Comparison of Conventional and Sustainable Lipid Extraction Methods for the Production of Oil and Protein Isolate from Edible Insect Meal
Abstract
:1. Introduction
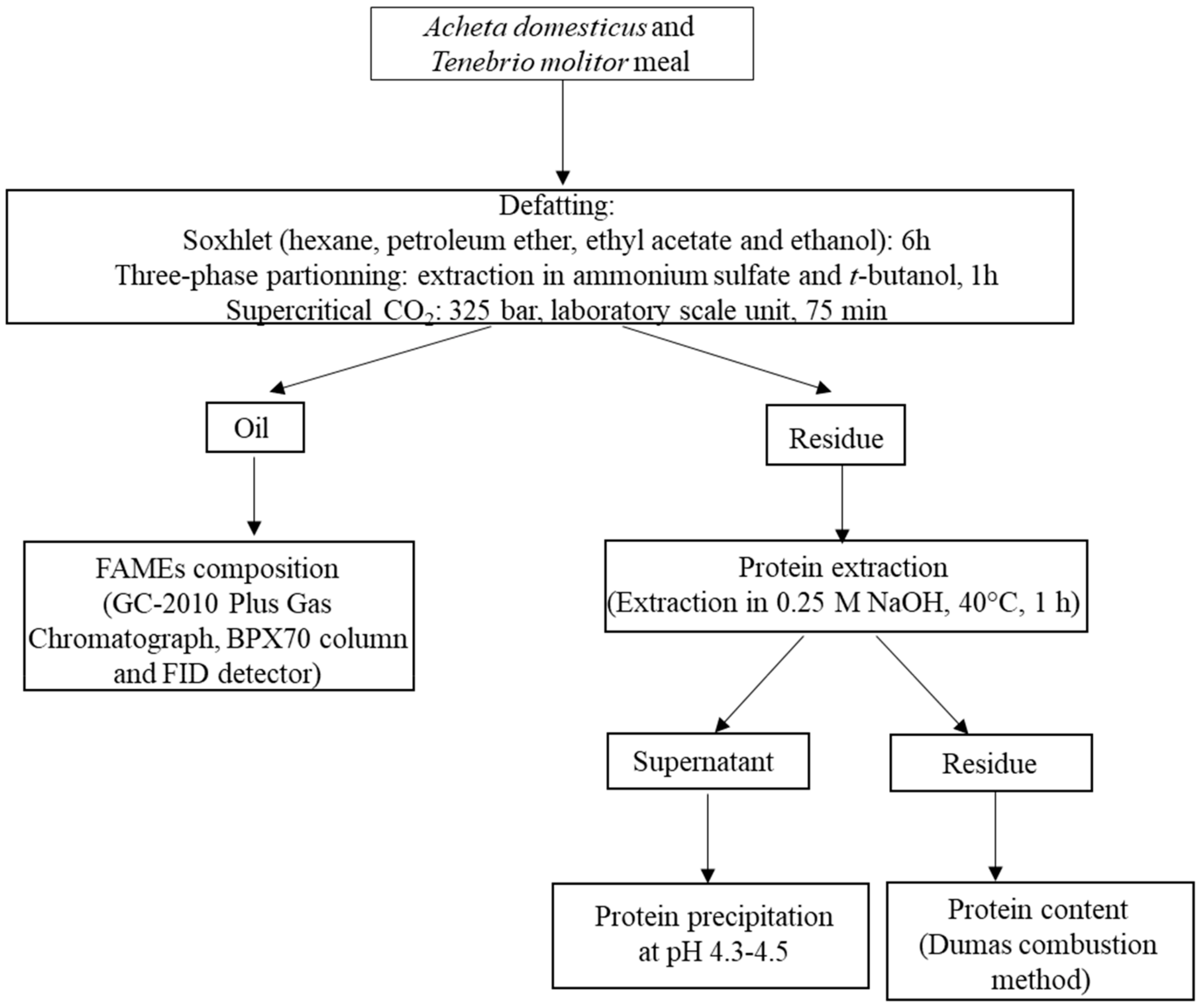
2. Materials and Methods
2.1. Insects
2.2. Lipid Extraction Methods
2.2.1. Soxhlet Method
2.2.2. Three-Phase Partitioning Method
2.2.3. Supercritical Carbon Dioxide
2.3. Characterization of Fatty Acid Methyl Esters (FAMEs) by Gas Chromatography
2.4. Protein Extraction
2.5. Extraction Yields
2.6. Statistical Analysis
3. Results and Discussion
3.1. Lipid Extraction Yield and Fatty Acid Composition
3.2. Protein Extraction and Purity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huis, A.V.; Itterbeeck, J.V.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects, Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; p. 187. Available online: http://www.fao.org/3/i3253e/i3253e.pdf (accessed on 30 September 2019).
