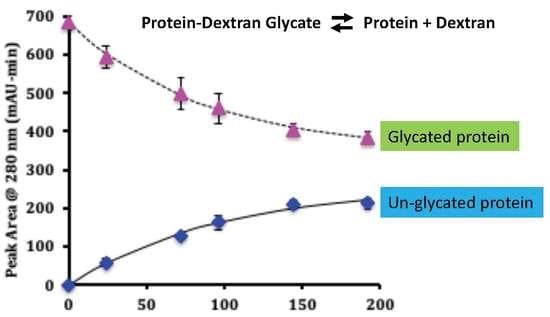
Hydrolysis of Whey Protein-Dextran Glycates Made Using the Maillard Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Glycated Proteins
2.2. Hydrolysis of Glycated Proteins
2.3. Chromatographic Analysis
2.4. Kinetic Model of the Hydrolysis Reaction
2.5. Statistical Analysis
3. Results
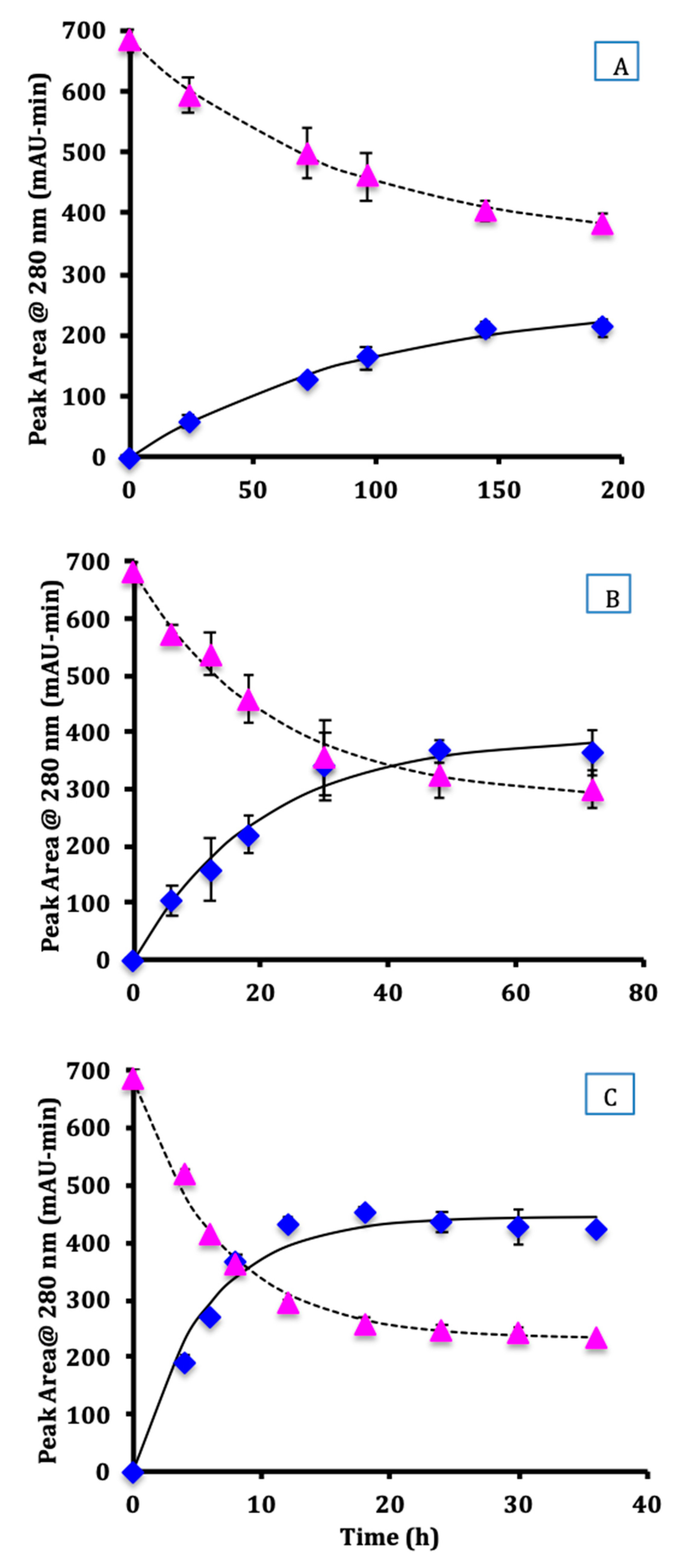
3.1. Kinetics of Hydrolysis of the Glycated Protein
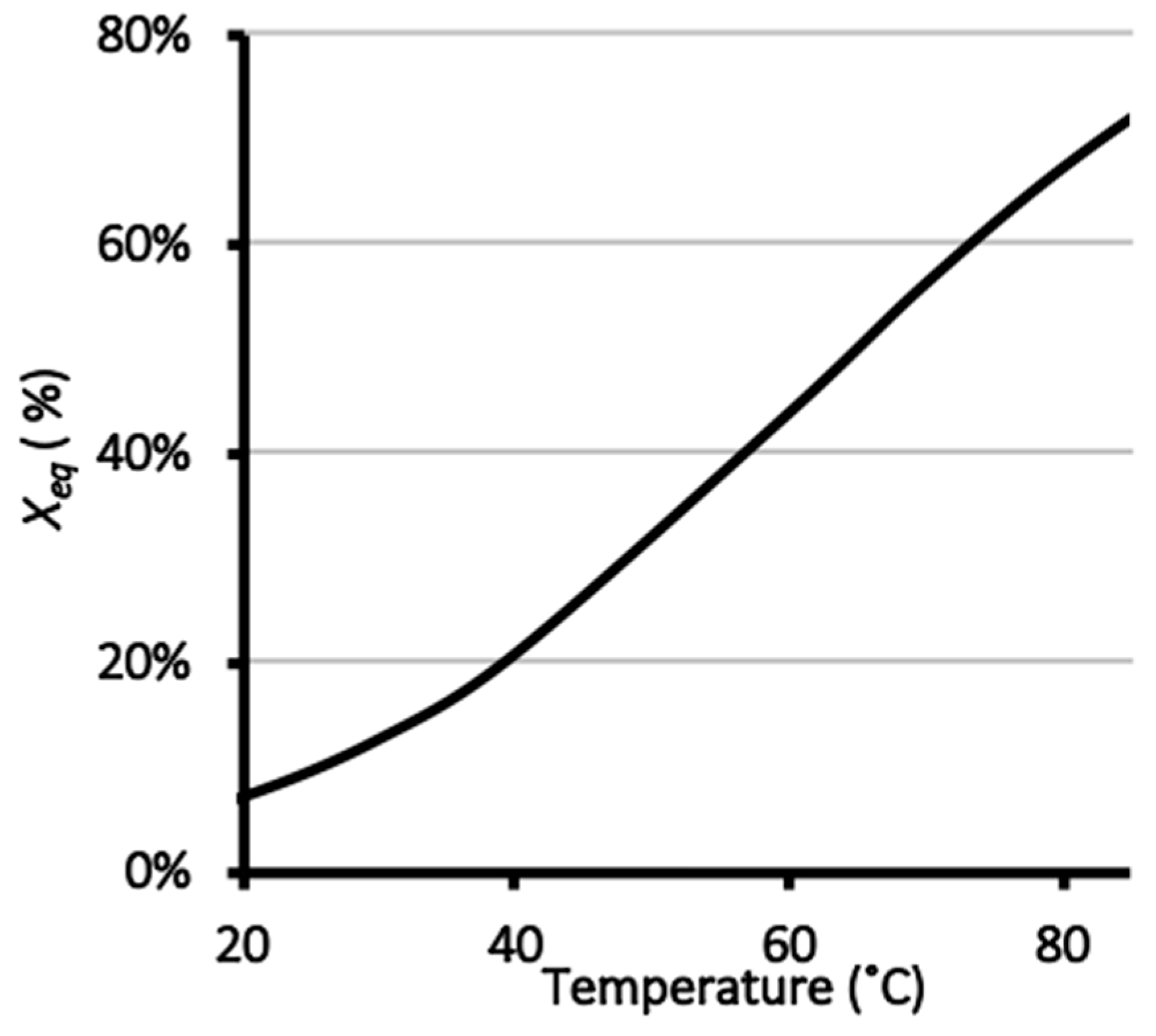
3.2. Thermodynamic Analysis of the Hydrolysis Reaction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Ru, Q.; Ding, Y. Glycation a promising method for food protein modification: Physicochemical properties and structure, a review. Food Res. Int. 2012, 49, 170–183. [Google Scholar] [CrossRef]
- De Oliveira, F.C.; Coimbra, J.S.d.R.; de Oliveira, E.B.; Zuñiga, A.D.G.; Rojas, E.E.G. Food protein-polysaccharide conjugates obtained via the Maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, E.M.; Park, C.W.; Drake, M.; Mulvihill, D.M.; O’Mahony, J.A. Enhancement of the functional properties of whey protein by conjugation with maltodextrin under dry-heating conditions. Int. J. Dairy Technol. 2018, 71, 216–225. [Google Scholar] [CrossRef]
- Shepherd, R.; Robertson, A.; Ofman, D. Dairy glycoconjugate emulsifiers: Casein–maltodextrins. Food Hydrocoll. 2000, 14, 281–286. [Google Scholar] [CrossRef]
- Jimenez-Castano, L.; Villamiel, M.; López-Fandiño, R. Glycosylation of individual whey proteins by Maillard reaction using dextran of different molecular mass. Food Hydrocoll. 2007, 21, 433–443. [Google Scholar] [CrossRef]
- Kato, A. Industrial applications of Maillard-type protein-polysaccharide conjugates. Food Sci. Technol. Res. 2002, 8, 193–199. [Google Scholar] [CrossRef]
- Akhtar, M.; Dickinson, E. Whey protein–maltodextrin conjugates as emulsifying agents: An alternative to gum arabic. Food Hydrocoll. 2007, 21, 607–616. [Google Scholar] [CrossRef]
