The Effect of the Ultra-High-Pressure Homogenization of Protein Encapsulants on the Survivability of Probiotic Cultures after Spray Drying
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Probiotic Cultures
2.3. Preparation of Probiotic Suspensions
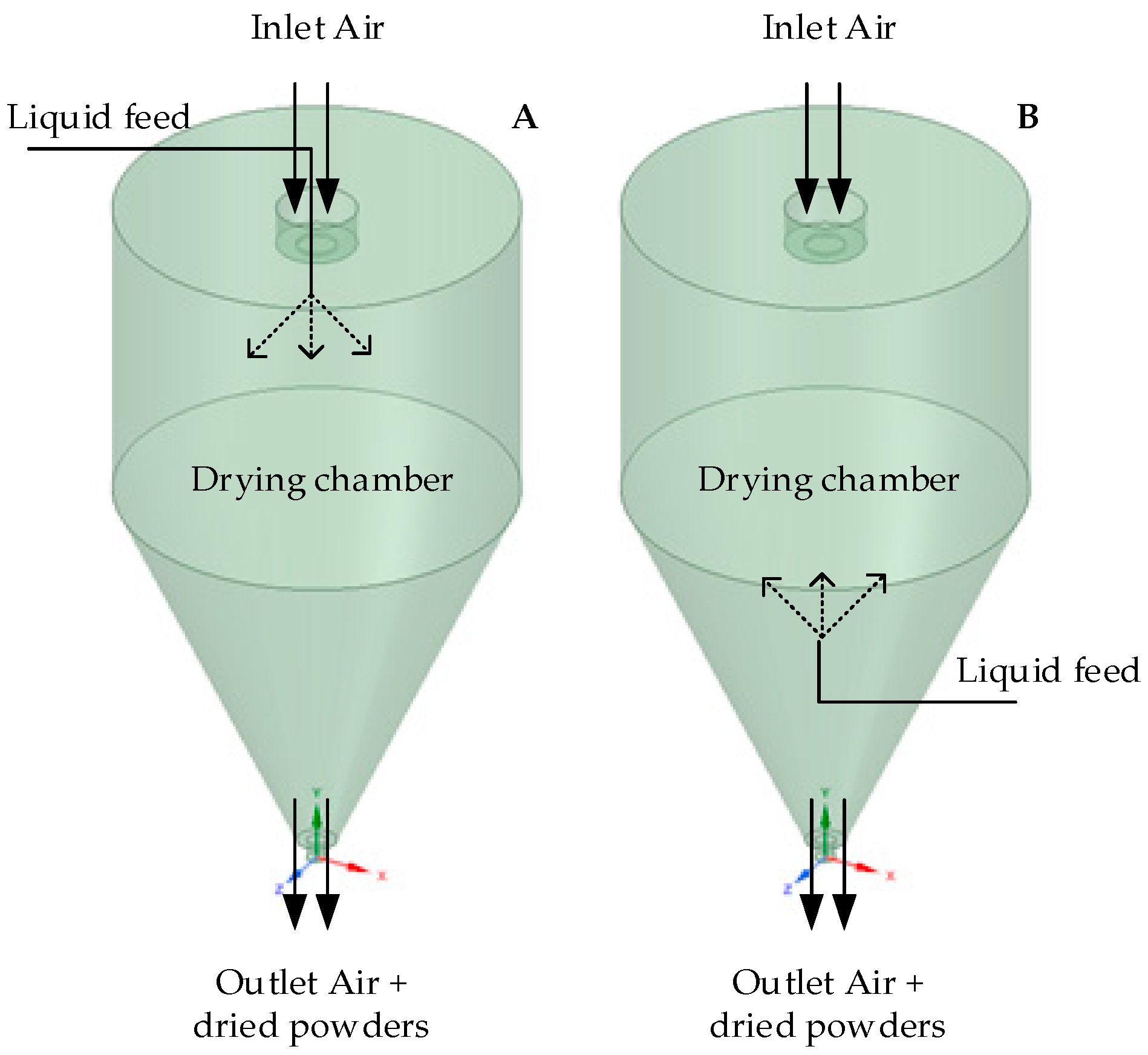
2.4. Drying of Samples
2.5. Enumeration of Probiotic Cultures
2.6. Physical Properties of Dried Powders
2.6.1. Moisture Content and Water Activity (aw)
2.6.2. Particle Size Distribution
2.7. Statistical Analysis
3. Results
3.1. Probiotic Survivablity
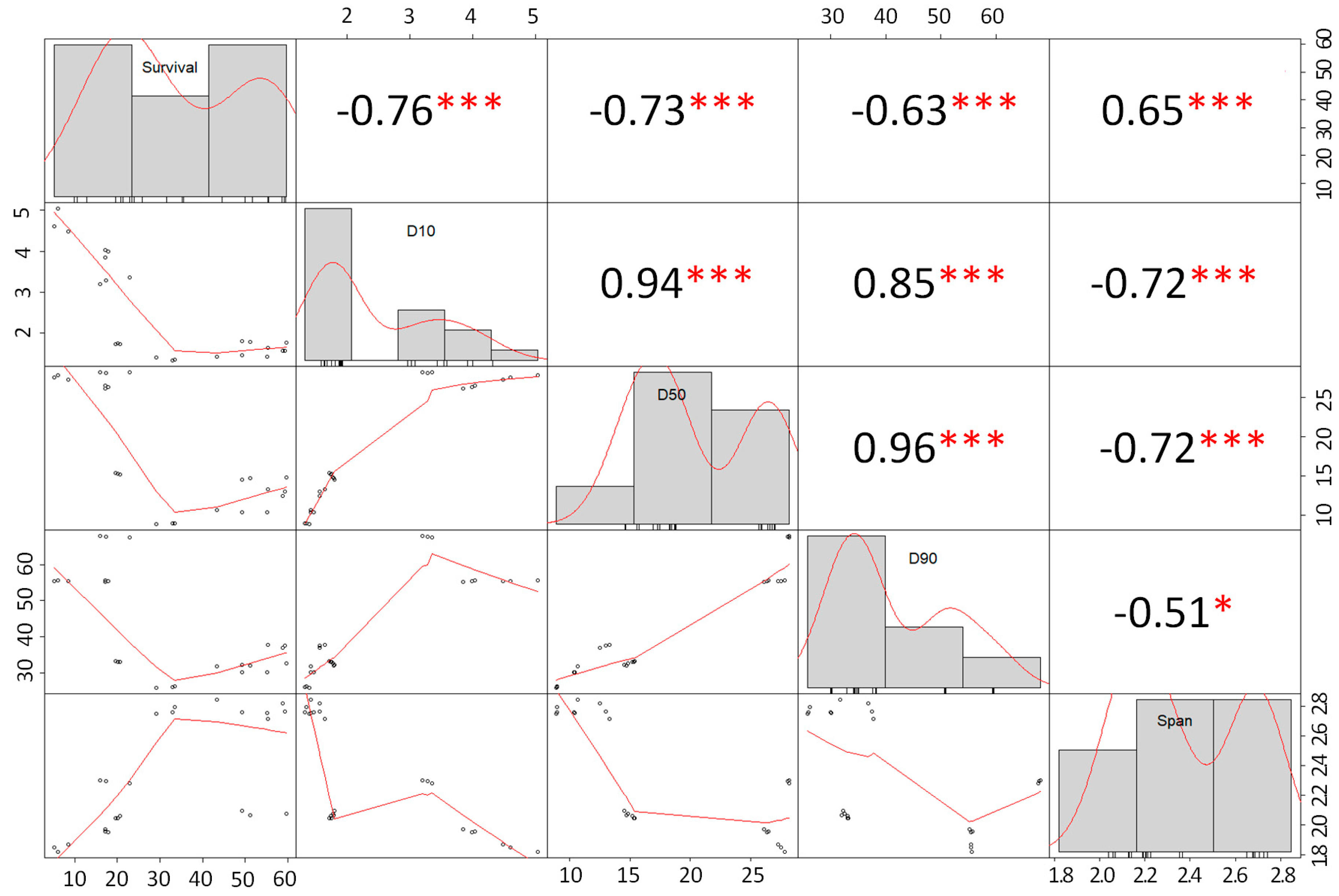
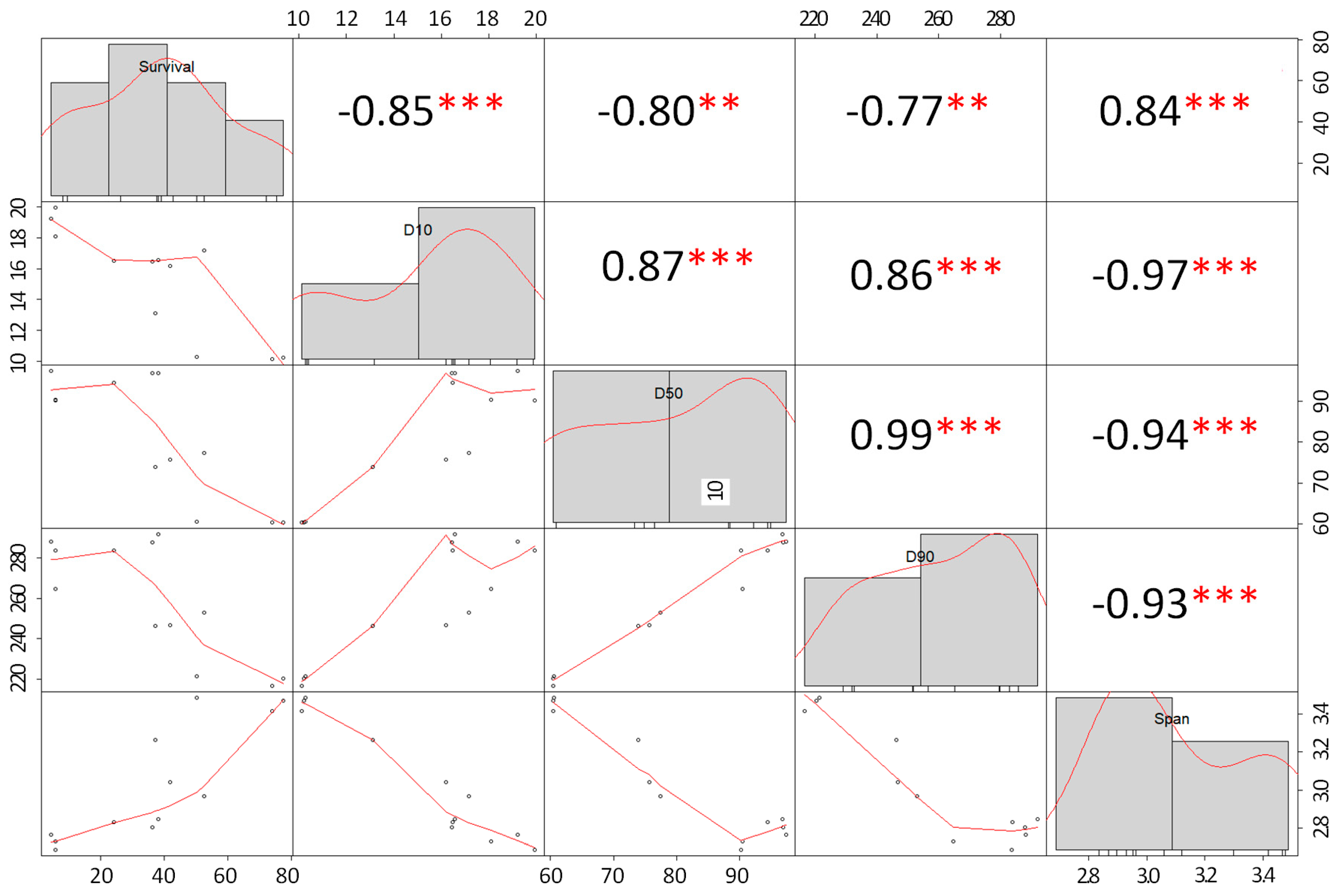
3.2. Particle Size Distribution
Effect of Particle Size and Survival of Probiotic Cells
3.3. Moisture Content and Water Activity
4. Discussion
4.1. Survivability of L. plantarum NRRL B-1927 Powders
4.2. Particle Size
4.3. Moisture Content and Water Activity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Champagne, C.P.; Gomes da Cruz, A.; Daga, M. Strategies to improve the functionality of probiotics in supplements and foods. Curr. Opin. Food Sci. 2018, 22, 160–166. [Google Scholar] [CrossRef]
- Bruhn, C.M.; Bruhn, J.C.; Cotter, A.; Garrett, C.; Klenk, M.; Powell, C.; Stanford, G.; Steinbring, Y.; West, E. Consumer Attitudes Toward Use of Probiotic Cultures. J. Food Sci. 2002, 67, 1969–1972. [Google Scholar] [CrossRef]
- Saarela, M.; Mogensen, G.; Fondén, R.; Mättö, J.; Mattila-Sandholm, T. Probiotic bacteria: Safety, functional and technological properties. J. Biotechnol. 2000, 84, 197–215. [Google Scholar] [CrossRef]
- Mattila-Sandholm, T.; Myllärinen, P.; Crittenden, R.; Mogensen, G.; Fondén, R.; Saarela, M. Technological challenges for future probiotic foods. Int. Dairy J. 2002, 12, 173–182. [Google Scholar] [CrossRef]
- Murugesan, R.; Orsat, V. Spray Drying for the Production of Nutraceutical Ingredients—A Review. Food Bioprocess Technol. 2012, 5, 3–14. [Google Scholar] [CrossRef]
