Optimization of Uruset Apple Vinegar Production Using Response Surface Methodology for the Enhanced Extraction of Bioactive Substances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Production of Uruset Apple (Cultivar with Red Penetralia) Vinegar
2.3. Ultrasound Treatments
2.4. Experimental Design
2.5. Determination of Total Phenolic Content and Total Flavonoid Content
2.6. Determination of Total Antioxidant Activity (DPPH and CUPRAC)
2.7. Color Analysis
2.8. Statistical Analysis
3. Results and Discussion
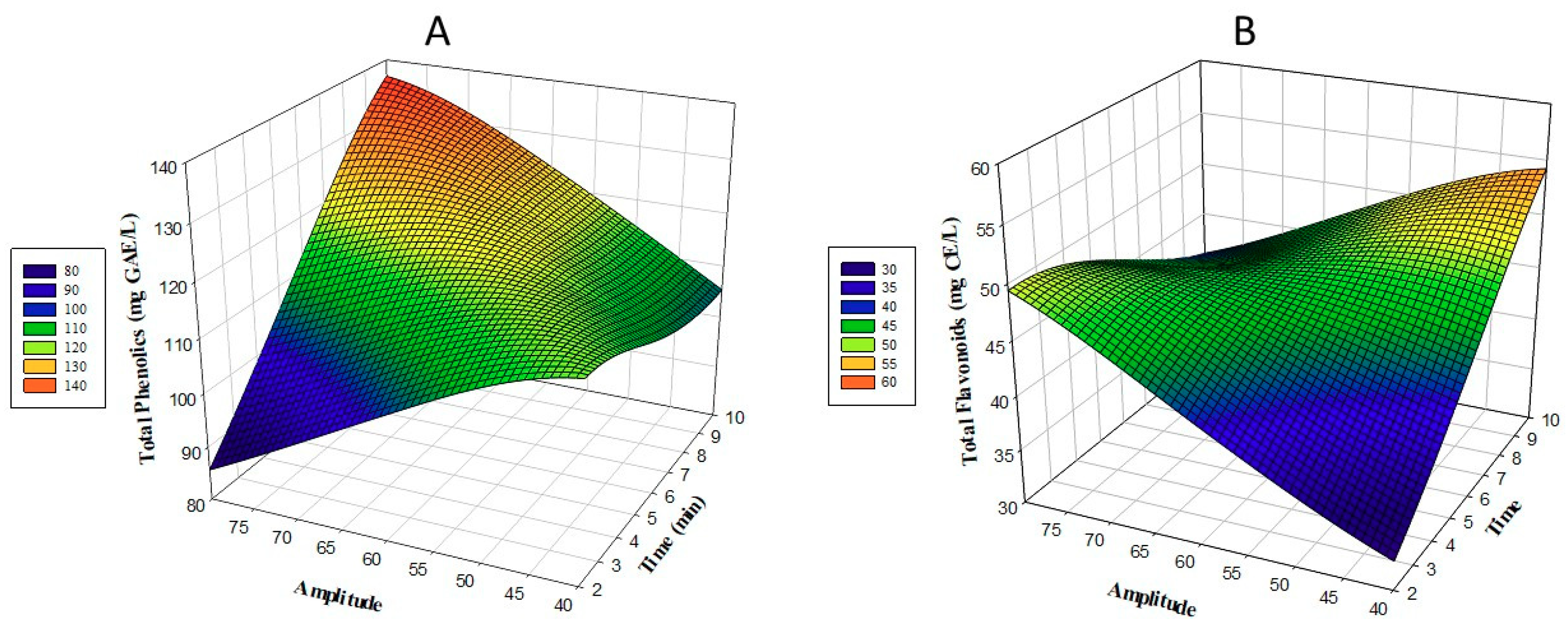
3.1. Total Phenolic Content and Total Flavonoid Content
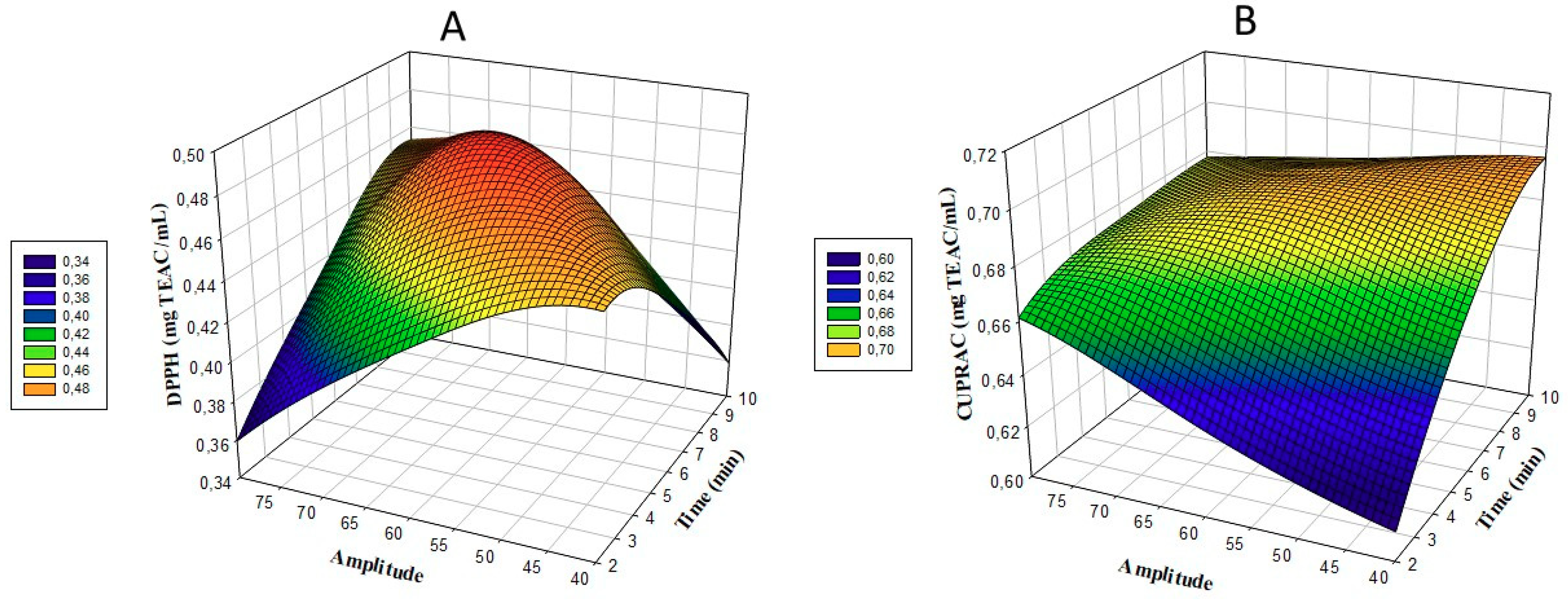
3.2. DPPH and CUPRAC (Cupric Reducing Antioxidant Capacity)
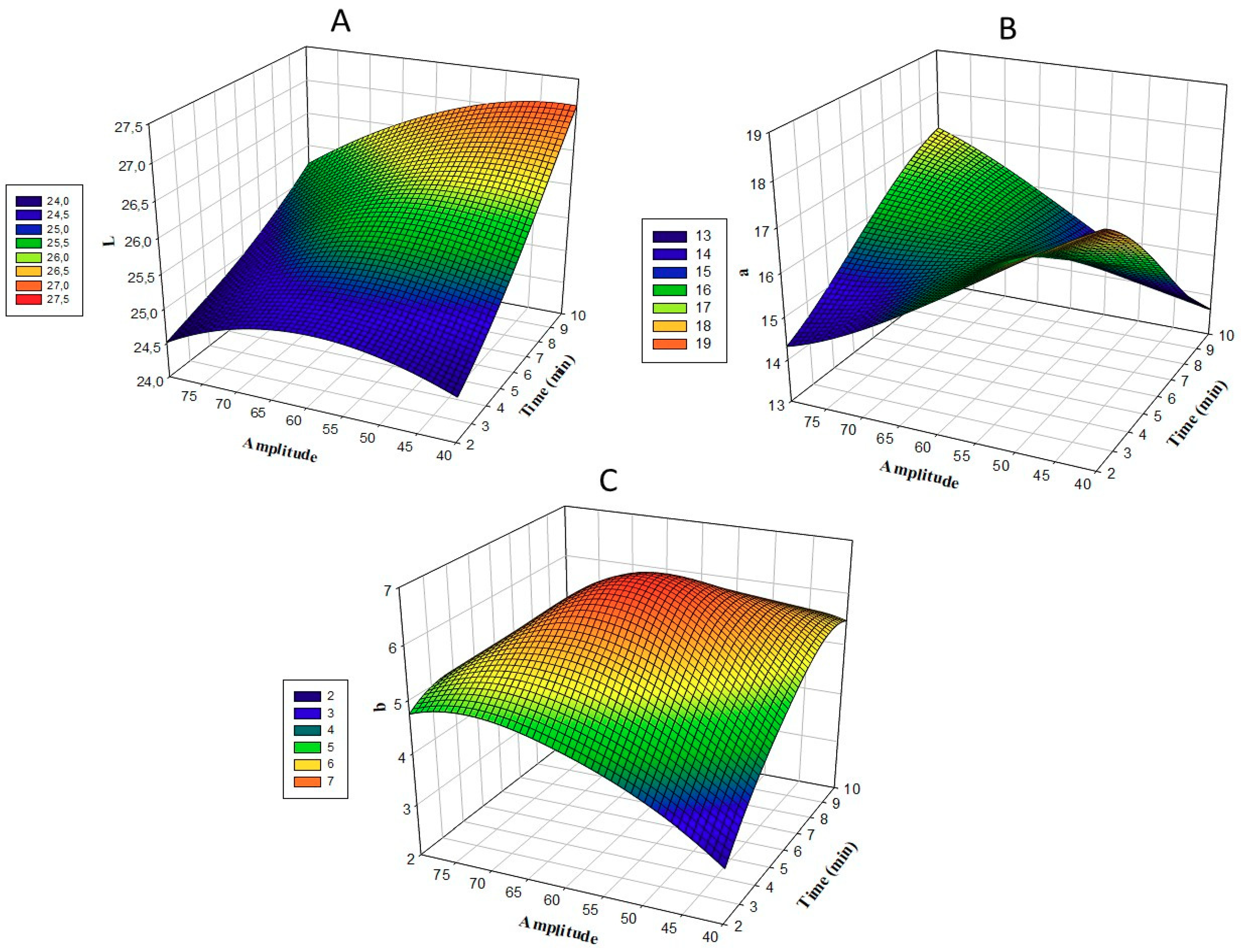
3.3. Color
4. Conclusions
Funding
Conflicts of Interest
References
- Johnston, C.S.; Gaas, C.A. Vinegar: Medicinal uses and antiglycemic effect. MedGenMed 2006, 8, 61. [Google Scholar]
- Karabiyikli, S.; Sengun, I.Y. Beneficial Effects of Acetic Acid Bacteria and Their Food Products. In Acetic Acid Bacteria: Fundamentals and Food Applications; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 221–242. [Google Scholar]
- Öztürk, A.; Özdemir, Y.; Göksel, Z. Apple Vinegar and its Therupotic Effects. Tarım Bilimleri Araştırma Dergisi 2009, 2, 155–158. [Google Scholar]
- Ramos, B.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Balsamic vinegar from Modena: An easy and effective approach to reduce Listeria monocytogenes from lettuce. Food Control 2014, 42, 38–42. [Google Scholar] [CrossRef]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Yolmeh, M.; Jafari, S.M. Applications of Response Surface Methodology in the Food Industry Processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Coşkun, S.; Aşkın, M.A. Determination of Pomological and Biochemical Characteristics of Some Local Apple Varieties. Süleyman Demirel Üniversitesi Ziraat Fakültesi Derg. 2016, 11, 120–131. [Google Scholar]
