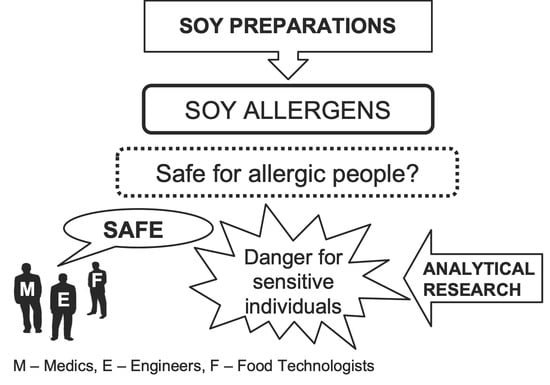
Soy Preparations Are Potentially Dangerous Factors in the Course of a Food Allergy
Abstract
1. Introduction
2. Aim of the Study
3. Materials and Methods
3.1. Questionnaire Survey
3.1.1. Individuals Participating in the Survey
3.1.2. Interview Questionnaire
3.2. Analytical Study
3.2.1. Materials
- Soy protein isolates:
- (1)
- IP 9000A, Gushen, China
- (2)
- Profam 648, ADM USA
- (3)
- IBS (Soy Protein Isolate), Guscen, China
- Soy protein concentrate: Sol Pro 110 Solbar, Israel
- Soy protein acid hydrolysates (SHB) from Polish company “PAULA Ingredients”:
- (1)
- SHB Paula,
- (2)
- SHB Paula Plus,
- (3)
- SHB KP,
- (4)
- SHB HVP.
3.2.2. Patients
- Serum A: Soy − 2, Birch and oak − 3, Alder and hazel − 2, Grass-mix − 2, Yeast − 2, Potatoes − 2, Hazelnuts 1
- Serum B: Soy − 2, D. pteron + D. farinae − 2, Bovine serum albumin − 1, α-Lactoalbumin − 1
- Serum C: Soy − 2, Timothy − 6, Asp. fumigatus − 3.
3.2.3. Protein Content
3.2.4. Protein Extraction
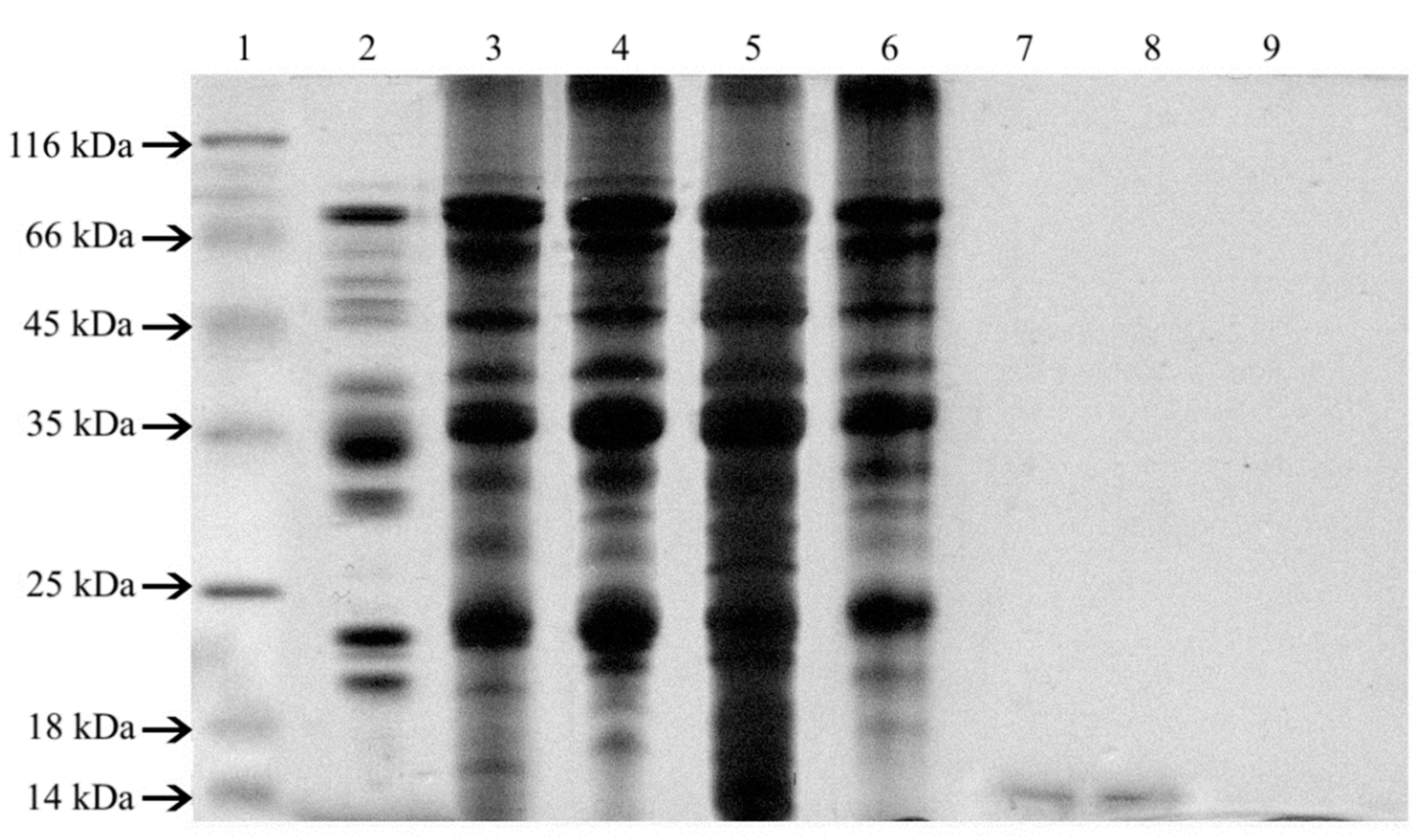
3.2.5. SDS-PAGE (Sodium Dodecyl Sulfate—Polyacrylamide Gel Electrophoresis)
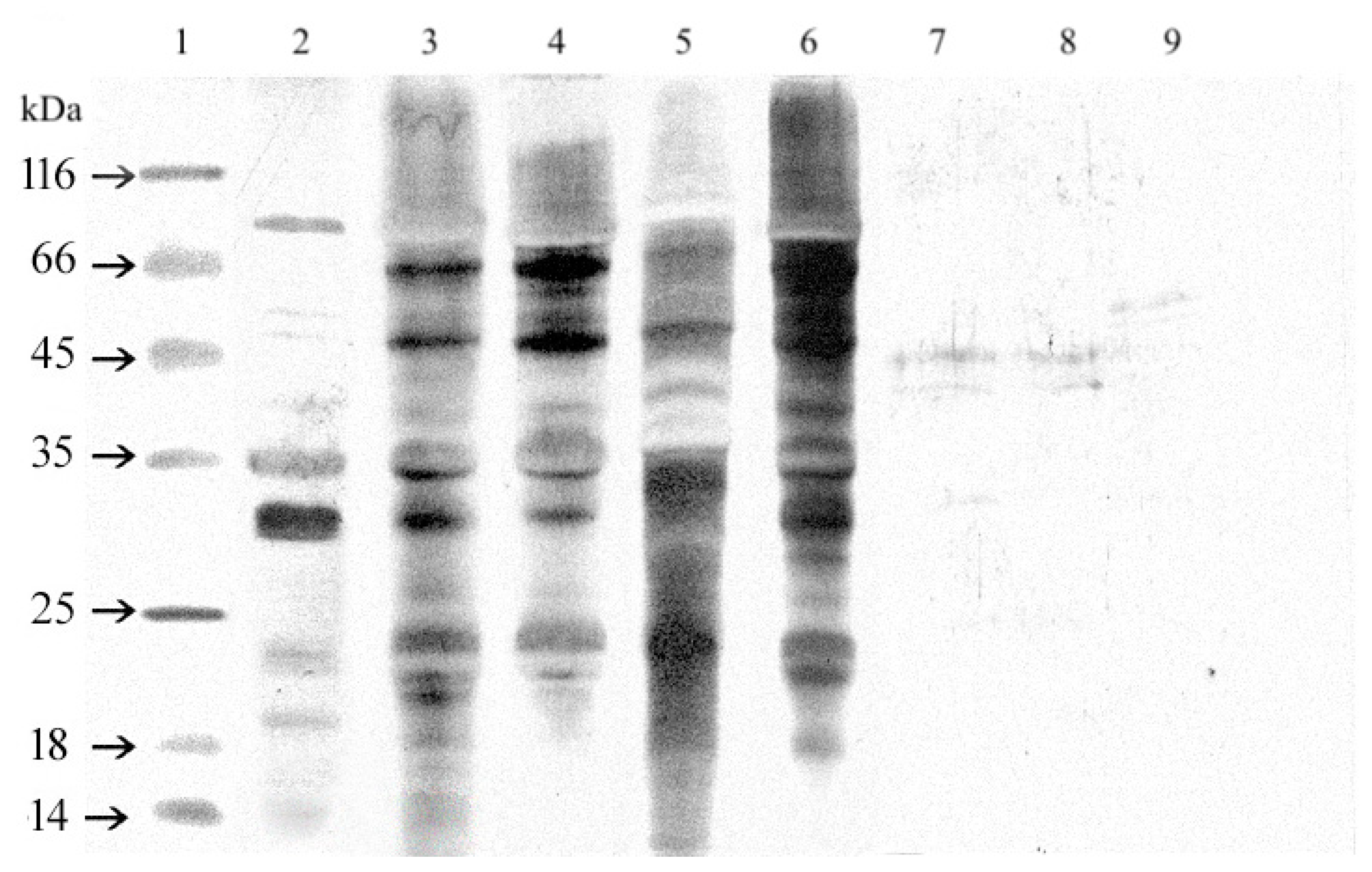
3.2.6. Western Blotting
3.2.7. Slot Blotting
3.3. Statistical Analysis
4. Results
4.1. Questionnaire Survey
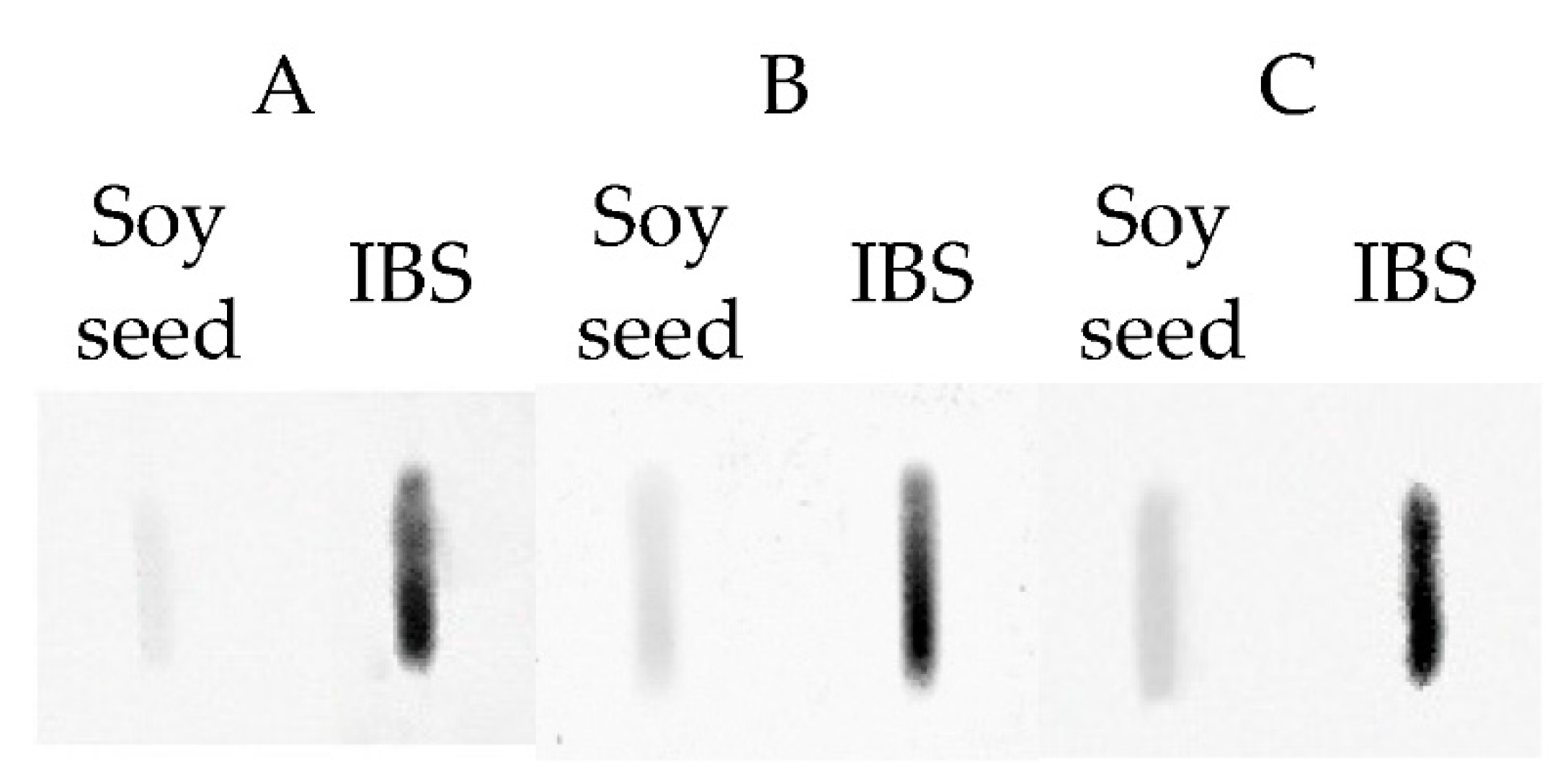
4.2. Analytical Study