- Hadzieva, J.; Mladenovska, K.; Crcarevska, M.S.; Dodov, M.G.; Dimchevska, S.; Geškovski, N.; Grozdanov, A.; Popovski, E.; Petruševski, G.; Chachorovska, M.; et al. Lactobacillus casei Encapsulated in Soy Protein Isolate and Alginate Microparticles Prepared by Spray Drying. Food Technol. Biotechnol. 2017, 55, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Huq, T.; Khan, A.; Khan, R.A.; Riedl, B.; Lacroix, M. Encapsulation of Probiotic Bacteria in Biopolymeric System. Crit. Rev. Food Sci. Nutr. 2013, 53, 909–916. [Google Scholar] [CrossRef]
- Oliver, C.M.; Augustin, M.A. Using dairy ingredients for encapsulation. In Dairy-Derived Ingredients; Corredig, M., Ed.; Woodhead Publishing: Sawston/Cambridge, UK, 2009; pp. 565–588. [Google Scholar] [CrossRef]
- Cal, K.; Sollohub, K. Spray drying technique. I: Hardware and process parameters. J. Pharm. Sci. 2010, 99, 575–586. [Google Scholar] [CrossRef]
- Reyes, V.; Chotiko, A.; Chouljenko, A.; Campbell, V.; Liu, C.; Theegala, C.; Sathivel, S. Influence of wall material on production of spray dried Lactobacillus plantarum NRRL B-4496 and its viability at different storage conditions. Dry. Technol. 2018, 36, 1738–1748. [Google Scholar] [CrossRef]
- Reyes, V.; Chotiko, A.; Chouljenko, A.; Sathivel, S. Viability of Lactobacillus acidophilus NRRL B-4495 encapsulated with high maize starch, maltodextrin, and gum arabic. LWT 2018, 96, 642–647. [Google Scholar] [CrossRef]
- Chávez, B.E.; Ledeboer, A.M. Drying of Probiotics: Optimization of Formulation and Process to Enhance Storage Survival. Dry. Technol. 2007, 25, 1193–1201. [Google Scholar] [CrossRef]
- Gong, P.; Di, W.; Yi, H.; Sun, J.; Zhang, L.; Han, X. Improved viability of spray-dried Lactobacillus bulgaricus sp1.1 embedded in acidic-basic proteins treated with transglutaminase. Food Chem. 2019, 281, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Kerr, W.L. Rheological properties and microstructure of tomato puree subject to continuous high pressure homogenization. J. Food Eng. 2015, 166, 45–54. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Singh, R.K. Ultra High Pressure Homogenization of Soy Milk: Effect on Quality Attributes during Storage. Beverages 2016, 2, 15. [Google Scholar] [CrossRef]