- Smetana, S.; Palanisamy, M.; Mathys, A.; Heinz, V. Sustainability of insect use for feed and food: Life Cycle Assessment perspective. J. Clean. Prod. 2016, 137, 741–751. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; De Boer, I.J.M. Environmental Impact of the Production of Mealworms as a Protein Source for Humans – A Life Cycle Assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.X.; Chong, G.H.; Hamzah, H.; Ghazali, H.M.; Xuan, T.C.; Hean, C.G. Comparison of subcritical CO2 and ultrasound-assisted aqueous methods with the conventional solvent method in the extraction of avocado oil. J. Supercrit. Fluids 2018, 135, 45–51. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Yi, L.; Van Valenberg, H.J.; Van Boekel, M.A.; Lakemond, C.M. Insect lipid profile: Aqueous versus organic solvent-based extraction methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.; Sagis, L.M.; Eisner-Schadler, V.; Van Huis, A.; Van Boekel, M.A. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Megido, R.C.; Gierts, C.; Blecker, C.; Brostaux, Y.; Haubruge, E.; Alabi, T.; Francis, F. Consumer acceptance of insect-based alternative meat products in Western countries. Food Qual. Prefer. 2016, 52, 237–243. [Google Scholar] [CrossRef]
- Bußler, S.; Rumpold, B.A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Recovery and techno-functionality of flours and proteins from two edible insect species: Meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon 2016, 2, e00218. [Google Scholar] [CrossRef]
- Schosler, H.; de Boer, J.; Boersema, J.J. Can we cut out the meat of the dish? Constructing consumer-oriented pathways towards meat substitution. Appetite 2012, 58, 39–47. [Google Scholar] [CrossRef]
- Panadare, D.; Rathod, V. Three phase partitioning for extraction of oil: A review. Trends Food Sci. Technol. 2017, 68, 145–151. [Google Scholar] [CrossRef]
- Dutta, R.; Sarkar, U.; Mukherjee, A. Process optimization for the extraction of oil from Crotalaria juncea using three phase partitioning. Ind. Crop. Prod. 2015, 71, 89–96. [Google Scholar] [CrossRef]
- Zhao, X.; Vázquez-Gutiérrez, J.L.; Johansson, D.P.; Landberg, R.; Langton, M. Yellow Mealworm Protein for Food Purposes - Extraction and Functional Properties. PLoS ONE 2016, 11, 0147791. [Google Scholar] [CrossRef] [PubMed]
- Purschke, B.; Stegmann, T.; Schreiner, M.; Jager, H. Pilot-scale supercritical CO2 extraction of edible insect oil from Tenebrio molitor L. larvae—Influence of extraction conditions on kinetics, defatting performance and compositional properties. Eur. J. Lipid Sc. Tech. 2017, 119, 1600134. [Google Scholar] [CrossRef]
- Simonne, A.; Simonne, E.; Eitenmiller, R.; Mills, H.; Cresman III, C. Could the dumas method replace the Kjeldahl digestion for nitrogen and crude protein. J. Sci. Food Agric. 1997, 73, 39–45. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.P.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Horowitz, W., Ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2008. [Google Scholar]