- Abacı, Z.T.; Sevindik, E. Determination of Bioactive Compounds and Total Antioxidant Capacity in Apple Varieties Grown in Ardahan Region. Yüzüncü Yıl Üniversitesi Tarım Bilim. Derg. 2014, 24, 175–184. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Esin Karademir, S.; Erçağ, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Dranca, F.; Oroian, M. Optimization of ultrasound-assisted extraction of total monomeric anthocyanin (TMA) and total phenolic content (TPC) from eggplant (Solanum melongena L.) peel. Ultrason. Sonochem. 2016, 31, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomas-Barberan, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in Food and Their Health Benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, P.; Gogate, P.R. Effect of novel ultrasound based processing on the nutrition quality of different fruit and vegetable juices. Ultrason. Sonochem. 2015, 27, 125–136. [Google Scholar] [CrossRef]
- Bhat, R.; Kamaruddin, N.S.B.C.; Min-Tze, L.; Karim, A.A. Sonication improves kasturi lime (Citrus microcarpa) juice quality. Ultrason. Sonochem. 2011, 18, 1295–1300. [Google Scholar] [CrossRef]
- Santhirasegaram, V.; Razali, Z.; Somasundram, C. Effects of thermal treatment and sonication on quality attributes of Chokanan mango (Mangifera indica L.) juice. Ultrason. Sonochem. 2013, 20, 1276–1282. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.-A.; Han, Z.; Sun, D.-W. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 2013, 141, 3201–3206. [Google Scholar] [CrossRef]
- Ubeda, C.; Hidalgo, C.; Torija, M.J.; Mas, A.; Troncoso, A.M.; Morales, M.L. Evaluation of antioxidant activity and total phenols index in persimmon vinegars produced by different processes. LWT Food Sci. Technol. 2011, 44, 1591–1596. [Google Scholar] [CrossRef]