- Paquin, P. Technological properties of high pressure homogenizers: The effect of fat globules, milk protiens and polysaccharides. Int. Dairy J. 1999, 9, 329–335. [Google Scholar] [CrossRef]
- Cavender, G.A.; Kerr, W.L. Microfluidization of Full-fat Ice Cream Mixes: Effects of Gum Stabilizer Choice on Physical and Sensory Changes. J. Food Process Eng. 2013, 36, 29–35. [Google Scholar] [CrossRef]
- Zamora, A.; Ferragut, V.; Jaramillo, P.D.; Guamis, B.; Trujillo, A.J. Effects of Ultra-High Pressure Homogenization on the Cheese-Making Properties of Milk. J. Dairy Sci. 2007, 90, 13–23. [Google Scholar] [CrossRef]
- Valdéz, J.C.; Peral, M.C.; Rachid, M.; Santana, M.; Perdigón, G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: The potential use of probiotics in wound treatment. Clin. Microbiol. Infect. 2005, 11, 472–479. [Google Scholar] [CrossRef]
- Khemariya, P.; Singh, S.; Jaiswal, N.; Chaurasia, S.N.S. Isolation and Identification of Lactobacillus plantarum from Vegetable Samples. Food Biotechnol. 2016, 30, 49–62. [Google Scholar] [CrossRef]
- Blackwood, B.P.; Yuan, C.Y.; Wood, D.R.; Nicolas, J.D.; Grothaus, J.S.; Hunter, C.J. Probiotic Lactobacillus Species Strengthen Intestinal Barrier Function and Tight Junction Integrity in Experimental Necrotizing Enterocolitis. J. Probiotics Health 2017, 5, 159. [Google Scholar] [CrossRef]
- USDA-ARS. Lactobacillus Plantarum NRRL B-1927. 2019. Available online: https://nrrl.ncaur.usda.gov/cgi-bin/usda/prokaryote/report.html?nrrlcodes=B%2d1927 (accessed on 15 June 2019).
- Dong, X.; Zhao, M.; Yang, B.; Yang, X.; Shi, J.; Jiang, Y. Effect of High-Pressure Homogenization on the functional Property of Peanut Protein. J. Food Process Eng. 2011, 34, 2191–2204. [Google Scholar] [CrossRef]