- Spinelli, J.; Lehman, L.; Wieg, D. Composition, processing, and utilization of red crab (Pleuroncodes planipes) as an aquacultural feed ingredient. J. Fish. Res. Board Can. 1974, 31, 1025–1029. [Google Scholar] [CrossRef]
- Azagoh, C.; Ducept, F.; Garcia, R.; Rakotozafy, L.; Cuvelier, M.-E.; Keller, S.; Lewandowski, R.; Mezdour, S. Extraction and physicochemical characterization of Tenebrio molitor proteins. Food Res. Int. 2016, 88, 24–31. [Google Scholar] [CrossRef]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Mariod, A.A.; Mirghani, M.E.S.; Hussein, I.H. Unconventional Oilseeds and Oil Sources, 1st ed.; Elsevier Associated Press: London, UK, 2017; p. 382. [Google Scholar]
- Rudyk, S.; Spirov, P.; Hussain, S. Effect of co-solvents on SC-CO2 extraction of crude oil by consistency test. J. Supercrit. Fluid. 2014, 91, 15–23. [Google Scholar] [CrossRef]
- Murali, H.S.; Mohan, M.S.; Manja, K.S.; Sankaran, R. Polar and nonpolar lipids and their fatty acid composition of a few Fusarium species. J. Am. Oil Chem. Soc. 1993, 70, 1039–1041. [Google Scholar] [CrossRef]
- Japir, A.A.-W.; Salimon, J.; Derawi, D.; Yahaya, B.H.; Bahadi, M.; Al-Shuja’a, S.; Yusop, M.R. A highly efficient separation and physicochemical characteristics of saturated fatty acids from crude palm oil fatty acids mixture using methanol crystallisation method. OCL J. 2018, 25, A203. [Google Scholar] [CrossRef]
- Meullemiestre, A.; Breil, C.; Abert-Vian, M.; Chemat, F. Modern Techniques and Solvents for the Extraction of Microbial Oils; Springer: New York, NY, USA, 2015; p. 52. [Google Scholar]
- Weihrauch, D.; Donini, A.; O’Donnell, M.J. Ammonia transport by terrestrial and aquatic insects. J. Insect Physiol. 2012, 58, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Finke, M.D. Estimate of chitin in raw whole insects. Zoo Biol. 2007, 26, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.J.; Hopkins, T.L.; Schaefer, J. Applications of solids NMR to the analysis of insect sclerotized structures. Insect Biochem. Mol. Biol. 1995, 25, 1067–1080. [Google Scholar] [CrossRef]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribereau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Perreault, V.; Henaux, L.; Bazinet, L.; Doyen, A. Pretreatment of flaxseed protein isolate by high hydrostatic pressure: Impacts on protein structure, enzymatic hydrolysis and final hydrolysate antioxidant capacities. Food Chem. 2017, 221, 1805–1812. [Google Scholar] [CrossRef]
| Insect Meal | Protein | Ash | Chitin | Other (Sugar, Lipid, etc.) |
|---|---|---|---|---|
| % w/w (dry basis) | ||||
| A. domesticus | 53.5 ± 0.3 | 5.5 ± 0.1 | 5.9 ± 0.6 | 35.1 ± 0.6 |
| T. molitor | 45.7 ± 0.02 | 4.3 ± 0.1 | 7.2 ± 0.5 | 42.9 ± 0.6 |
| Insect Meal | Defatting Method | Extracted Fat (% w/w of Sample Mass) |
|---|---|---|
| A. domesticus | Soxhlet (Hexane) | 14.6 ± 0.1 c |
| Soxhlet (Petroleum ether) | 14.7 ± 0.2 c | |
| Soxhlet (Ethyl acetate) | 15.1 ± 0.3 b | |
| Soxhlet (Ethanol) | 22.7 ± 2.9 a | |
| TPP | 19.3 ± 2.0 ab | |
| SC-CO2 | 11.9 ± 1.4 c | |
| T. molitor | Soxhlet (Hexane) | 25.5 ± 0.1 a |
| Soxhlet (Petroleum ether) | 24.3 ± 1.2 a | |
| Soxhlet (Ethyl acetate) | 25.7 ± 0.3 a | |