- Srdić-Rajić, T.; Konić Ristić, A. Antioxidants: Role on Health and Prevention. In The Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 227–233. [Google Scholar]
- Nadeem, M.; Ubaid, N.; Qureshi, T.M.; Munir, M.; Mehmood, A. Effect of ultrasound and chemical treatment on total phenol, flavonoids and antioxidant properties on carrot-grape juice blend during storage. Ultrason. Sonochem. 2018, 45, 1–6. [Google Scholar] [CrossRef]
- Zafra-Rojas, Q.Y.; Cruz-Cansino, N.; Ramírez-Moreno, E.; Delgado-Olivares, L.; Villanueva-Sánchez, J.; Alanís-García, E. Effects of ultrasound treatment in purple cactus pear (Opuntia ficus-indica) juice. Ultrason. Sonochem. 2013, 20, 1283–1288. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Alternatives to conventional thermal treatments in fruit-juice processing. Part 2: Effect on composition, phytochemical content, and physicochemical, rheological, and organoleptic properties of fruit juices. Crit. Rev. Food Sci. Nutr. 2017, 57, 637–652. [Google Scholar] [CrossRef]
- Rawson, A.; Tiwari, B.K.; Patras, A.; Brunton, N.; Brennan, C.; Cullen, P.J.; O’Donnell, C. Effect of thermosonication on bioactive compounds in watermelon juice. Food Res. Int. 2011, 44, 1168–1173. [Google Scholar] [CrossRef]
- Tiwari, B.K.; O’Donnell, C.P.; Patras, A.; Cullen, P.J. Anthocyanin and Ascorbic Acid Degradation in Sonicated Strawberry Juice. J. Agric. Food Chem. 2008, 56, 10071–10077. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Avoiding non-enzymatic browning by high-intensity pulsed electric fields in strawberry, tomato and watermelon juices. J. Food Eng. 2009, 92, 37–43. [Google Scholar] [CrossRef]
| Factor Levels | |||||
|---|---|---|---|---|---|
| Independent variable | Lowest | Low | Center | High | Highest |
| (−1.41) | (−1) | 0 | (+1) | (1.41) | |
| Time (Factor 1, X1) | 2 | 4 | 6 | 8 | 10 |
| Amplitude (Factor 2, X2) | 40 | 50 | 60 | 70 | 80 |
