Prior Exposure to Dry-Cured Meat Promotes Resistance to Simulated Gastric Fluid in Salmonella Typhimurium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Contamination of Brazilian Dry-Cured Loin (BDL) Samples
2.3. Acid Tolerance Response Assessment
2.3.1. Strain Pre-Adaptation
2.3.2. Acid Challenge Trial
2.4. Salmonella Inactivation Modeling in BDL and in Simulated Gastric Fluid (SGF)
2.4.1. Experimental Design
2.4.2. Salmonella Survival in the BDL Matrix and Resistance to SGF
2.4.3. Model Fitting and Performance Evaluation
2.5. Statistical Data Analysis
3. Results and Discussion
3.1. Salmonella Acid Tolerance Response
3.2. Salmonella Typhimurium Inactivation Modelling and Model Performance Evaluation
3.3. Salmonella Typhimurium Inactivation in BDL and SGF
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morales, R.; Guerrero, L.; Aguiar, A.P.S.; Guàrdia, M.D.; Gou, P. Factors affecting dry-cured ham consumer acceptability. Meat Sci. 2013, 95, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Bosse, R.; Müller, A.; Gibis, M.; Weiss, A.; Schmidt, H.; Weiss, J. Recent advances in cured raw ham manufacture. Crit. Rev. Food Sci. Nutr. 2018, 58, 610–630. [Google Scholar] [CrossRef] [PubMed]
- Rosario, D.K.; Bernardo, Y.A.; Mutz, Y.S.; Tiwari, B.; Rajkovic, A.; Bernardes, P.C.; Conte-Junior, C.A. Modelling inactivation of Staphylococcus spp. on sliced Brazilian dry-cured loin with thermosonication and peracetic acid combined treatment. Int. J. Food Microbiol. 2019, 309, 108328. [Google Scholar] [CrossRef] [PubMed]
- Flores, J. Mediterranean vs. northern European meat products. Processing technologies and main differences. Food Chem. 1997, 59, 505–510. [Google Scholar] [CrossRef]
- Toldrá, F. The role of muscle enzymes in dry-cured meat products with different drying conditions. Trends Food Sci. Technol. 2006, 17, 164–168. [Google Scholar] [CrossRef]
- Barbuti, S.; Parolari, G. Validation of manufacturing process to control pathogenic bacteria in typical dry fermented products. Meat Sci. 2002, 62, 323–329. [Google Scholar] [CrossRef]
- Andreoli, G.; Merla, C.; Valle, C.D.; Corpus, F.; Morganti, M.; D’incau, M.; Carra, E. Foodborne Salmonellosis in Italy: Characterization of Salmonella enterica serovar Typhimurium and monophasic variant 4,[5], 12: I−Isolated from salami and human patients. J. Food Prot. 2017, 80, 632–639. [Google Scholar] [CrossRef]
- Gossner, C.M.; Van Cauteren, D.; Le Hello, S.; Weill, F.X.; Terrien, E.; Tessier, S.; Janin, C.; Brisabois, A.; Dusch, V.; Vaillant, V. Nationwide outbreak of Salmonella enterica serotype2012, 4,[5], 12: I:-infection associated with consumption of dried pork sausage, France, November to December. Eur. Surveill. 2011, 15, 19592. [Google Scholar]
- Omer, M.K.; Álvarez-Ordoñez, A.; Prieto, M.; Skjerve, E.; Asehun, T.; Alvseike, O.A. A systematic review of bacterial foodborne outbreaks related to red meat and meat products. Foodborne Pathog. Dis. 2018, 15, 598–611. [Google Scholar] [CrossRef]
- Scavia, G.; Ciaravino, G.; Luzzi, I.; Lenglet, A.; Ricci, A.; Barco, L.; Dionisi, A.M. A multistate epidemic outbreak of Salmonella Goldcoast infection in humans, June 2009 to March 2010: The investigation in Italy. Eurosurveillance 2013, 18, 20424. [Google Scholar] [CrossRef]
- CDC (Center for Disease Control and Prevention). Surveillance for foodborne disease outbreaks United States, 2016: Annual Report; CDC: Atlanta, GA, USA, 2016. Available online: https://www.cdc.gov/fdoss/pdf/2016_FoodBorneOutbreaks_508.pdf (accessed on 24 February 2019).