- O’Mahony, J.A.; Drapala, K.P.; Mulcahy, E.M.; Mulvihill, D.M. Whey Protein—Carbohydrate Conjugates. In Whey Proteins; Elsevier: Amsterdam, The Netherlands, 2019; pp. 249–280. [Google Scholar]
- Xu, L.; Gong, Y.; Gern, J.E.; Ikeda, S.; Lucey, J.A. Glycation of whey protein with dextrans of different molar mass: Effect on immunoglobulin E–binding capacity with blood sera obtained from patients with cow milk protein allergy. J. Dairy Sci. 2018, 101, 6823–6834. [Google Scholar] [CrossRef]
- Zhu, D.; Damodaran, S.; Lucey, J.A. Formation of whey protein isolate (WPI)−dextran conjugates in aqueous solutions. J. Agric. Food Chem. 2008, 56, 7113–7118. [Google Scholar] [CrossRef]
- Zhu, D.; Damodaran, S.; Lucey, J.A. Physicochemical and emulsifying properties of whey protein isolate (WPI)−dextran conjugates produced in aqueous solution. J. Agric. Food Chem. 2010, 58, 2988–2994. [Google Scholar] [CrossRef]
- Oliver, C.M.; Melton, L.D.; Stanley, R.A. Creating proteins with novel functionality via the Maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Spotti, M.J.; Perduca, M.J.; Piagentini, A.; Santiago, L.G.; Rubiolo, A.C.; Carrara, C.R. Gel mechanical properties of milk whey protein–dextran conjugates obtained by Maillard reaction. Food Hydrocoll. 2013, 31, 26–32. [Google Scholar] [CrossRef]
- Martinez-Alvarenga, M.; Martinez-Rodriguez, E.; Garcia-Amezquita, L.; Olivas, G.; Zamudio-Flores, P.; Acosta-Muniz, C.; Sepulveda, D. Effect of Maillard reaction conditions on the degree of glycation and functional properties of whey protein isolate–Maltodextrin conjugates. Food Hydrocoll. 2014, 38, 110–118. [Google Scholar] [CrossRef]
- O’Mahony, J.A.; Drapala, K.P.; Mulcahy, E.M.; Mulvihill, D.M. Controlled glycation of milk proteins and peptides: Functional properties. Int. Dairy J. 2017, 67, 16–34. [Google Scholar] [CrossRef]
