5082. [Google Scholar] [CrossRef] [PubMed]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J. Agric. Food Chem. 2010, 58, 4712–4718. [Google Scholar] [CrossRef]
- Corrêa, A.P.F.; Daroit, D.J.; Coelho, J.; Meira, S.M.M.; Lopes, F.C.; Segalin, J. Antioxidant, antihypertensive and antimicrobial properties of ovine milk caseinate hydrolyzed with a microbial protease. J. Sci. Food Agric. 2011, 91, 2247–2254. [Google Scholar] [CrossRef]
- Meisel, H.; FitzGerald, R.J. Biofunctional peptides from milk proteins: Mineral binding and cytomodulatory effects. Curr. Pharm. Des. 2003, 9, 1289–1295. [Google Scholar]
- Ferraretto, A.; Gravaghi, C.; Fiorilli, A.; Tettamanti, G. Casein-derived bioactive phosphopeptides: Role of phosphorylation and primary structure in promoting calcium uptake by HT-29 tumor cells. FEBS Lett. 2003, 551, 92–98. [Google Scholar] [CrossRef]
- Bütikofer, U.; Meyer, J.; Sieber, R.; Wechsler, D. Quantification of the angiotensin-converting enzyme-inhibiting tripeptides Val-Pro-Pro and Ile-Pro-Pro in hard, semi-hard and soft cheeses. Int. Dairy J. 2007, 17, 968–975. [Google Scholar] [CrossRef]
- Sieber, R.; Bütikofer, U.; Egger, C.; Portmann, R.; Walther, B.; Wechsler, D. ACE inhibitory activity and ACE-inhibiting peptides in different cheese varieties. Dairy Sci. Technol. 2010, 90, 47–73. [Google Scholar] [CrossRef]
- Parrot, S.; Degraeve, P.; Curia, C.; Martial-Gros, A. In vitro study on digestion of peptides in Emmental cheese:Analytical evaluation and influence on angiotensin I converting enzyme inhibitory peptides. Nahrung 2003, 47, 87–94. [Google Scholar] [CrossRef]
- Silva, S.V.; Malcata, F.X. Caseins as source of bioactive peptides. Int. Dairy J. 2005, 15, 1–15. [Google Scholar] [CrossRef]
- Stuknyte, M.; Cattaneo, S.; Masotti, F.; De Noni, I. Occurrence and fate of ACE-inhibitor peptides in cheeses and in their digestates following in vitro static gastrointestinal digestion. Food Chem. 2015, 168, 27–33. [Google Scholar] [CrossRef]
- Aluko, R. Functional Foods and Nutraceuticals; Springer: New York, NY, USA, 2012; pp. 37–61. [Google Scholar]
- Quereshi, T.M.; Vegarud, G.E.; Abrahamsen, R.K.; Skeie, S. Angiotensin I-converting enzyme-inhibitory activity of the Norwegian autochthonous cheeses Gamalost and Norvegia after in vitro human gastrointestinal digestion. J. Dairy Sci. 2013, 96, 838–853. [Google Scholar] [CrossRef]