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jideani, V.A. Functional properties of soybean food ingredients in food systems. In Soybean—Biochemistry, Chemistry and Physiology; Ng, T.B., Ed.; IntechOpen: London, UK, 2011; pp. 345–366. ISBN 978-953-307-219-7. [Google Scholar]
- Verhoeckx, K.C.M.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumar, R.; Sabapathy, S.N.; Bawa, A.S. Functional and edible uses of soy protein products. Compr. Rev. Food Sci. Food Saf. 2008, 7, 14–28. [Google Scholar] [CrossRef]
- Chen, M.; Sutherland, A.; Birrueta, G.; Laubach, S.; Leonard, S.; Peters, B.; Schulten, V. Analysis of allergen-specific T cell and IgE reactivity to different preparations of cow’s milk-containing food extracts. Cells 2019, 8, 667. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Fang, Y.; Nagano, T.; Guo, S.; Wang, R. Soy as a food ingredient. In Proteins in Food Processing; Yada, R.Y., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Elsevier: Amsterdam, The Netherlands, 2017; pp. 149–186. [Google Scholar]
- Otegbayo, B.; Bolaji, O.; Adebiyi, O.M.; Olunlade, B.A. Effect of soy enrichment on bread quality. Int. Food Res. J. 2018, 25, 1120–1125. [Google Scholar]
- Anibarro, B.; Seoane, F.J.; Mugica, M.V. Involvement of hidden allergens on food allergic reactions. J. Investig. Allergol. Clin. Immunol. 2007, 17, 168–172. [Google Scholar] [PubMed]