- EFSA (European Food Safety Agency); ECDC (European Centre for Disease Prevention and Control). The European Union summary report on trends of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2016, 14, 4634. [Google Scholar]
- Cabral, C.C.; Panzenhagen, P.H.N.; Delgado, K.F.; Silva, G.R.A.; Dos Prazeres Rodrigues, D.; Franco, R.M.; Conte, C.A. Contamination of carcasses and utensils in small swine slaughterhouses by Salmonella in the northwestern region of the state of Rio de Janeiro, Brazil. J. Food Prot. 2017, 80, 1128–1132. [Google Scholar] [CrossRef]
- Bonardi, S.; Bruini, I.; Bolzoni, L.; Cozzolino, P.; Pierantoni, M.; Brindani, F.; Bellotti, P.; Renzi, M.; Pongolini, S. Assessment of Salmonella survival in dry-cured Italian salami. Int. J. Food Microbiol. 2017, 262, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Bover-Cid, S.; Belletti, N.; Garriga, M.; Aymerich, T. Response surface methodology to investigate the effect of high pressure processing on Salmonella inactivation on dry-cured ham. Food Res. Int. 2012, 45, 1111–1117. [Google Scholar] [CrossRef]
- Leistner, L. Basic aspects of food preservation by hurdle technology. Int. J. Food Microbiol. 2000, 55, 181–186. [Google Scholar] [CrossRef]
- Foster, J.W.; Hall, H.K. Adaptive acidification tolerance response of Salmonella Typhimurium. J. Bacteriol. 1990, 172, 771–778. [Google Scholar] [CrossRef]
- Gruzdev, N.; Pinto, R.; Sela, S. Effect of desiccation on tolerance of Salmonella enterica to multiple stresses. Appl. Environ. Microbiol. 2011, 77, 1667–1673. [Google Scholar] [CrossRef]
- Sadeghi-Mehr, A.; Lautenschlaeger, R.; Drusch, S. Behavior of Salmonella spp. and Listeria monocytogenes throughout the manufacture and shelf-life of dry-cured formed ham. Food Control 2016, 64, 22–28. [Google Scholar] [CrossRef]
- Fàbrega, A.; Vila, J. Salmonella enterica serovar Typhimurium skills to succeed in the host: Virulence and regulation. Clin. Microbiol. Rev. 2013, 26, 308–341. [Google Scholar] [CrossRef]