- Cruz, N.S.; Capellas, M.; Jaramillo, D.P.; Trujillo, A.J.; Guamis, B.; Ferragut, V. Soymilk treated by ultra high-pressure homogenization: Acid coagulation properties and characteristics of a soy-yogurt product. Food Hydrocoll. 2009, 23, 490–496. [Google Scholar] [CrossRef]
- Floury, J.; Desrumaux, A.; Legrand, J. Effect of Ultra-high-pressure Homogenization on Structure and on Rheological Properties of Soy Protein-stabilized Emulsions. J. Food Sci. 2002, 67, 3388–3395. [Google Scholar] [CrossRef]
- Lapsiri, W.; Bhandari, B.; Wanchaitanawong, P. Viability of Lactobacillus plantarum TISTR 2075 in Different Protectants during Spray Drying and Storage. Dry. Technol. 2012, 30, 1407–1412. [Google Scholar] [CrossRef]
- Barbosa, J.; Borges, S.; Amorim, M.; Pereira, M.J.; Oliveira, A.; Pintado, M.E.; Teixeira, P. Comparison of spray drying, freeze drying and convective hot air drying for the production of a probiotic orange powder. J. Funct. Foods 2015, 17, 340–351. [Google Scholar] [CrossRef]
- Mis Solval, K.; Bankston, J.D.; Bechtel, P.J.; Sathivel, S. Physicochemical Properties of Microencapsulated ω-3 Salmon Oil with Egg White Powder. J. Food Sci. 2016, 81, E600–E609. [Google Scholar] [CrossRef] [PubMed]
- Karam, M.C.; Petit, J.; Zimmer, D.; Baudelaire Djantou, E.; Scher, J. Effects of drying and grinding in production of fruit and vegetable powders: A review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Laneuville, S.I.; Paquin, P.; Turgeon, S.L. Effect of preparation conditions on the characteristics of whey protein--xanthan gum complexes. Food Hydrocoll. 2000, 14, 305–314. [Google Scholar] [CrossRef]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Yonekura, L.; Parmenter, C.; Fisk, I. Impact of Milk Protein Type on the Viability and Storage Stability of Microencapsulated Lactobacillus acidophilus NCIMB 701748 Using Spray Drying. Food Bioprocess Technol. 2014, 7, 1255–1268. [Google Scholar] [CrossRef]