- Cabanillas, B.; Jappe, U.; Novak, N. Allergy to peanut, soybean, and other legumes: Recent advances in allergen characterization, stability to processing and IgE cross-reactivity. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.E.; Lizaso, M.T. Cross-reactivity syndromes in food allergy. J. Investig. Allergol. Clin. Immunol. 2011, 21, 162–170. [Google Scholar]
- Tomczak, A.; Zielińska-Dawidziak, M.; Piasecka-Kwiatkowska, D.; Springer, E.; Lampart-Szczapa, E. Cross-reaction between proteins isolated from new narrow-leafed lupine breeding lines and antibodies present on sera of patients sensitized to soybeans and peanuts. Eur. Food Res. Technol. 2019, 245, 433–441. [Google Scholar] [CrossRef]
- Lima-Cabello, E.; Alché, J.D.; Jimenez-Lopez, J.C. Narrow-Leafed Lupin Main Allergen β-Conglutin (Lup an 1) Detection and Quantification Assessment in Natural and Processed Foods. Foods 2019, 8, 513. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, W.; Yung, E.; Zhong, N.; Wong, G.W.; Li, J. Frequency of food group consumption and risk of allergic disease and sensitization in schoolchildren in urban and rural China. Clin. Exp. Allergy 2015, 45, 1823–1832. [Google Scholar] [CrossRef]
- Hao, G.D.; Zheng, Y.W.; Wang, Z.X.; Kong, X.A.; Song, Z.J.; Lai, X.X.; Spangfort, M.D. High correlation of specific IgE sensitization between birch pollen, soy and apple allergens indicates pollen-food allergy syndrome among