| Soxhlet (Ethanol) | 28.8 ± 5.9 a | |
| TPP | 23.7 ± 2.4 a | |
| SC-CO2 | 22.1 ± 0.6 a |
| Fatty Acid | Relative Abundance (u.a.) | ||||||
|---|---|---|---|---|---|---|---|
| SH | SP | SA | SE | TPP | SC-CO2 | ||
| Saturated | C14:0 | 0.81 ± 0.03 b | 0.83 ± 0.04 b | 0.81 ± 0.05 b | 0.69 ± 0.03 c | 0.60 ± 0.10 c | 0.95 ± 0.03 a |
| C16:0 | 27.70 ± 1.00 a,b | 27.50 ± 0.30 a,b | 26.90 ± 1.10 a,b | 24.60 ± 0.70 b | 25.10 ± 2.40 b | 29.60 ± 0.30 a | |
| C17:0 | 0.29 ± 0.01 a | 0.25 ± 0.02 a | 0.24 ± 0.03 a,b | 0.21 ± 0.01 a,b | 0.10 ± 0.10 b | 0.22 ± 0.00 a,b | |
| C18:0 | 10.30 ± 0.20 a | 10.14 ± 0.03 a,b | 10.00 ± 0.30 a,b | 9.60 ± 0.40 b | 10.50 ± 0.10 a | 9.92 ± 0.08 a,b | |
| C20:0 | 0.60 ± 0.01 a | 0.57 ± 0.03 a,b | 0.57 ± 0.02 a,b | 0.46 ± 0.04 c | 0.43 ± 0.06 c | 0.50 ± 0.02 b,c | |
| MUFA | C16:1 | 1.17 ± 0.01 a | 1.14 ± 0.03 a | 1.12 ± 0.00 a,b | 1.10 ± 0.03 a,b | 1.02 ± 0.08 b | 1.22 ± 0.05 a |
| C17:1 | - | - | - | - | - | - | |
| C18:1T | 0.15 ± 0.01 a | 0.15 ± 0.01 a | 0.14 ± 0.01 a | 0.12 ± 0.00 b | 0.12 ± 0.01 b | 0.11 ± 0.00 b | |
| C18:1P | 0.20 ± 0.01 b,c | 0.19 ± 0.03 b,c | 0.18 ± 0.02 c | 0.16 ± 0.02 c | 0.40 ± 0.10 a,b | 0.52 ± 0.08 a | |
| C18:1V | 21.40 ± 0.70 a,b | 21.10 ± 0.50 a,b | 20.40 ± 0.40 b | 19.50 ± 0.40 b | 20.30 ± 1.30 b | 22.50 ± 0.40 a | |
| C18:1 | 0.58 ± 0.02 a | 0.54 ± 0.04 a,b | 0.54 ± 0.04 a,b | 0.50 ± 0.01 a,b | 0.47 ± 0.06 b | 0.60 ± 0.02 a | |
| C20:1 | - | - | - | - | 0.10 ± 0.00 b | 0.11 ± 0.00 a | |
| PUFA | C18:2 | 30.10 ± 1.10 b,c | 29.50 ± 0.50 c | 29.30 ± 0.30 c | 33.00 ± 0.70 a,b | 33.60 ± 2.20 a | 27.40 ± 0.50 c |
| C18:3 | 1.52 ± 0.01 a | 1.48 ± 0.05 a,b | 1.46 ± 0.03 a,b | 1.47 ± 0.05 a,b | 1.39 ± 0.08 b | 1.48 ± 0.03 a.b | |
| C20:3 | 0.29 ± 0.01 b,c | 0.28 ± 0.02 c | 0.30 ± 0.00 b,c | 0.43 ± 0.04 a | 0.35 ± 0.04 b | 0.15 ± 0.01 d | |
| C22:6 | 0.14 ± 0.01 b | 0.14 ± 0.01 b | 0.15 ± 0.02 b | 0.15 ± 0.01 b | 0.17 ± 0.01 a,b | 0.19 ± 0.01 a | |
| Fatty Acid | Relative Abundance (u.a.) | ||||||
|---|---|---|---|---|---|---|---|
| SH | SP | SA | SE | TPP | SC-CO2 | ||
| Saturated | C14:0 | 1.65 ± 0.05 b | 1.64 ± 0.02 b | 1.60 ± 0.10 b | 1.67 ± 0.00 b | 1.58 ± 0.01 b | 1.72 ± 0.04 a |
| C16:0 | 18.74 ± 0.00 a,b | 18.77 ± 0.03 a,b | 18.67 ± 0.05 a,b | 17.78 ± 0.05 c | 18.20 ± 0.40 b,c | 19.10 ± 0.20 a | |
| C17:0 | 0.42 ± 0.02 a | 0.40 ± 0.02 a | 0.41 ± 0.00 a | 0.41 ± 0.01 a | 0.44 ± 0.01 a | 0.41 ± 0.00 a | |
| C18:0 | 2.30 ± 0.01c | 2.30 ± 0.01 c | 2.33 ± 0.01 b,c | 2.80 ± 0.08 a | 2.62 ± 0.08 a,b | 2.20 ± 0.20 c | |
| MUFA | C16:1 | 0.93 ± 0.00 a | 0.94 ± 0.02 a | 0.96 ± 0.00 a | 0.95 ± 0.05 a | 0.93 ± 0.03 a | 0.97 ± 0.02 a |
| C17:1 | 0.13 ± 0.00 a | 0.13 ± 0.01 a | - | - | 0.11 ± 0.00 a | 0.12 ± 0.00 a | |
| C18:1V | 39.74 ± 0.00 a | 39.74 ± 0.07 a | 39.70 ± 0.03 a | 36.94 ± 0.02 b | 38.10 ± 0.60 b | 39.80 ± 0.30 a | |
| C18:1 | 0.31 ± 0.01 a | 0.29 ± 0.00 a | 0.31 ± 0.01 a | 0.30 ± 0.02 a | 0.30 ± 0.01 a | 0.29 ± 0.01 a | |
| PUFA | C18:2 | 33.76 ± 0.06 c | 33.70 ± 0.05 c | 33.80 ± 0.09 c | 37.00 ± 0.10 a | 34.70 ± 0.50 b | 33.40 ± 0.10 c |