- Creamer, L.K.; Bienvenue, A.; Nilsson, H.; Paulsson, M.; van Wanroij, M.; Lowe, E.K.; Anema, S.G.; Boland, M.J.; Jiménez-Flores, R. Heat-Induced Redistribution of Disulfide Bonds in Milk Proteins. 1. Bovine β-Lactoglobulin. J. Agric. Food Chem. 2004, 52, 7660–7668. [Google Scholar] [CrossRef]
- González-Ferrero, C.; Irache, J.M.; González-Navarro, C.J. Soybean protein-based microparticles for oral delivery of probiotics with improved stability during storage and gut resistance. Food Chem. 2018, 239, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, M.; Sujatno, M.; Sutadipura, N.; Faried, A. β-Conglycinin Content Obtained from Two Soybean Varieties Using Different Preparation and Extraction Methods. HAYATI J. Biosci. 2011, 18, 37–42. [Google Scholar] [CrossRef]
- Thanh, V.H.; Shibasaki, K. Major proteins of soybean seeds. Subunit structure of beta-conglycinin. J. Agric. Food Chem. 1978, 26, 692–695. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Wu, C.; Teng, F.; Qi, B.; Zhang, X.; Zhou, L.; Yu, G.; Wang, H.; Zhang, S.; et al. Stability Mechanism of Two Soybean Protein-Phosphatidylcholine Nanoemulsion Preparation Methods from a Structural Perspective: A Raman Spectroscopy Analysis. Sci. Rep. 2019, 9, 6985. [Google Scholar] [CrossRef] [PubMed]
- Guraya, H.S.; James, C. Deagglomeration of Rice Starch-Protein Aggregates by High-Pressure Homogenization. Starch Stärke 2002, 54, 108–116. [Google Scholar] [CrossRef]
- Sharma, M.; Kadam, D.M.; Chadha, S.; Wilson, R.A.; Gupta, R.K. Influence of particle size on physical and sensory attributes of mango pulp powder. Int. Agrophys. 2013, 27, 323–328. [Google Scholar] [CrossRef][Green Version]
- Zhao, R.; Sun, J.; Torley, P.; Wang, D.; Niu, S. Measurement of particle diameter of Lactobacillus acidophilus microcapsule by spray drying and analysis on its microstructure. World J. Microbiol. Biotechnol. 2008, 24, 1349–1354. [Google Scholar] [CrossRef]