birch pollen allergic patients in northern China. J. Zhejiang Univ. Sci. B 2016, 17, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Fukutomi, Y.; Saito, A.; Sekiya, K.; Tsuburai, T.; Taniguchi, M.; Akiyama, K. Frequent episodes of adult soybean allergy during and following the pollen season. J. Allergy Clin. Immunol. Pract. 2015, 3, 441. [Google Scholar] [CrossRef] [PubMed]
- Skypala, I.J. Food-induced anaphylaxis: Role of hidden allergens and cofactors. Front. Immunol. 2019, 10, 673. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci. Technol. 2016, 49, 24–34. [Google Scholar] [CrossRef]
- Wang, Z.; Li, L.; Yuan, D.; Zhao, X.; Cui, S.; Hu, J.; Wang, J. Reduction of the allergenic protein in soybean meal by enzymatic hydrolysis. Food Agric. Immunol. 2014, 25, 301–310. [Google Scholar] [CrossRef]
- Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Beyer, K.; Defernez, M.; Sperrin, M.; Mackie, A.R.; Salt, L.J.; Hourihane, J.O.; Asero, R.; Belohlavkova, S.; et al. How much is too much? Threshold dose distributions for 5 food allergens. J. Allergy Clin. Immunol. 2015, 135, 964–971. [Google Scholar] [CrossRef]
- Popping, B.; Diaz-Amigo, C. European regulations for labeling requirements for food allergens and substances causing intolerances: History and future. J. Assoc. Off. Agric. Chem. 2018, 101, 2–7. [Google Scholar] [CrossRef]