- Benkerroum, N. Antimicrobial peptides generated from milk proteins: A survey and prospects for application in the food industry. A review. Int. J. Dairy Technol. 2010, 63, 320–338. [Google Scholar] [CrossRef]
- López-Expósito, I.; Quiros, A.; Amigo, L.; Recio, I. Casein hydrolysates as a source of antimicrobial, antioxidant and antihypertensive peptides. Le Lait 2007, 87, 241–249. [Google Scholar] [CrossRef]
- López-Expósito, I.; Minervini, F.; Amigo, L.; Recio, I. Identification of antibacterial peptides from bovine kappacasein. J. Food Prot. 2006, 69, 2992–2997. [Google Scholar] [CrossRef] [PubMed]
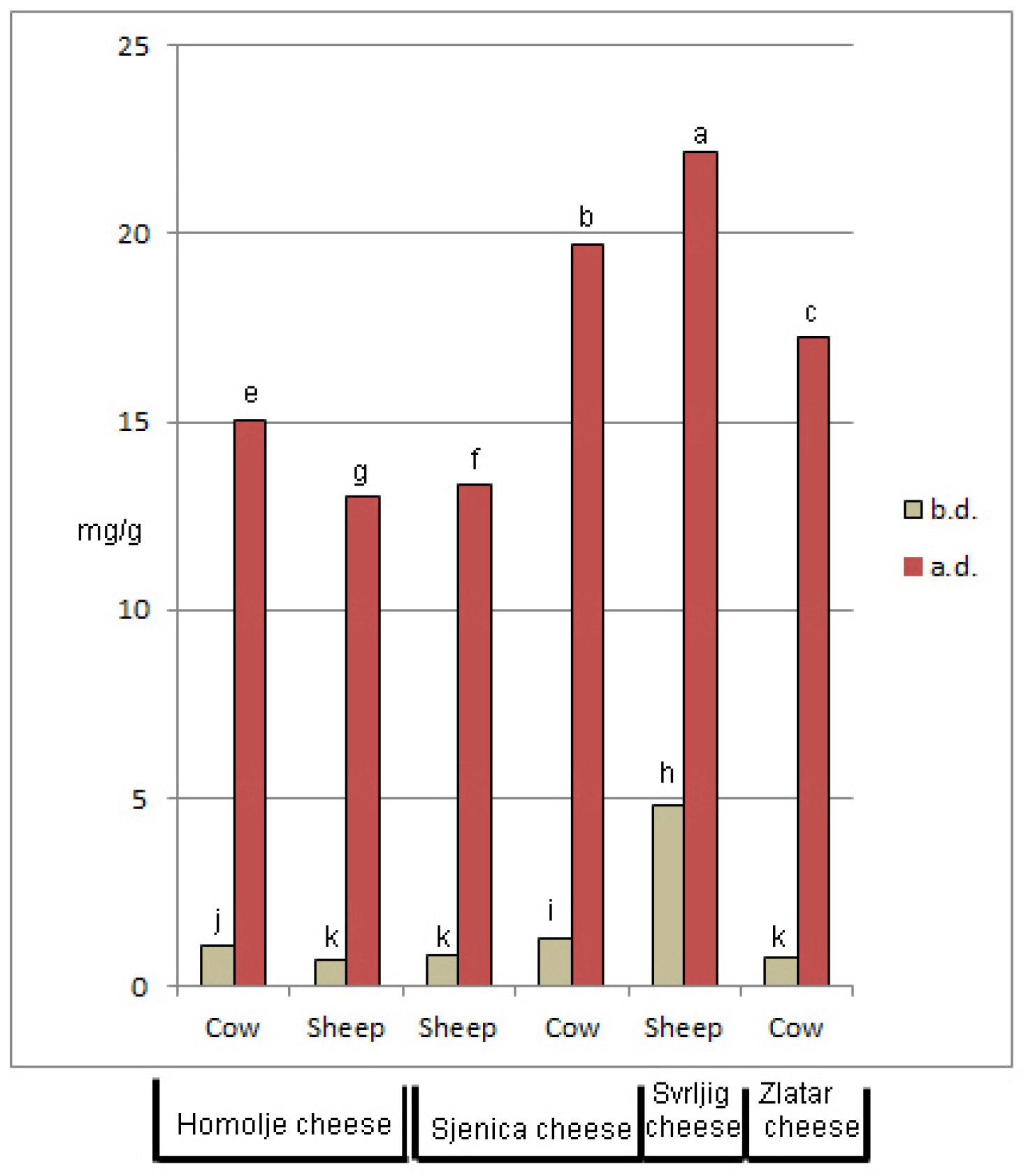

| Parameter | Cow Cheeses | Sheep Cheese | ||||
|---|---|---|---|---|---|---|
| Homolje | Sjenica | Zlatar | Homolje | Sjenica | Svrljig | |
| DM (%) | 59.09 ± 0.05 a | 46.81 ± 0.11 d | 43.08 ± 5.88 d,e | 57.49 ± 0.12 b | 51.50 ± 0.01 c | 51.82 ± 5.35 c,e |
| Fat (%) | 31.25 ± 0.35 a | 27.5 ± 0.07 c,f | 22.33 ± 4.80 f | 30.50 ± 0.05 b | 31.50 ± 0.05 a | 29.38 ± 3.90 a,b,c |
| FDM (%) | 52.89 ± 0.55 e,d | 58.75 ± 0.13 b | 51.31 ± 4.02 e,d | 53.06 ± 0.11 d | 61.17 ± 0.01 a | 56.56 ± 1.78 b,c |
| MNFS (%) | 59.51 ± 0.23 e | 73.37 ± 0.15 a | 73.09 ± 3.16 a,b | 61.17 ± 0,17 d | 70.81 ± 0.01 b | 68.07 ± 3.84 c |
| TN (%) | 3.2338 ± 0.2209 a | 2.8223 ± 0.0107 c | 2.5022 ± 0.2573 d | 2.8976 ± 0.0245 b,c | 2.9305 ± 0.0418 b | 2.7522 ± 0.0699 c |
| TP (%) | 20.63 ± 1.41 a | 18.01 ± 0.06 c | 16.11 ± 1.78 c,d | 18.49 ± 0.16 b | 18.70 ± 0.27 b | 17.56 ± 0.45 c,d |
| WSN (%) | 0.2376 ± 0.011 b | 0.3319 ± 0.0072 a | 0.3650 ± 0.0725 a | 0.2330 ± 0.0136 b | 0.3752 ± 0.0092 a | 0.3327 ± 0.0646 a |
| RI (%) | 7.35 ± 0.29 b | 11.76 ± 0.30 a | 14.59 ± 2.56 a | 8.04 ± 0.46 b | 12.80 ± 0.50 a | 12.09 ± 2.32 a |
| PTA-SN (%) | 0.0752 ± 0.0002 c | 0.0511 ± 0.0013 e | 0.0217 ± 0.0065 g | 0.0359 ± 0.0002 f | 0.1050 ± 0.0047 a | 0.0761 ± 0.0070 c |
| TCA-SN (%) | 0.1756 ± 0.0046 f | 0.2597 ± 0.0046 c | 0.1981 ± 0.0114 e | 0.2315 ± 0.0011 d | 0.3246 ± 0.034 a | 0.2351 ± 0.0332 d |
| NaCl (%) | 5.43 ± 0.01 c | 3.69 ± 0.03 f | 3.48 ± 0.27 f | 6.49 ± 0.31 a | 4.13 ± 0.01 e | 5.95 ± 0.01 b |
| pH | 4.71 ± 0.03 a | 4.51 ± 0.09 b | 4.69 ± 0.19 a | 5.18 ± 0.24 a | 4.29 ± 0.11 b | 4.88 ± 0.29 a |