| Sample | Encoded Independent Variables | Dependent Variables | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (X1) | Amplitude (X2) | Response 1 | Response 2 | Response 3 | Response 4 | Response 5 | Response 6 | Response 7 | |
| Total Phenolics Compound (mg GAE/L) | Total flavonoids (mg CE/L) | DPPH (mg TEAC/mL) | CUPRAC (mg TEAC/mL) | L | a | b | |||
| CV | 69.17 | 11.58 | 0.134 | 0.213 | 35.60 | 5.18 | 15.48 | ||
| PV | 86.14 | 24.12 | 0.415 | 0.586 | 24.36 | 15.24 | 5.65 | ||
| C | 108.70 | 43.14 | 0.449 | 0.623 | 25.36 | 16.04 | 6.06 | ||
| 1 | 8 (+1) | 50(−1) | 115.26 | 49.443 | 0.438 | 0.692 | 26.48 | 14.93 | 6.19 |
| 2 | 8 (+1) | 70 (+1) | 127.62 | 44.650 | 0.473 | 0.686 | 25.85 | 16.24 | 5.54 |
| 3 | 6 (0) | 60 (0) | 121.22 | 46.700 | 0.495 | 0.687 | 25.93 | 15.75 | 6.66 |
| 4 | 2 (−1.41) | 60 (0) | 103.01 | 40.760 | 0.424 | 0.633 | 25.06 | 16.02 | 4.56 |
| 5 | 6 (0) | 60 (0) | 119.65 | 46.143 | 0.493 | 0.685 | 25.91 | 15.82 | 6.74 |
| 6 | 6 (0) | 60 (0) | 120.92 | 46.210 | 0.494 | 0.688 | 25.85 | 15.85 | 6.77 |
| 7 | 6 (0) | 60 (0) | 121.42 | 46.180 | 0.491 | 0.687 | 25.86 | 15.77 | 6.76 |
| 8 | 10 (+1.41) | 60 (0) | 123.82 | 48.150 | 0.422 | 0.687 | 26.76 | 15.43 | 5.20 |
| 9 | 4 (−1) | 70 (+1) | 105.68 | 47.150 | 0.438 | 0.674 | 25.17 | 15.28 | 5.82 |
| 10 | 6 (0) | 80 (+1.41) | 112.67 | 46.140 | 0.418 | 0.674 | 24.99 | 15.85 | 4.86 |
| 11 | 4 (−1) | 50 (−1) | 117.04 | 40.456 | 0.476 | 0.654 | 25.31 | 16.75 | 5.18 |
| 12 | 6 (0) | 60 (0) | 119.33 | 46.010 | 0.492 | 0.689 | 25.82 | 15.78 | 6.74 |
| 13 | 6 (0) | 40 (−1.41) | 109.02 | 43.280 | 0.425 | 0.671 | 25.73 | 15.95 | 4.92 |
| Source | Total Phenolics Compound (mg GAE/L) | Total Flavonoids (mg CE/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DF | SS 1 | MS | F-Value | p-Value | SS 1 | MS | F-Value | p-Value | |
| Model | 5 | 627.396 | 125.479 | 180.33 | 0.0000 | 817.695 | 163.539 | 201.73 | 0.0000 |