- Kutzli, I.; Gibis, M.; Baier, S.K.; Weiss, J. Formation of whey protein isolate (WPI)–maltodextrin conjugates in fibers produced by needleless electrospinning. J. Agric. Food Chem. 2018, 66, 10283–10291. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ismail, B. Effect of Maillard-induced glycosylation on the nutritional quality, solubility, thermal stability and molecular configuration of whey proteinv. Int. Dairy J. 2012, 25, 112–122. [Google Scholar] [CrossRef]
- Böttger, F.H.; Etzel, M.R.; Lucey, J.A. In vitro infant digestion of whey protein–dextran glycates. Food Dig. 2013, 4, 76–84. [Google Scholar] [CrossRef]
- Mulcahy, E.M.; Mulvihill, D.M.; O’Mahony, J.A. Physicochemical properties of whey protein conjugated with starch hydrolysis products of different dextrose equivalent values. Int. Dairy J. 2016, 53, 20–28. [Google Scholar] [CrossRef]
- Cordes, E.; Jencks, W. On the mechanism of Schiff base formation and hydrolysis. J. Am. Chem. Soc. 1962, 84, 832–837. [Google Scholar] [CrossRef]
- Allelein, S.; Arunkumar, A.; Etzel, M.R. Method for chromatographic analysis of whey protein-dextran glycation products. J. Chromatogr. A 2012, 1270, 330–333. [Google Scholar] [CrossRef]
- Engel, T.; Reid, P. Physical chemistry. Pearson Benjamin-Cummings 2006, 473, 477–479. [Google Scholar]
- Silbey, R.; Alberty, R. Physical Chemistry, 3rd ed.; John Willey & Sons. Inc.: New York, NY, USA, 2001. [Google Scholar]
- Chang, R. General Chemistry, 3rd ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Cordes, E.H.; Jencks, W.P. The mechanism of hydrolysis of Schiff bases derived from aliphatic amines. J. Am. Chem. Soc. 1963, 85, 2843–2848. [Google Scholar] [CrossRef]
- Borch, R.F.; Bernstein, M.D.; Durst, H.D. Cyanohydridoborate anion as a selective reducing agent. J. Am. Chem. Soc. 1971, 93, 2897–2904. [Google Scholar] [CrossRef]
