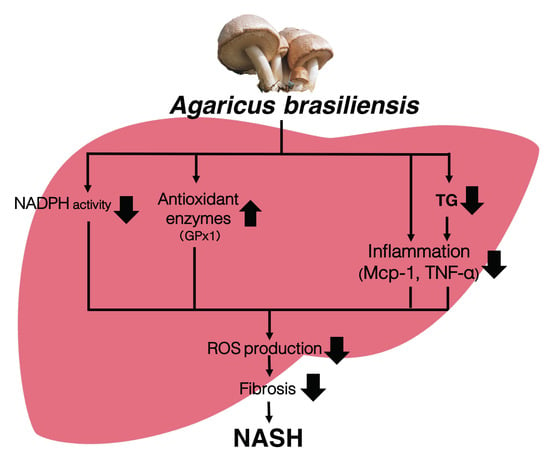
Agaricus brasiliensis KA21 May Prevent Diet-Induced Nash Through Its Antioxidant, Anti-Inflammatory, and Anti-Fibrotic Activities in the Liver
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Studies
2.3. mRNA Expression Analysis by Quantitative RT-PCR
2.4. Blood Chemistry and Metabolite Analysis
2.5. Liver and Feces Lipid Analysis and Extraction
2.6. TBARS Measurement
2.7. Histology and Staining Analysis