- Audia, J.P.; Webb, C.C.; Foster, J.W. Breaking through the acid barrier: An orchestrated response to proton stress by enteric bacteria. Int. J. Med. Microbiol. 2001, 291, 97–106. [Google Scholar] [CrossRef]
- Coroller, L.; Jeuge, S.; Couvert, O.; Christieans, S.; Ellouze, M. Extending the gamma concept to non-thermal inactivation: A dynamic model to predict the fate of Salmonella during the dried sausages process. Food Microbiol. 2015, 45, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Beumer, R.R.; De Vries, J.; Rombouts, F.M. Campylobacter jejuni non-culturable coccoid cells. Int. J. Food Microbiol. 1992, 15, 153–163. [Google Scholar] [CrossRef]
- Samara, A.; Koutsoumanis, K.P. Effect of treating lettuce surfaces with acidulants on the behaviour of Listeria monocytogenes during storage at 5 and 20 °C and subsequent exposure to simulated gastric fluid. Int. J. food Microbiol. 2009, 129, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Khoo, W.J.; Zheng, Q.; Chung, H.J.; Yuk, H.G. Growth temperature alters Salmonella Enteritidis heat/acid resistance, membrane lipid composition and stress/virulence related gene expression. Int. J. Food Microbiol. 2014, 172, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Baranyi, J.; Pin, C.; Ross, T. Validating and comparing predictive models. Int. J. Food Microbiol. 1999, 48, 159–166. [Google Scholar] [CrossRef]
- Álvarez-Ordóñez, A.; Prieto, M.; Bernardo, A.; Hill, C.; López, M. The acid tolerance response of Salmonella spp.: An adaptive strategy to survive in stressful environments prevailing in foods and the host. Food Res. Int. 2012, 45, 482–492. [Google Scholar] [CrossRef]
- Lee, I.S.; Slonczewski, J.L.; Foster, J.W. A low-pH-inducible, stationary-phase acid tolerance response in Salmonella Typhimurium. J. Bacteriol. 1994, 176, 1422–1426. [Google Scholar] [CrossRef]
- Lianou, A.; Koutsoumanis, K.P. Evaluation of the strain variability of Salmonella enterica acid and heat resistance. Food Microbiol. 2013, 34, 259–267. [Google Scholar] [CrossRef]
- Fong, K.; Wang, S. Heat resistance of Salmonella enterica is increased by pre-adaptation to peanut oil or sub-lethal heat exposure. Food Microbiol. 2016, 58, 139–147. [Google Scholar] [CrossRef]