- Soon, J.M.; Manning, L. “May Contain” allergen statements: Facilitating or frustrating consumers? J. Consum. Policy 2017, 40, 447–472. [Google Scholar] [CrossRef]
- Allen, K.J.; Turner, P.J.; Pawankar, R.; Taylor, S.; Sicherer, S.; Lack, G.; Rosario, N.; Ebisawa, M.; Wong, G.; Mills, E.N.C.; et al. Precautionary labelling of foods for allergen content: Are we ready for a global framework? World Allergy Organ. J. 2014, 7, 10. [Google Scholar] [CrossRef]
- Zurzolo, G.A.; Mathai, M.L.; Koplin, J.J.; Allen, K.J. Hidden allergens in foods and implications for labelling and clinical care of food allergic patients. Curr. Allergy Asthma Rep. 2012, 12, 292–296. [Google Scholar] [CrossRef]
- AOAC Authors Crude protein in cereal grains and oilseeds: Generic combustion method; First action 1992. AOAC Official Method 992.23. 2005. Available online: http://www.wdfxw.net/Fulltext72449940.htm (accessed on 7 December 2019).
- Bandara, B.E.S.; De Silva, D.A.M.; Maduwanthi, B.C.H.; Warunasinghe, W.A.A. Impact of food labeling information on consumer purchasing decision: With special reference to faculty of Agricultural Sciences. Procedia Food Sci. 2016, 6, 309–313. [Google Scholar] [CrossRef]
- Goyal, R.; Deshmukh, N. Food label reading: Read before you eat. J. Educ. Health Promot. 2018, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.allergen.org Allergen Nomenclature (accessed on 6 November 2019).