| Linear | 2 | 323.797 | 161.898 | 232.67 | 0.0000 | 425.301 | 21.265 | 262.31 | 0.0000 |
| X1 | 1 | 318.069 | 318.069 | 457.11 | 0.0000 | 376.899 | 376.899 | 464.91 | 0.0000 |
| X2 | 1 | 5.728 | 5.728 | 8.23 | 0.024 | 48.402 | 48.402 | 59.7 | 0.0001 |
| Square | 2 | 163.005 | 81.503 | 117.13 | 0.0000 | 62.525 | 31.262 | 38.56 | 0.0002 |
| X1*X1 | 1 | 71.957 | 71.957 | 103.41 | 0.0000 | 46.037 | 46.037 | 56.79 | 0.0001 |
| X2*X2 | 1 | 133.584 | 133.584 | 191.98 | 0.0000 | 33.873 | 33.873 | 41.78 | 0.0003 |
| 2-Way Interaction | 1 | 140.594 | 140.594 | 202.05 | 0.0000 | 32.987 | 32.987 | 406.9 | 0.0000 |
| X1*X2 | 1 | 140.594 | 140.594 | 202.05 | 0.0000 | 32.987 | 32.987 | 406.9 | 0.0000 |
| Error | 7 | 4.871 | 0.696 | 0.5675 | 0.0811 | ||||
| Lack-of-Fit | 3 | 1.247 | 0.416 | 0.46 | 0.7258 | 0.2894 | 0.0965 | 1.39 | 0.3681 |
| Pure Error | 4 | 3.624 | 0.906 | 0.2781 | 0.0695 | ||||
| Total | 12 | 632.267 | 82.337 | ||||||
| R2 | 0.9923 | 0.9931 | |||||||
| Adj R2 | 0.9868 | 0.9882 | |||||||
| Pred R2 | 0.9716 | 0.9593 | |||||||
| Source | DPPH (mg TEAC/mL) | CUPRAC (mg TEAC/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DF | S 1 | MS | F-Value | p-Value | SS 1 | MS | F-Value | p-Value | |
| Model | 5 | 0.01258 | 0.002516 | 1041.04 | 0.0000 | 0.003383 | 0.000677 | 150.16 | 0.0000 |
| Linear | 2 | 0.000028 | 0.000014 | 5.77 | 0.0331 | 0.002069 | 0.001035 | 229.59 | 0.0000 |
| X1 | 1 | 0.000004 | 0.000004 | 1.65 | 0.24 | 0.00204 | 0.00204 | 452.67 | 0.0000 |
| X2 | 1 | 0.000024 | 0.000024 | 9.89 | 0.0163 | 0.000029 | 0.000029 | 6.51 | 0.038 |
| Square | 2 | 0.011188 | 0.005594 | 2314.77 | 0.0000 | 0.001141 | 0.00057 | 126.59 | 0.0000 |
| X1*X1 | 1 | 0.00703 | 0.00703 | 2909.06 | 0.0000 | 0.001069 | 0.001069 | 237.2 | 0.0000 |
| X2*X2 | 1 | 0.007334 | 0.007334 | 3034.53 | 0.0000 | 0.000303 | 0.000303 | 67.33 | 0.0001 |
| Two-way Interaction | 1 | 0.001363 | 0.001363 | 564.1 | 0.0000 | 0.000173 | 0.000173 | 38.43 | 0.0004 |