- Ibanez-Ruiz, M.; Robbe-Saule, V.; Hermant, D.; Labrude, S.; Norel, F. Identification of RpoS (sigma(S))-regulated genes in Salmonella enterica serovar Typhimurium. J. Bacteriol. 2000, 182, 5749–5756. [Google Scholar] [CrossRef]
- McMeechan, A.; Roberts, M.; Cogan, T.A.; Jørgensen, F.; Stevenson, A.; Lewis, C.; Rowley, G.; Humphrey, T.J. Role of the alternative sigma factors σE and σS in survival of Salmonella enterica serovar Typhimurium during starvation, refrigeration and osmotic shock. Microbiology 2007, 153, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Mutz, Y.S.; Rosario, D.K.A.; Paschoalin, V.M.F.; Conte-Junior, C.A. Salmonella enterica: A hidden risk for dry-cured meat consumption? Crit. Rev. Food Sci. Nutr. 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lianou, A.; Nychas, G.J.E.; Koutsoumanis, K.P. Variability in the adaptive acid tolerance response phenotype of Salmonella enterica strains. Food Microbiol. 2017, 62, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ross, T. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Bacteriol. 1996, 81, 501–508. [Google Scholar] [CrossRef]
- Vignolo, G.; Fontana, C.; Fadda, S. Semidry and dry fermented sausages. Handb. Meat Process. 2010, 21, 379–398. [Google Scholar] [CrossRef]
- Granato, D.; de Araújo Calado, V.M.; Jarvis, B. Observations on the use of statistical methods in food science and technology. Food Res. Int. 2014, 55, 137–149. [Google Scholar] [CrossRef]
- Smith, E.P.; Rose, K.A. Model godness-of-fit analysis using regression and related techniques. Ecol. Model. 1985, 77, 49–64. [Google Scholar] [CrossRef]
- Giffel, M.C.; Zwietering, M.H. Validation of predictive models describing the growth of Listeria monocytogenes. Int. J. Food Microbiol. 1999, 46, 135–149. [Google Scholar] [CrossRef]