- Ciron, C.I.E.; Gee, V.L.; Kelly, A.L.; Auty, M.A.E. Comparison of the effects of high-pressure microfluidization and conventional homogenization of milk on particle size, water retention and texture of non-fat and low-fat yoghurts. Int. Dairy J. 2010, 20, 314–320. [Google Scholar] [CrossRef]
- Song, X.; Zhou, C.; Fu, F.; Chen, Z.; Wu, Q. Effect of high-pressure homogenization on particle size and film properties of soy protein isolate. Ind. Crops Prod. 2013, 43, 538–544. [Google Scholar] [CrossRef]
- Würth, R.; Foerst, P.; Kulozik, U. Effects of skim milk concentrate dry matter and spray drying air temperature on formation of capsules with varying particle size and the survival microbial cultures in a microcapsule matrix. Dry. Technol. 2018, 36, 93–99. [Google Scholar] [CrossRef]
- Ananta, E.; Birkeland, S.E.; Corcoran, B.; Fitzgerald, G.; Hinz, S.; Klijn, A.; Mättö, J.; Mercernier, A.; Nilsson, U.; Nyman, M.; et al. Processing effects on the nutritional advancement of probiotics and prebiotics. Microb. Ecol. Health Dis. 2004, 16, 113–124. [Google Scholar] [CrossRef][Green Version]
- Picot, A.; Lacroix, C. Encapsulation of bifidobacteria in whey protein-based microcapsules and survival in simulated gastrointestinal conditions and in yoghurt. Int. Dairy J. 2004, 14, 505–515. [Google Scholar] [CrossRef]
- Abe, F.; Miyauchi, H.; Uchijima, A.; Yaeshima, T.; Iwatsuki, K. Effects of storage temperature and water activity on the survival of bifidobacteria in powder form. Int. J. Dairy Technol. 2009, 62, 234–239. [Google Scholar] [CrossRef]
- Weinbreck, F.; Bodnár, I.; Marco, M.L. Can encapsulation lengthen the shelf-life of probiotic bacteria in dry products? Int. J. Food Microbiol. 2010, 136, 364–367. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L. Fennema’s Food Chemistry; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
| Drying Method | Encap. Mat. | NO-UHPH | UHPH (150 Mpa) | ||||