- Pomés, A.; Davies, J.M.; Gadermaier, G.; Hilger, C.; Holzhauser, T.; Lidholm, J.; Lopata, A.L.; Mueller, G.A.; Nandy, A.; Radauer, C.; et al. WHO IUIS Allergen Nomenclature Sub-Committee: WHO/IUIS Allergen Nomenclature: Providing a common language. Mol. Immunol. 2018, 100, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Roychaudhuri, R.; Sarath, G.; Zeece, M.; Markwell, J. Stability of the allergenic soybean Kunitz trypsin inhibitor. Biochem. Biophys. Acta 2004, 1699, 207–212. [Google Scholar] [CrossRef]
- Wilson, S.; Blaschek, K.; de Mejia, E. Allergenic proteins in soybean: Processing and reduction of P34 allergenicity. Nutr. Rev. 2005, 63, 47–58. [Google Scholar] [CrossRef]
- Campos, S.; Doxey, J.; Hammond, D. Nutrition labels on pre-packaged foods: A systematic review. Public Health Nutr. 2011, 14, 1496–1506. [Google Scholar] [CrossRef]
- Marra, C.A.; Harvard, S.; Grubisic, M.; Galo, J.; Clarke, A.; Elliott, S.; Lynd, L.D. Consumer preferences for food alergen labeling. Allergyasthma Clin. Immunol. 2017, 13, 19–30. [Google Scholar]
- Baker, M.G.; Saf, S.; Tsuang, A.; Nowak-Wegrzyn, A. Hidden allergens in food allergy. Ann. Allergyasthma Immunol. 2018, 121, 285–292. [Google Scholar] [CrossRef]
- Jędrusek-Golińska, A.; Zielińska-Dawidziak, M.; Zielińska, P.; Kowalski, R.; Piasecka-Kwiatkowska, D. Analysis of risk and consumers’ awareness regarding the gluten content in meat products on the example of frankfurter type sausages. Qual. Assur. Saf. Crop. Foods 2019, 12, 1–10. [Google Scholar] [CrossRef]
- Miller, L.M.S.; Cassady, D.L. The effects of nutrition knowledge on food label use. A review of the literature. Appetite 2015, 92, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Hieke, S.; Taylor, C.R. A critical review of the literature on nutritional labeling. J. Consum. Aff. 2012, 46, 120–156. [Google Scholar] [CrossRef]
- Lin, C.-T.; Yen, S.T. Knowledge of dietary fats among us consumers. J. Am. Diet. Assoc. 