- Ross, T.; Dalgaard, P.; Tienungoon, S. Predictive modelling of the growth and survival of Listeria in fishery products. Int. J. Food Microbiol. 2000, 62, 231–245. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Kendall, P.A.; Sofos, J.N. Modeling the boundaries of growth of Salmonella Typhimurium in broth as a function of temperature, water activity and pH. J. Food Prot. 2004, 67, 53–59. [Google Scholar] [CrossRef]
- Hwang, C.A.; Porto-Fett, A.C.S.; Juneja, V.K.; Ingham, S.C.; Ingham, B.H.; Luchansky, J.B. Modeling the survival of Escherichia coli O157:H7, Listeria monocytogenes and Salmonella Typhimurium during fermentation, drying, and storage of soudjouk-style fermented sausage. Int. J. Food Microbiol. 2009, 129, 244–252. [Google Scholar] [CrossRef]
- Stollewerk, K.; Jofré, A.; Comaposada, J.; Arnau, J.; Garriga, M. NaCl-free processing, acidification, smoking and high pressure: Effects on growth of Listeria monocytogenes and Salmonella enterica in QDS processed® dry-cured ham. Food Control 2014, 35, 56–64. [Google Scholar] [CrossRef]
- Corkrey, R.; Olley, J.; Ratkowsky, D.; McMeekin, T.; Ross, T. Universality of thermodynamic constants governing biological growth rates. PLoS ONE 2012, 7, e32003. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC). Regulation (EC) 853/2004 of the European Parliament and of the Council of 29 April 2004, laying down specific hygiene rules for on the hygiene of foodstuffs. Off. J. Eur. Union L 2004, 139, 59. [Google Scholar]
- Ana, A.S.S.; Barbosa, M.S.; Destro, M.T.; Landgraf, M.; Franco, B.D. Growth potential of Salmonella spp. and Listeria monocytogenes in nine types of ready-to-eat vegetables stored at variable temperature conditions during shelf-life. Int. J. food Microbial. 2012, 157, 52–58. [Google Scholar]
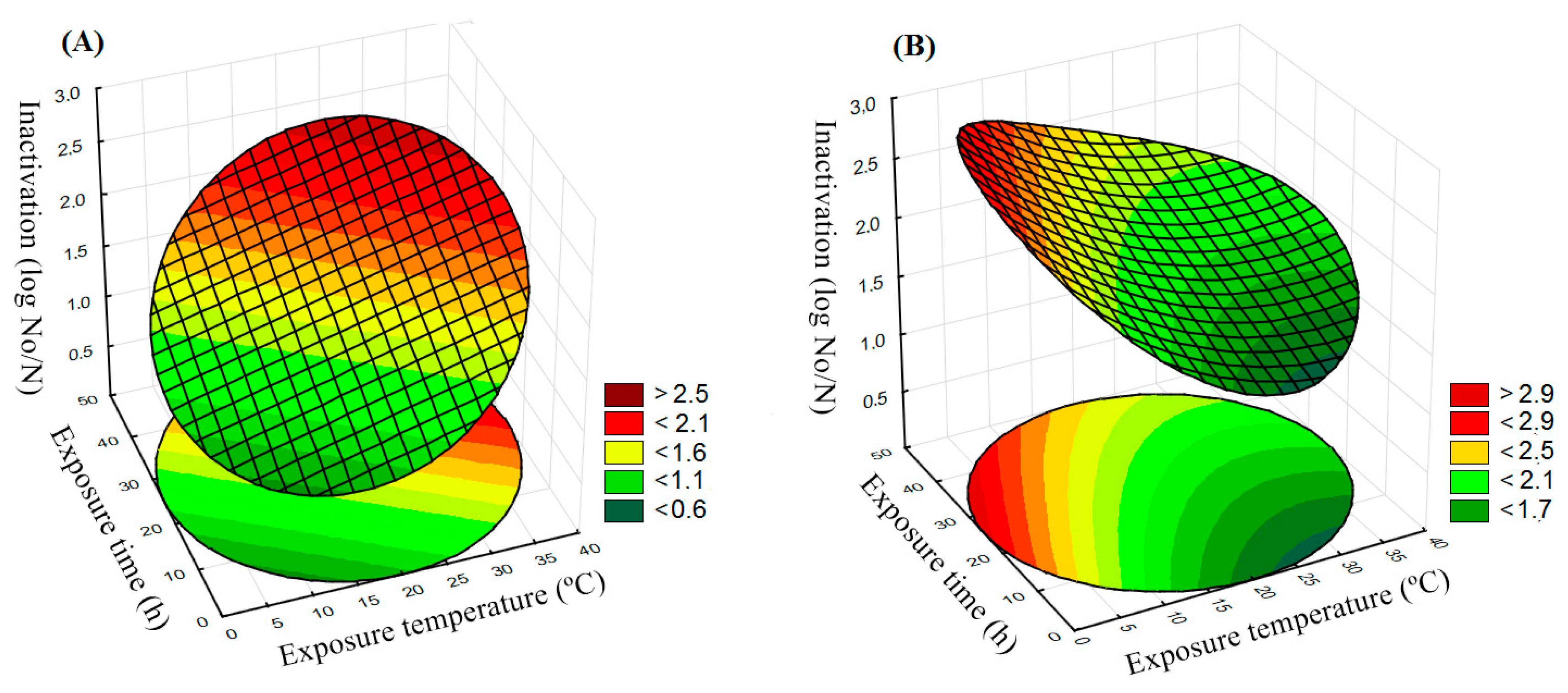
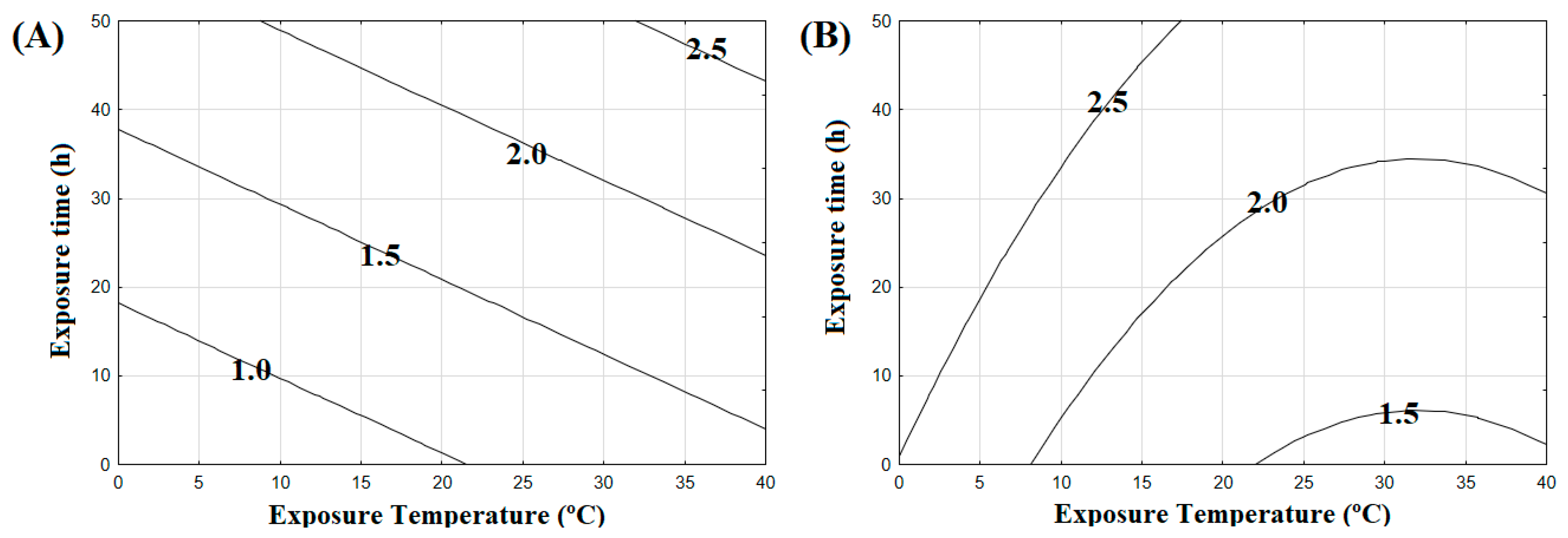
| Run | Exposure Temperature (°C) | Exposure Time (h) | Inactivation in BDL (log N0/N) | Inactivation in SGF (log N0/N) |
|---|---|---|---|---|
| 1 | 10.0 | 7.1 | 1.08 | 1.92 |
| 2 | 10.0 | 41.0 | 1.63 | 2.78 |
| 3 | 33.0 | 7.1 | 1.36 | 1.58 |
| 4 | 33.0 | 41.0 | 2.09 | 2.09 |
| 5 | 5.7 | 24.0 | 1.21 | 2.53 |
| 6 | 38.2 | 24.0 | 2.08 | 1.84 |
| 7 | 22.0 | 0.0 | 1.03 | 1.50 |