|---|---|---|---|---|---|---|---|
| Log CFU/g Solids | Cell Survival (%) | Log CFU/g Solids | Cell Survival (%) | ||||
| Before Drying | After Drying | Before Drying | After Drying | ||||
| CC | SPI | 8.67 ± 0.32 | 8.43 ± 0.08 | 6.56 ± 1.75 d,B | 9.27 ± 010 | 8.47 ± 0.17 | 20.10 ± 0.56 d,A |
| CC | WPI | 9.22 ± 0.02 | 8.46 ± 0.01 | 17.37 ± 0.37 c,B | 9.20 ± 0.08 | 8.93 ± 0.04 | 53.39 ± 5.58 a,b,A |
| MX | SPI | 9.16 ± 0.30 | 8.43 ± 0.08 | 18.68 ± 3.66 c,B | 9.27 ± 010 | 8.77 ± 0.03 | 31.86 ± 2.39 c,d,A |
| MX | WPI | 9.02 ± 0.02 | 8.78 ± 0.02 | 57.87 ± 2.16 a,A | 9.20 ± 0.08 | 8.87 ± 0.10 | 49.19 ± 5.90 a,b,c,A |
| FD | SPI | 9.16 ± 0.30 | 7.87 ± 0.07 | 5.13 ± 0.81 d,B | 9.27 ± 010 | 8.78 ± 0.11 | 32.77 ± 7.65 b,c,d,A |
| FD | WPI | 9.02 ± 0.02 | 8.66 ± 0.08 | 43.87 ± 7.98 b,A | 9.20 ± 0.08 | 9.02 ± 0.10 | 67.29 ± 14.97 a,A |
| Drying Method | Encap. Mat. | NO-UHPH | UHPH (150 Mpa) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Particle Size Distribution | Particle Size Distribution | ||||||||
| D10 (µm) | D50 (µm) | D90 (µm) | Span | D10 (µm) | D50 (µm) | D90 (µm) | Span | ||
| CC | SPI | 4.71 ± 0.29 c,A | 27.54 ± 0.26 c,A | 55.52 ± 0.13 c,A | 1.85 ± 0.02 d,B | 1.74 ± 0.02 c,B | 15.30 ± 0.10 c,B | 33.09 ± 0.09 c,B | 2.05 ± 0.01 c,A |
| CC | WPI | 3.96 ± 0.10 c,d,A | 26.32 ± 0.17 c,A | 55.46 ± 0.23 c,A | 1.96 ± 0.01 d,B | 1.79 ± 0.01 c,B | 14.69 ± 0.15 c,B | 32.28 ± 0.25 c,B | 2.08 ± 0.02 c,A |
| MX | SPI | 3.28 ± 0.08 c,d,A | 28.15 ± 0.05 c,A | 67.81 ± 0.17 c,A | 2.29 ± 0.01 c,B | 1.36 ± 0.03 d,B | 8.92 ± 0.02 d,B | 26.07 ± 0.22 d,B | 2.77 ± 0.02 b,A |
| MX | WPI | 1.59 ± 0.04 d,A | 12.94 ± 0.41 d,A | 37.39 ± 0.50 d,A | 2.77 ± 0.05 b,A | 1.44 ± 0.03 d,B | 10.47 ± 0.17 d,B | 30.66 ± 0.97 c,d,B | 2.79 ± 0.05 b,A |
| FD | SPI | 19.09 ± 0.93 a,A | 92.62 ± 4.07 a,A | 278.85 ± 12.46 a,A | 2.73 ± 0.04 b,B | 16.50 ± 0.06 a,B | 96.02 ± 1.37 a,A | 287.91 ± 3.98 a,A | 2.83 ± 0.02 b,A |