2010, 110, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Wills, J.; Schmidt, D.; Pillo-Blocka, F.; Cairns, G. Exploring global consumer attitudes toward nutrition information on food labels. Nutr. Rev. 2009, 67, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Vagadia, B.H.; Vanga, S.K.; Singh, A.; Gariepy, Y.; Raghavan, V. Comparison of Conventional and Microwave Treatment on Soymilk for Inactivation of Trypsin Inhibitors and In Vitro Protein Digestibility. Foods 2018, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Martinez-Villaluenga, C.; De Meijia, E.G. Quantification of human IgE immunoreactive soybean proteins in commercial soy ingredients and products. J. Food Sci. 2008, 73, 90–99. [Google Scholar] [CrossRef]
- Tsumura, K. Improvement of the physicochemical properties of soybean proteins by enzymatic hydrolysis. Food Sci. Technol. Res. 2009, 15, 381–388. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, G.X.; Sun, Z.W.; Zhang, B.; Wang, T. Stability and immunoreactivity of glycinin and β-conglycinin to hydrolysis in vitro. Food Agric. Immunol. 2010, 21, 253–263. [Google Scholar] [CrossRef]
- Amigo-Benavent, M.; Silván, J.M.; Moreno, F.J.; Villamiel, M.; Del Castillo, M.D. Protein quality, antigenicity, and antioxidant activity of soy-based foodstuffs. J. Agric. Food Chem. 2008, 13, 6498–6505. [Google Scholar] [CrossRef]
- Gomaa, A.; Boye, J.I. Impact of thermal processing time and cookie size on the detection of casein, egg, gluten and soy allergens in food. Food Res. Int. 2013, 52, 483–489. [Google Scholar] [CrossRef]
- Van Boxtel, E.L.; van den Broek, L.A.; Koppelman, S.J.; Gruppen, H. Legumin allergens from peanuts and soybeans: Effects of denaturation and aggregation on allergenicity. Mol. Nutr. Food Res. 2008, 52, 674–682. [Google Scholar] [CrossRef] [PubMed]
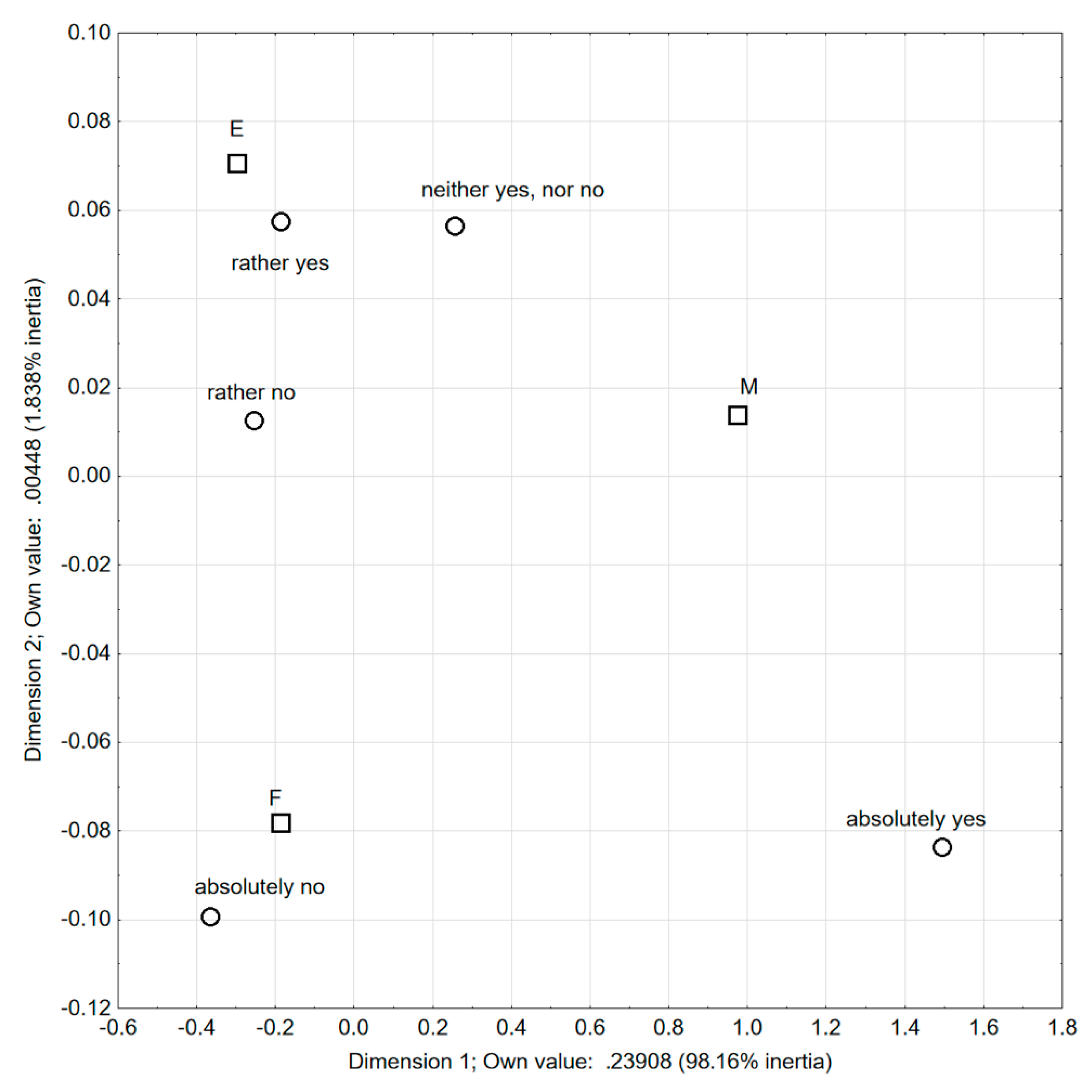
| Factors | Categories of Features | E | M | F | Three Groups Together | p-Value |
|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | |||
| Sex | Women | 37.6 | 34.0 | 24.0 | 32.3 | NS |
| Men | 62.4 | 66.0 | 76.0 | 67.7 | ||
| Age | 23–24 years | 33.0 | 40.0 | 31.0 | 35.0 | NS |
| 25–26 years | 37.0 | 36.0 | 38.0 | 37.0 | ||
| 27–28 years | 30.0 | 24.0 | 31.0 | 28.0 | ||
| Place of residence | Village | 23.7 | 24.0 | 24.0 | 23.9 | NS |
| city < 200.000 citizens | 42.3 | 52.0 | 44.0 | 45.0 | ||
| city ≥ 200.000 citizens | 33.7 | 24.0 | 32.0 | 31.1 | ||
| Food allergy | 0 | 0 | 0 | 0 | NS | |
| Questions | % of Answers | Chi-Square Independence Test p Value | ||||
|---|---|---|---|---|---|---|
| E | M | F | Three Groups Together | |||