| Cheese | TEAC mmol Trolox (Eq/kg) | Reducing Power A700 | Chelating Properties IC50 (mg/mL) | |||
|---|---|---|---|---|---|---|
| B.D. | A.D. | B.D. | A.D. | B.D. | A.D. | |
| Sjenica | ||||||
| sheep | 17.13 ± 1.36 b,B | 400.1 ± 9.44 a,A | 0.143 e,B | 0.285 c,A | 42.34 ± 1.3 a,A | 39.34 ± 0.95 b,A |
| cow | 16.54 ± 0.81 b,B | 209.53 ± 5.42 e,c,A | 0.296 a,B | 0.349 a,A | 36.15 ± 0.89 b,B | 40.31 ± 1.10 b,A |
| Homolje | ||||||
| sheep | 5.82 ± 2.02 g,B | 348.80 ± 15.17 b,A | 0.150 d,e,B | 0.269 d,A | 31.34 ± 0.45 c,B | 38.59 ± 1.42 b,A |
| cow | 8.81 ± 1.96 e,f,B | 262.3 ± 9.01 d,A | 0.156 c,d,B | 0.194 g,A | 15.19 ± 0.67 e,B | 29.87 ± 1.21 c,A |
| Svrljig | ||||||
| Sheep | 36.43 ± 0.15 a,B | 306.94 ± 13.16 c,A | 0.136 f,B | 0.255 e,A | 29.71 ± 1.2 c,A | 27.53 ± 1.15 c,A |
| Zlatar | ||||||
| Cow | 10.28 ± 0.27 d,f,B | 250.40 ± 4.92 d,A | 0.176 b,B | 0.285 b,A | 34.22 ± 1.20 b,B | 42.80 ± 0.63 a,A |
| Protein Fraction | ACE-Inhibitor Activity * IC50 (mg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Sheep Cheeses | Cow Cheeses | ||||||
| Svrljig | Homolje | Sjenica | Homolje | Sjenica | Zlatar | ||
| WSF | b.d. | 3.87 ± 0.05 g | 4.50 ± 0.24 e | 4.61 ± 0.04 e | 2.95 ± 0.01 i | 3.79 ± 0.06 g | 2.26 ± 0.02 j |
| a.d. | 7.09 ± 0.11 b | 8.96 ± 0.014 a | 4.71 ± 0.01 e | 4.73 ± 0.08 e | 4.26 ± 0.02 f | 5.63 ± 0.11 d | |
| WINF | a.d. | 6.39 ± 0.21 c | 5.84 ± 0.07 d | 3.28 ± 0.12 h | 4.20 ± 0.23 e | 3.17 ± 0.07 h | 1.31 ± 0.07 k |
| ACE inhibition (%) ** | |||||||
| WSF | b.d. | 64.23 ± 0.10 e | 55.10 ± 0.21 g | 54.23 ± 0.54 g | 69.20 ± 0.42 d | 64.96 ± 0.35 e | 75.26 ± 0.41 b |
| a.d. | 35.27 ± 0.05 m | 32.40 ± 0.35 k | 53.53 ± 0.67 g,h | 52.85 ± 0.36 h | 57.68 ± 0.24 f | 46.40 ± 0.18 i | |
| WINF | a.d. | 39.45 ± 0.15 l | 44.80 ± 0.22 j | 71.22 ± 0.11 c | 54.52 ± 0.54 g | 71.86 ± 0.52 c | 84.23 ± 0.32 a |
| Sjenica Cow Cheese | Svrljig Sheep Cheese | Homolje Cow Cheese | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WINF | WSF | Cheese | WINF | WSF | Cheese | WINF | WSF | Cheese | ||
| mg/mL | ||||||||||
| Micromycetes | ||||||||||
| Aspergillus fumigatus | MIC | 4.80 | n.d. | 4.40 | 4.00 | 4.00 | 4.00 | 4.16 | 4.64 | 4.00 |
| MFC | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Aspergillus versicolor | MIC | 4.80 | 5.00 | 4.40 | 2.00 | 4.00 | 2.00 | 2.08 | 4.64 | 4.00 |