- Tuma, D.J.; Donohue, T.M., Jr.; Medina, V.A.; Sorrell, M.F. Enhancement of acetaldehyde-protein adduct formation by L-ascorbate. Arch. Biochem. Biophys. 1984, 234, 377–381. [Google Scholar] [CrossRef]
- Hornsey, V.S.; Prowse, C.V.; Pepper, D.S. Reductive amination for solid-phase coupling of protein: A practical alternative to cyanogen bromide. J. Immunol. Methods 1986, 93, 83–88. [Google Scholar] [CrossRef]
- Li, N.; Arunkumar, A.; Etzel, M.R. Kinetics of whey protein glycation using dextran and the dry-heating method. Foods 2019, 8, 528. [Google Scholar] [CrossRef]
| Temperature (°C) | 60 | 70 | 80 |
|---|---|---|---|
| Glycated protein k (h−1) | 0.0115 ± 0.0007 | 0.046 ± 0.006 | 0.15 ± 0.01 |
| Un-glycated protein k (h−1) | 0.010 ± 0.002 | 0.051 ± 0.007 | 0.18 ± 0.02 |
| [PD]eq (mAU-min) | 345 ± 9 | 280 ± 19 | 231 ± 9 |
| [P]eq (mAU-min) | 255 ± 18 | 392 ± 21 | 447 ± 16 |
| K | 0.74 ± 0.06 | 1.4 ± 0.1 | 1.9 ± 0.1 |
| k1 (h−1) | 0.00463 ± 0.00001 | 0.0283 ± 0.0003 | 0.107 ±0.003 |
| k2 (h−1) | 0.006 ± 0.001 | 0.020 ± 0.004 | 0.055 ± 0.008 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Etzel, M.R. Hydrolysis of Whey Protein-Dextran Glycates Made Using the Maillard Reaction. Foods 2019, 8, 686. https://doi.org/10.3390/foods8120686
Li N, Etzel MR. Hydrolysis of Whey Protein-Dextran Glycates Made Using the Maillard Reaction. Foods. 2019; 8(12):686. https://doi.org/10.3390/foods8120686
Chicago/Turabian StyleLi, Na, and Mark R. Etzel. 2019. "Hydrolysis of Whey Protein-Dextran Glycates Made Using the Maillard Reaction" Foods 8, no. 12: 686. https://doi.org/10.3390/foods8120686
APA StyleLi, N., & Etzel, M. R. (2019). Hydrolysis of Whey Protein-Dextran Glycates Made Using the Maillard Reaction. Foods, 8(12), 686. https://doi.org/10.3390/foods8120686