2.8. Statistical Analysis
3. Results
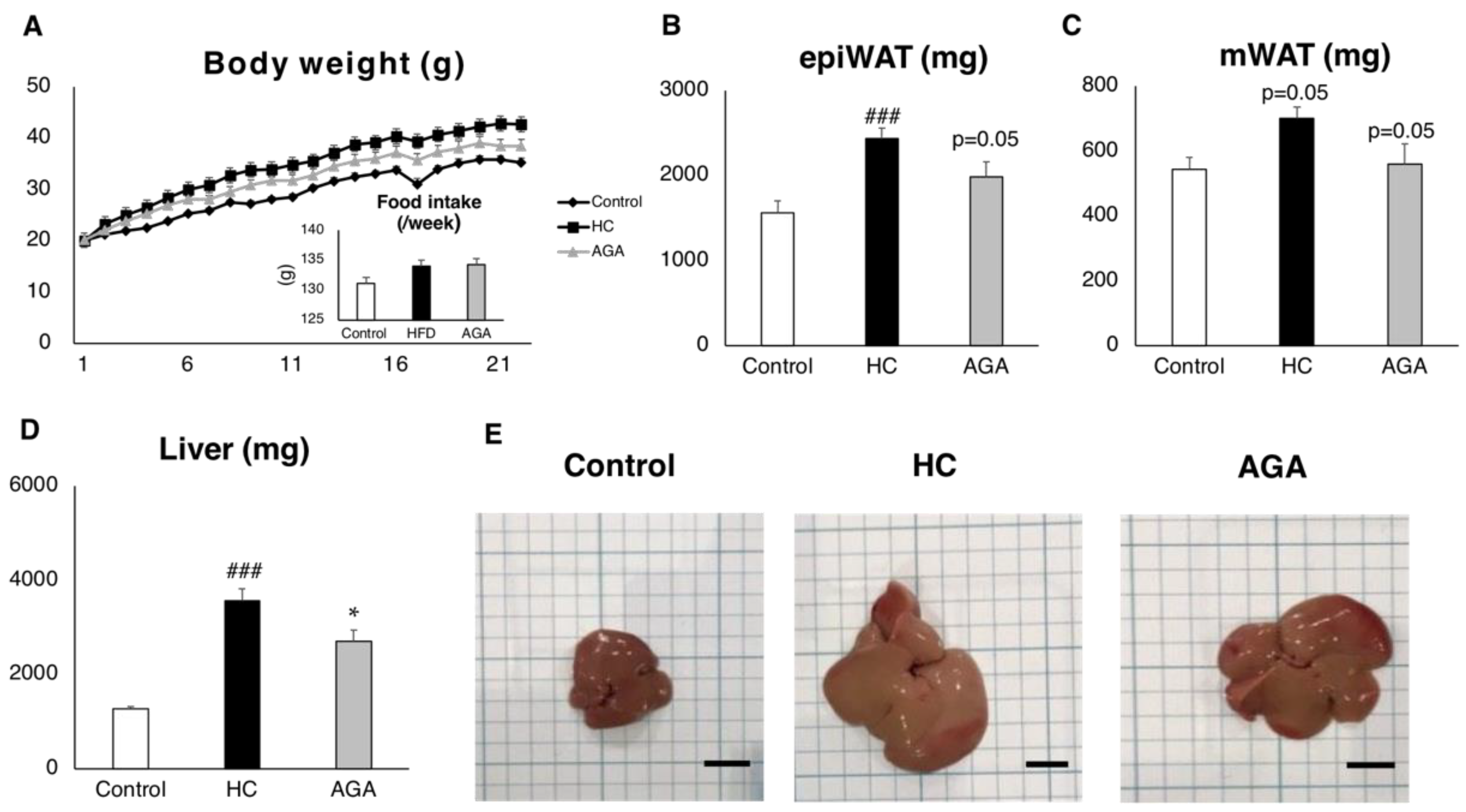
3.1. Agaricus Prevents Dietary-Induced NASH
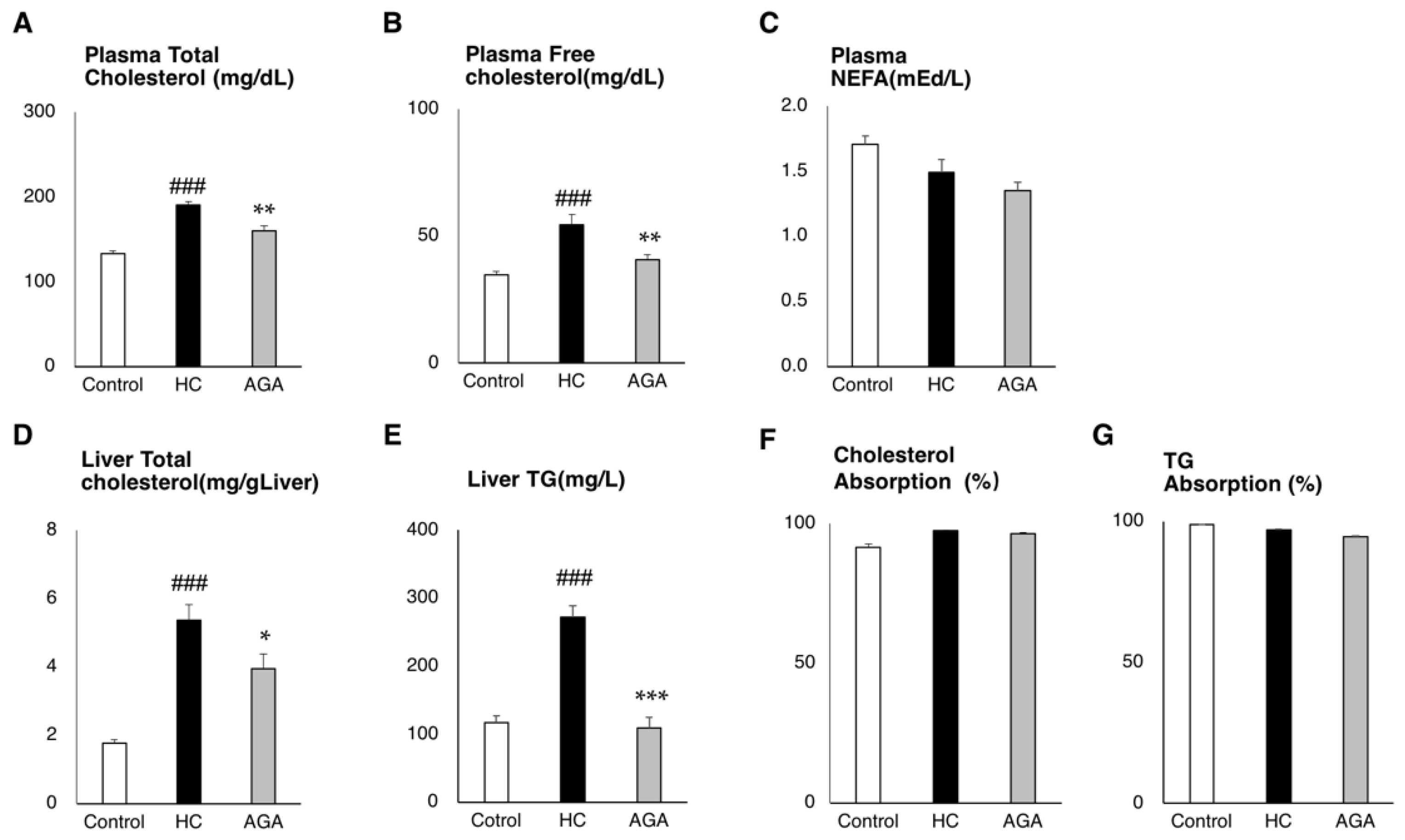
3.2. Agaricus Reduces the Liver Lipids Parameter in HC-Induced Model Mice