| C18:3 | 1.25 ± 0.01 a | 1.24 ± 0.01 a | 1.28 ± 0.01 a | 1.30 ± 0.08 a | 1.21 ± 0.02 a | 1.27 ± 0.01 a | |
| Insect Meal | Defatting Method | Protein Extraction Yield (%) | Protein Purity (%) |
|---|---|---|---|
| A. domesticus | Whole meal (without defatting) | 38.9 ± 1.7 a | 58.3 ± 0.5 d |
| Soxhlet (Hexane) | 32.4 ± 3.8 ab | 74.7 ± 0.3 ab | |
| Soxhlet (Petroleum ether) | 33.1 ± 1.0 ab | 74.3 ± 2.0 b | |
| Soxhlet (Ethyl acetate) | 31.6 ± 3.0 b | 74.4 ± 1.2 b | |
| Soxhlet (Ethanol) | 31.0 ± 4.0 b | 78.5 ± 2.0 a | |
| TPP | ND | ND | |
| SC-CO2 | 33.7 ± 1.1 ab | 70.1 ± 1.9 c | |
| T. molitor | Whole meal (without defatting) | 39.3 ± 0.8 a | 48.7 ± 0.1 b |
| Soxhlet (Hexane) | 33.7 ± 1.6 b | 74.0 ± 2.2 a | |
| Soxhlet (Petroleum ether) | 33.5 ± 1.2 b | 72.7 ± 1.5 a | |
| Soxhlet (Ethyl acetate) | 33.2 ± 0.6 b | 75.4 ± 0.5 a | |
| Soxhlet (Ethanol) | 33.9 ± 3.7 b | 75.3 ± 0.8 a | |
| TPP | ND | ND | |
| SC-CO2 | 36.4 ± 1.5 ab | 72.7 ± 4.1 a |
| Protein Source | Extraction Method | Protein Extraction Yield (%) | Protein Purity (%) | Reference |
|---|---|---|---|---|
| A. domesticus1 | Alkaline solubilization and isoelectric precipitation | 31.0–38.9 | 58.3–78.5 | |
| T. molitor1 | Alkaline solubilization and isoelectric precipitation | 33.2–39.3 | 48.7–75.4 | |
| Pea | Alkali extraction-isoelectric precipitation | 62.6–76.7 | 83.3–86.9 | [29] |
| Salt extraction | 68.2–74.8 | 71.5–79.3 | ||
| Micellar precipitation | 30.7–31.1 | 81.9–87.8 | ||
| Pea | Isoelectric precipitation | 55.0 | 81.7 | [30] |
| Ultrafiltration | 57.1 | 83.9 | ||
| Lentil | Isoelectric precipitation | 50.3–62.8 | 78.2–79.1 | |
| Ultrafiltration | 51.9–60.5 | 82.7–88.6 | ||
| Chickpea | Isoelectric precipitation | 53.7–69.1 | 63.9–73.6 | |
| Ultrafiltration | 50.3–54.7 | 68.5–76.5 | ||
| Flaxseed | Hydrolysis with cellulase followed by isoelectric precipitation | ND | 82 | [31] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laroche, M.; Perreault, V.; Marciniak, A.; Gravel, A.; Chamberland, J.; Doyen, A. Comparison of Conventional and Sustainable Lipid Extraction Methods for the Production of Oil and Protein Isolate from Edible Insect Meal. Foods 2019, 8, 572. https://doi.org/10.3390/foods8110572
Laroche M, Perreault V, Marciniak A, Gravel A, Chamberland J, Doyen A. Comparison of Conventional and Sustainable Lipid Extraction Methods for the Production of Oil and Protein Isolate from Edible Insect Meal. Foods. 2019; 8(11):572. https://doi.org/10.3390/foods8110572
Chicago/Turabian StyleLaroche, Myriam, Véronique Perreault, Alice Marciniak, Alexia Gravel, Julien Chamberland, and Alain Doyen. 2019. "Comparison of Conventional and Sustainable Lipid Extraction Methods for the Production of Oil and Protein Isolate from Edible Insect Meal" Foods 8, no. 11: 572. https://doi.org/10.3390/foods8110572
APA StyleLaroche, M., Perreault, V., Marciniak, A., Gravel, A., Chamberland, J., & Doyen, A. (2019). Comparison of Conventional and Sustainable Lipid Extraction Methods for the Production of Oil and Protein Isolate from Edible Insect Meal. Foods, 8(11), 572. https://doi.org/10.3390/foods8110572