| FD | WPI | 15.49 ± 2.11 b,A | 75.66 ± 1.75 b,A | 248.69 ± 3.70 b,A | 3.09 ± 0.15 a,B | 10.20 ± 0.07 b,B | 60.50 ± 0.03 b,B | 219.43 ± 2.49 b,B | 3.46 ± 0.04 a,A |
| Drying Method | Encapsulating Material | NO-UHPH | UHPH (150 Mpa) | ||
|---|---|---|---|---|---|
| Moisture (%) | Water Activity (aw) | Moisture (%) | Water Activity (aw) | ||
| CC | SPI | 4.35 ± 0.09 b,A | 0.22 ± 0.00 a,B | 3.81 ± 0.43 a,B | 0.23 ± 0.00 a,A |
| CC | WPI | 5.18 ± 0.16 a,A | 0.22 ± 0.00 a,A | 3.89 ± 0.42 a,B | 0.21 ± 0.01 a,B |
| MX | SPI | 4.16 ± 0.22 b,A | 0.20 ± 0.00 b,A | 2.25 ± 0.11 b,A | 0.15 ± 0.01 b,B |
| MX | WPI | 4.83 ± 0.25 a,A | 0.22 ± 0.00 a,A | 3.79 ± 0.07 a,B | 0.21 ± 0.02 a,A |
| FD | SPI | 1.03 ± 0.05 d,A | 0.03 ± 0.00 d,A | 0.41 ± 0.07 c,B | 0.04 ± 0.00 c,A |
| FD | WPI | 2.04 ± 0.12 c,A | 0.06 ± 0.00 c,A | 0.24 ± 0.09 c,B | 0.03 ± 0.00 c,B |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mis-Solval, K.E.; Jiang, N.; Yuan, M.; Joo, K.H.; Cavender, G.A. The Effect of the Ultra-High-Pressure Homogenization of Protein Encapsulants on the Survivability of Probiotic Cultures after Spray Drying. Foods 2019, 8, 689. https://doi.org/10.3390/foods8120689
Mis-Solval KE, Jiang N, Yuan M, Joo KH, Cavender GA. The Effect of the Ultra-High-Pressure Homogenization of Protein Encapsulants on the Survivability of Probiotic Cultures after Spray Drying. Foods. 2019; 8(12):689. https://doi.org/10.3390/foods8120689
Chicago/Turabian StyleMis-Solval, Kevin E., Nan Jiang, Meilan Yuan, Kay H. Joo, and George A. Cavender. 2019. "The Effect of the Ultra-High-Pressure Homogenization of Protein Encapsulants on the Survivability of Probiotic Cultures after Spray Drying" Foods 8, no. 12: 689. https://doi.org/10.3390/foods8120689
APA StyleMis-Solval, K. E., Jiang, N., Yuan, M., Joo, K. H., & Cavender, G. A. (2019). The Effect of the Ultra-High-Pressure Homogenization of Protein Encapsulants on the Survivability of Probiotic Cultures after Spray Drying. Foods, 8(12), 689. https://doi.org/10.3390/foods8120689