| X1*X2 | 1 | 0.001363 | 0.001363 | 564.1 | 0.0000 | 0.000173 | 0.000173 | 38.43 | 0.0004 |
| Error | 7 | 0.000017 | 0.000002 | 0.000032 | 0.000005 | ||||
| Lack-of-Fit | 3 | 0.000007 | 0.000002 | 0.86 | 0.5306 | 0.000022 | 0.000007 | 3.11 | 0.1506 |
| Pure Error | 4 | 0.00001 | 0.000003 | 0.000009 | 0.000002 | ||||
| Total | 12 | 0.012597 | 0.003415 | ||||||
| R2 | 0.9987 | 0.9908 | |||||||
| Adj R2 | 0.9987 | 0.9842 | |||||||
| Pred R2 | 0.9944 | 0.9302 | |||||||
| Source | L* | a* | b* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | SS 1 | MS | F-Value | p-Value | SS 1 | MS | F-Value | p-Value | SS 1 | MS | F-Value | p-Value | |
| Model | 5 | 3.22643 | 0.64529 | 241.98 | 0 | 2.32207 | 0.46441 | 168.28 | 0 | 8.45916 | 1.69183 | 217.92 | 0 |
| Linear | 2 | 2.72531 | 1.36265 | 510.98 | 0 | 0.35788 | 0.17894 | 64.84 | 0 | 0.33535 | 0.16768 | 21.6 | 0.001 |
| X1 | 1 | 2.30493 | 2.30493 | 864.33 | 0 | 0.34711 | 0.34711 | 125.78 | 0 | 0.33403 | 0.33403 | 43.02 | 0.0003 |
| X2 | 1 | 0.42038 | 0.42038 | 157.64 | 0 | 0.01077 | 0.01077 | 3.9 | 0.0888 | 0.00132 | 0.00132 | 0.17 | 0.6923 |
| Square | 2 | 0.44012 | 0.22006 | 82.52 | 0 | 0.0314 | 0.0157 | 5.69 | 0.0341 | 7.70772 | 3.85386 | 496.39 | 0 |
| X1*X1 | 1 | 0.00148 | 0.00148 | 0.56 | 0.48 | 0.00688 | 0.00688 | 2.49 | 0.1583 | 4.98007 | 4.98007 | 641.45 | 0 |
| X2*X2 | 1 | 0.38949 | 0.38949 | 146.06 | 0 | 0.01602 | 0.01602 | 5.81 | 0.0468 | 4.91618 | 4.91618 | 633.22 | 0 |
| Two-way Interaction | 1 | 0.06101 | 0.06101 | 22.88 | 0.002 | 1.9328 | 1.9328 | 700.36 | 0 | 0.41609 | 0.41609 | 53.59 | 0.0002 |
| X1*X2 | 1 | 0.06101 | 0.06101 | 22.88 | 0.002 | 1.9328 | 1.9328 | 700.36 | 0 | 0.41609 | 0.41609 | 53.59 | 0.0002 |
| Error | 7 | 0.01867 | 0.00267 | 0.01932 | 0.00276 | 0.05435 | 0.00776 | ||||||
| Lack-of-Fit | 3 | 0.01109 | 0.0037 | 1.95 | 0.2635 | 0.0128 | 0.00427 | 2.62 | 0.1878 | 0.04683 | 0.01561 | 8.3 | 0.0342 |