| How often do you read the labels of the products you buy? | always | 26.7 | 52.0 | 62.0 | 45.8 | p < 0.001 |
| usually | 55.4 | 44.0 | 38 | 46.2 | ||
| never | 17.9 | 4.0 | 0.0 | 8.0 | ||
| What is the sensitizing ingredient in soy? | protein | 59.4 | 38.0 | 93.0 | 68.5 | p < 0.001 |
| lipid | 2.0 | 0.0 | 0.0 | 0.8 | ||
| carbohydrates | 5.0 | 22.0 | 2.0 | 7.2 | ||
| I do not know | 33.7 | 40.0 | 5.0 | 23.5 | ||
| Processes That Can Reduce Soy’s Immunoreactivity | % of Answers | Chi-Square Independence Test p Value | ||||
|---|---|---|---|---|---|---|
| E | M | F | Three Groups Together | |||
| Hydrolysis | yes | 31.7 | 48 | 62 | 47 | p < 0.001 |
| no | 68.3 | 52 | 38 | 53 | ||
| Thermal treatment | yes | 34.7 | 70 | 48 | 46 | 0.002 |
| no | 65.3 | 30 | 52 | 54 | ||
| Fragmentation | yes | 12.9 | 26 | 7 | 7 | 0.005 |
| no | 87.1 | 74 | 93 | 93 | ||
| Genetic modification | yes | 16.8 | 16 | 32 | 22.7 | 0.017 |
| no | 83.2 | 84 | 68 | 77.3 | ||
| Pre-treatment (soaking) | yes | 2 | 12 | 6 | 5.6 | 0.040 |
| no | 98 | 88 | 94 | 94.4 | ||
| I do not know | yes | 52.5 | 24 | 15 | 31.9 | p < 0.001 |
| no | 47.5 | 76 | 85 | 68.1 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrusek-Golińska, A.; Piasecka-Kwiatkowska, D.; Zielińska, P.; Zielińska-Dawidziak, M.; Szymandera-Buszka, K.; Hęś, M. Soy Preparations Are Potentially Dangerous Factors in the Course of a Food Allergy. Foods 2019, 8, 655. https://doi.org/10.3390/foods8120655
Jędrusek-Golińska A, Piasecka-Kwiatkowska D, Zielińska P, Zielińska-Dawidziak M, Szymandera-Buszka K, Hęś M. Soy Preparations Are Potentially Dangerous Factors in the Course of a Food Allergy. Foods. 2019; 8(12):655. https://doi.org/10.3390/foods8120655
Chicago/Turabian StyleJędrusek-Golińska, Anna, Dorota Piasecka-Kwiatkowska, Paulina Zielińska, Magdalena Zielińska-Dawidziak, Krystyna Szymandera-Buszka, and Marzanna Hęś. 2019. "Soy Preparations Are Potentially Dangerous Factors in the Course of a Food Allergy" Foods 8, no. 12: 655. https://doi.org/10.3390/foods8120655
APA StyleJędrusek-Golińska, A., Piasecka-Kwiatkowska, D., Zielińska, P., Zielińska-Dawidziak, M., Szymandera-Buszka, K., & Hęś, M. (2019). Soy Preparations Are Potentially Dangerous Factors in the Course of a Food Allergy. Foods, 8(12), 655. https://doi.org/10.3390/foods8120655