| 8 | 22.0 | 48.0 | 2.57 | 2.22 |
| 9 | 22.0 | 24.0 | 1.57 | 2.01 |
| 10 | 22.0 | 24.0 | 1.54 | 1.91 |
| 11 | 22.0 | 24.0 | 1.65 | 1.93 |
| Adaptation | Strain | Decimal Reduction |
|---|---|---|
| Non-adapted cells (NA) | Panama | 1.55 ± 0.09 a |
| Derby | 1.40 ± 0.11 a,b | |
| Typhimurium | 1.31 ± 0.08 b | |
| Meat-stressed cells (MS) | Panama | 0.96 ± 0.09 c |
| Derby | 0.77 ± 0.20 c,d | |
| Typhimurium | 0.77 ± 0.26 c,d | |
| Acid-adapted cells (AA) | Panama | 0.67 ± 0.09 d |
| Derby | 0.11 ± 0.02 e | |
| Typhimurium | 0.03 ± 0.02 e |
| Inactivation Model | Distribution Normality * | Residual Distribution Normality * | R2adj | Mse | Lack-of-Fit Test | Af | Bf |
|---|---|---|---|---|---|---|---|
| Inactivation in BDL | Normal (0.48) | Normal (0.47) | 0.87 | 0.021 | 0.08 | 1.12 | 1.03 |
| Inactivation in SGF | Normal (0.50) | Normal (0.55) | 0.93 | 0.006 | 0.20 | 1.10 | 1.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutz, Y.S.; Rosario, D.K.A.; Castro, V.S.; Bernardes, P.C.; Paschoalin, V.M.F.; Conte-Junior, C.A. Prior Exposure to Dry-Cured Meat Promotes Resistance to Simulated Gastric Fluid in Salmonella Typhimurium. Foods 2019, 8, 603. https://doi.org/10.3390/foods8120603
Mutz YS, Rosario DKA, Castro VS, Bernardes PC, Paschoalin VMF, Conte-Junior CA. Prior Exposure to Dry-Cured Meat Promotes Resistance to Simulated Gastric Fluid in Salmonella Typhimurium. Foods. 2019; 8(12):603. https://doi.org/10.3390/foods8120603
Chicago/Turabian StyleMutz, Yhan S., Denes K. A. Rosario, Vinicius S. Castro, Patricia C. Bernardes, Vania M. F. Paschoalin, and Carlos A. Conte-Junior. 2019. "Prior Exposure to Dry-Cured Meat Promotes Resistance to Simulated Gastric Fluid in Salmonella Typhimurium" Foods 8, no. 12: 603. https://doi.org/10.3390/foods8120603
APA StyleMutz, Y. S., Rosario, D. K. A., Castro, V. S., Bernardes, P. C., Paschoalin, V. M. F., & Conte-Junior, C. A. (2019). Prior Exposure to Dry-Cured Meat Promotes Resistance to Simulated Gastric Fluid in Salmonella Typhimurium. Foods, 8(12), 603. https://doi.org/10.3390/foods8120603