| Pure Error | 4 | 0.00758 | 0.00189 | 0.00652 | 0.00163 | 0.00752 | 0.00188 | ||||||
| Total | 12 | 3.2451 | 2.34139 | 8.51351 | |||||||||
| R2 | 0.9942 | 0.9917 | 0.9936 | ||||||||||
| Adj R2 | 0.9901 | 0.9859 | 0.9891 | ||||||||||
| Pred R2 | 0.9692 | 0.9403 | 0.9604 | ||||||||||
| Variable | Setting | |||
|---|---|---|---|---|
| X1 | 7.4 | |||
| X2 | 62.2 | |||
| Response | Fit | SE Fit | 95% CI | 95% PI |
| b* | 6.51 | 0.04 | (6.4196; 6.5960) | (6.2816; 6.7341) |
| a* | 15.77 | 0.02 | (15.7154; 15.8206) | (15.6331; 15.9029) |
| L* | 26.11 | 0.02 | (26.0537; 26.1571) | (25.9728; 26.2380) |
| CUPRAC (mg TEAC/mL) | 0.69 | 0.00 | (0.690020; 0.694270) | (0.686694; 0.697596) |
| DPPH (mg TEAC/mL) | 0.49 | 0.00 | (0.483650; 0.486761) | (0.481214; 0.489197) |
| Total Flavonoids (mg CE/L) | 46.95 | 0.12 | (46.666; 47.236) | (46.220; 47.682) |
| Total Phenolics (mg GAE/L) | 124.25 | 0.35 | (123.416; 125.086) | (122.109; 126.393) |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yıkmış, S. Optimization of Uruset Apple Vinegar Production Using Response Surface Methodology for the Enhanced Extraction of Bioactive Substances. Foods 2019, 8, 107. https://doi.org/10.3390/foods8030107
Yıkmış S. Optimization of Uruset Apple Vinegar Production Using Response Surface Methodology for the Enhanced Extraction of Bioactive Substances. Foods. 2019; 8(3):107. https://doi.org/10.3390/foods8030107
Chicago/Turabian StyleYıkmış, Seydi. 2019. "Optimization of Uruset Apple Vinegar Production Using Response Surface Methodology for the Enhanced Extraction of Bioactive Substances" Foods 8, no. 3: 107. https://doi.org/10.3390/foods8030107
APA StyleYıkmış, S. (2019). Optimization of Uruset Apple Vinegar Production Using Response Surface Methodology for the Enhanced Extraction of Bioactive Substances. Foods, 8(3), 107. https://doi.org/10.3390/foods8030107