| MFC | n.d. | n.d. | n.d. | 4.00 | n.d. | 4.00 | 4.16 | n.d. | n.d. | |
| Aspergillus ochraceus | MIC | 4.80 | 5.00 | 4.40 | 4.00 | 4.00 | 4.00 | 4.16 | 4.64 | 4.00 |
| MFC | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Aspergillus niger | MIC | n.d. | n.d. | n.d. | n.d. | n.d. | 4.00 | n.d. | 4.64 | n.d. |
| MFC | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Trichoderma viride | MIC | 4.80 | n.d. | n.d. | n.d. | n.d. | 4.00 | 4.16 | n.d. | n.d. |
| MFC | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Penicillium funiculosum | MIC | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 4.16 | 2.32 | n.d. |
| MFC | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 4.64 | n.d. | |
| Penicillium ochrochloron | MIC | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2.32 | n.d. |
| MFC | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 4.64 | n.d. | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barac, M.; Vucic, T.; Zilic, S.; Pesic, M.; Sokovic, M.; Petrovic, J.; Kostic, A.; Sredovic Ignjatovic, I.; Milincic, D. The Effect of In Vitro Digestion on Antioxidant, ACE-Inhibitory and Antimicrobial Potentials of Traditional Serbian White-Brined Cheeses. Foods 2019, 8, 94. https://doi.org/10.3390/foods8030094
Barac M, Vucic T, Zilic S, Pesic M, Sokovic M, Petrovic J, Kostic A, Sredovic Ignjatovic I, Milincic D. The Effect of In Vitro Digestion on Antioxidant, ACE-Inhibitory and Antimicrobial Potentials of Traditional Serbian White-Brined Cheeses. Foods. 2019; 8(3):94. https://doi.org/10.3390/foods8030094
Chicago/Turabian StyleBarac, Miroljub, Tanja Vucic, Sladjana Zilic, Mirjana Pesic, Marina Sokovic, Jovana Petrovic, Aleksandar Kostic, Ivana Sredovic Ignjatovic, and Danijel Milincic. 2019. "The Effect of In Vitro Digestion on Antioxidant, ACE-Inhibitory and Antimicrobial Potentials of Traditional Serbian White-Brined Cheeses" Foods 8, no. 3: 94. https://doi.org/10.3390/foods8030094
APA StyleBarac, M., Vucic, T., Zilic, S., Pesic, M., Sokovic, M., Petrovic, J., Kostic, A., Sredovic Ignjatovic, I., & Milincic, D. (2019). The Effect of In Vitro Digestion on Antioxidant, ACE-Inhibitory and Antimicrobial Potentials of Traditional Serbian White-Brined Cheeses. Foods, 8(3), 94. https://doi.org/10.3390/foods8030094