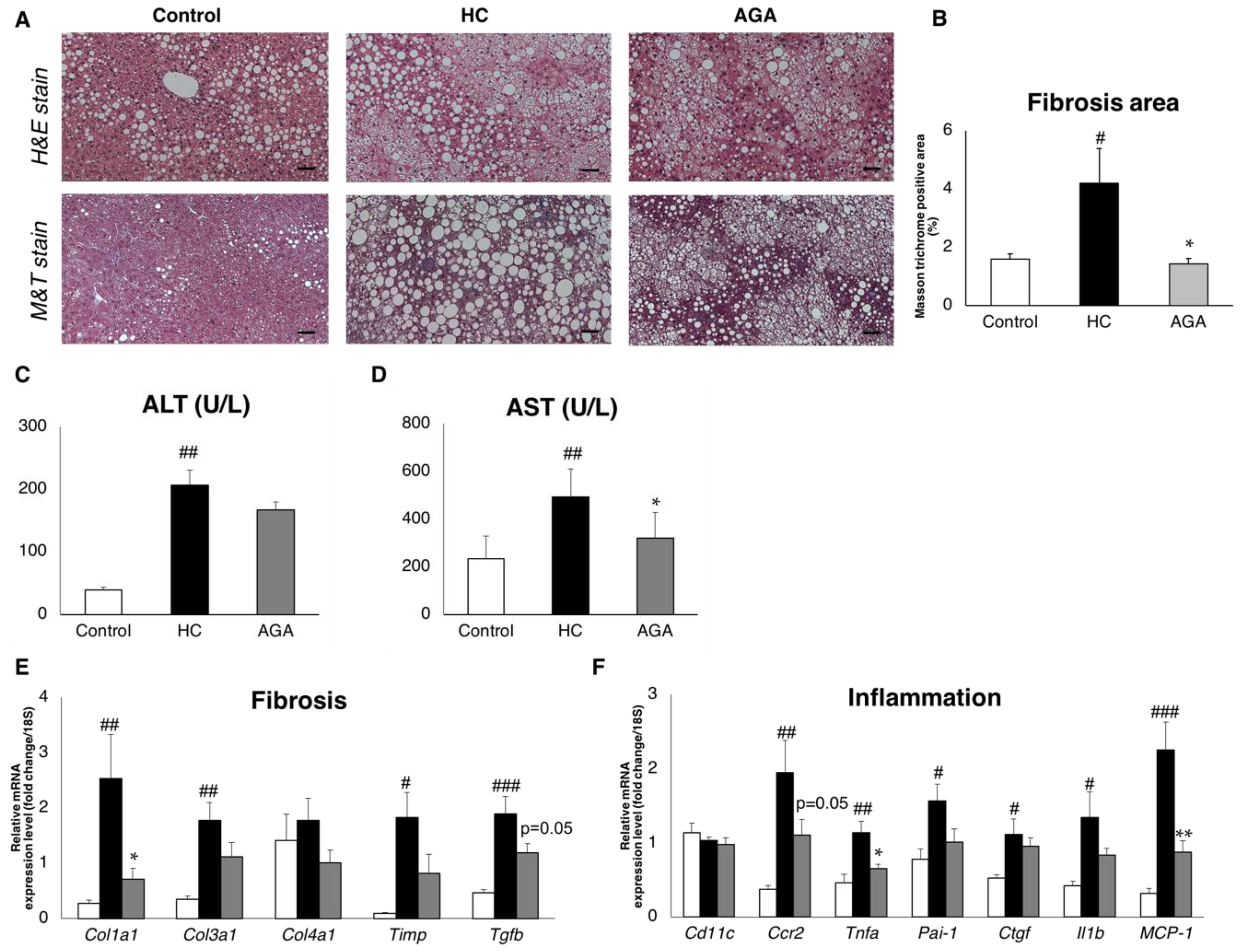
3.3. Agaricus Prevents NASH Progression at the Level of Gene Expression and Histological Analysis
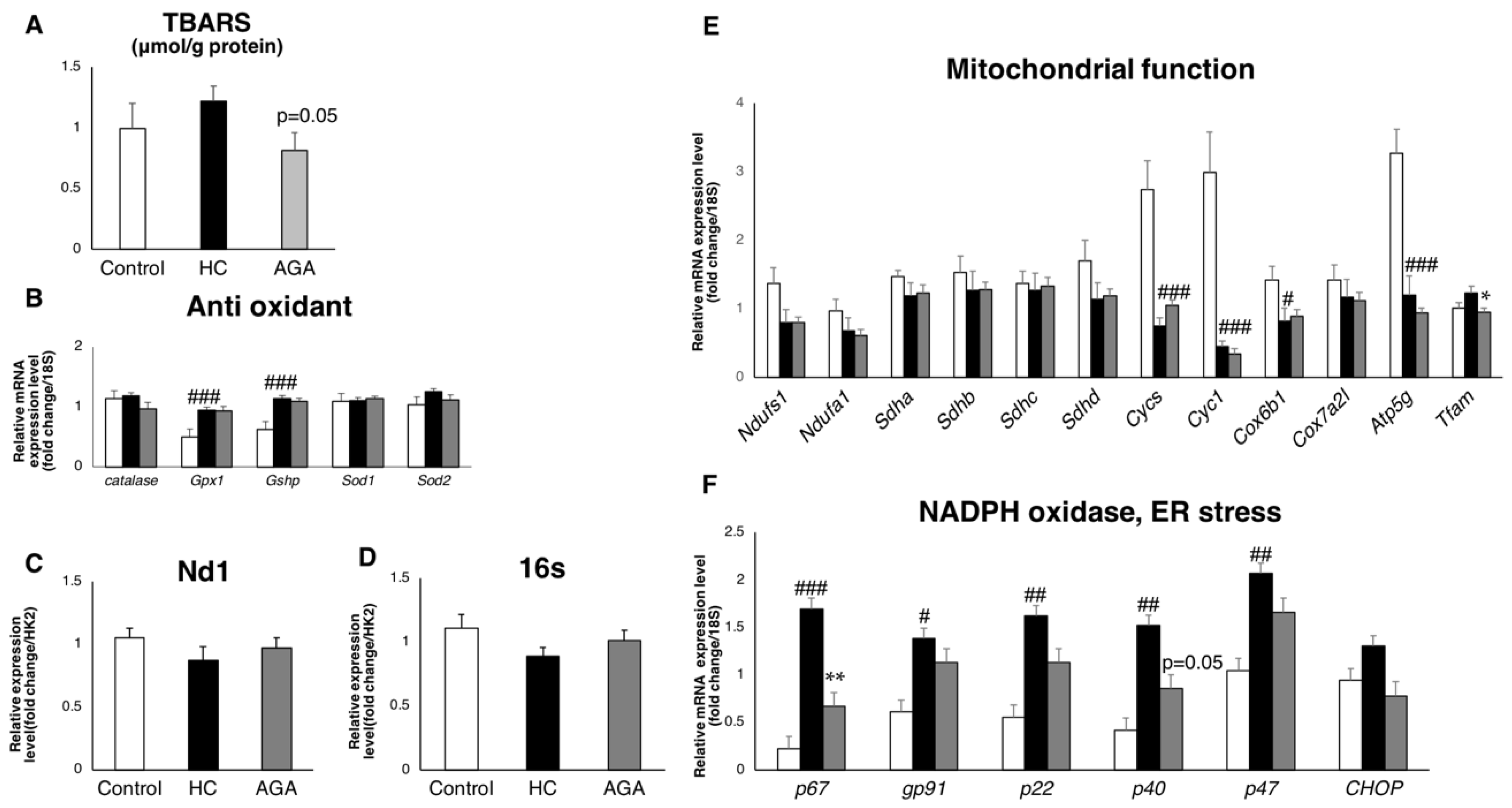
3.4. Agaricus Reduces Oxidative Stress
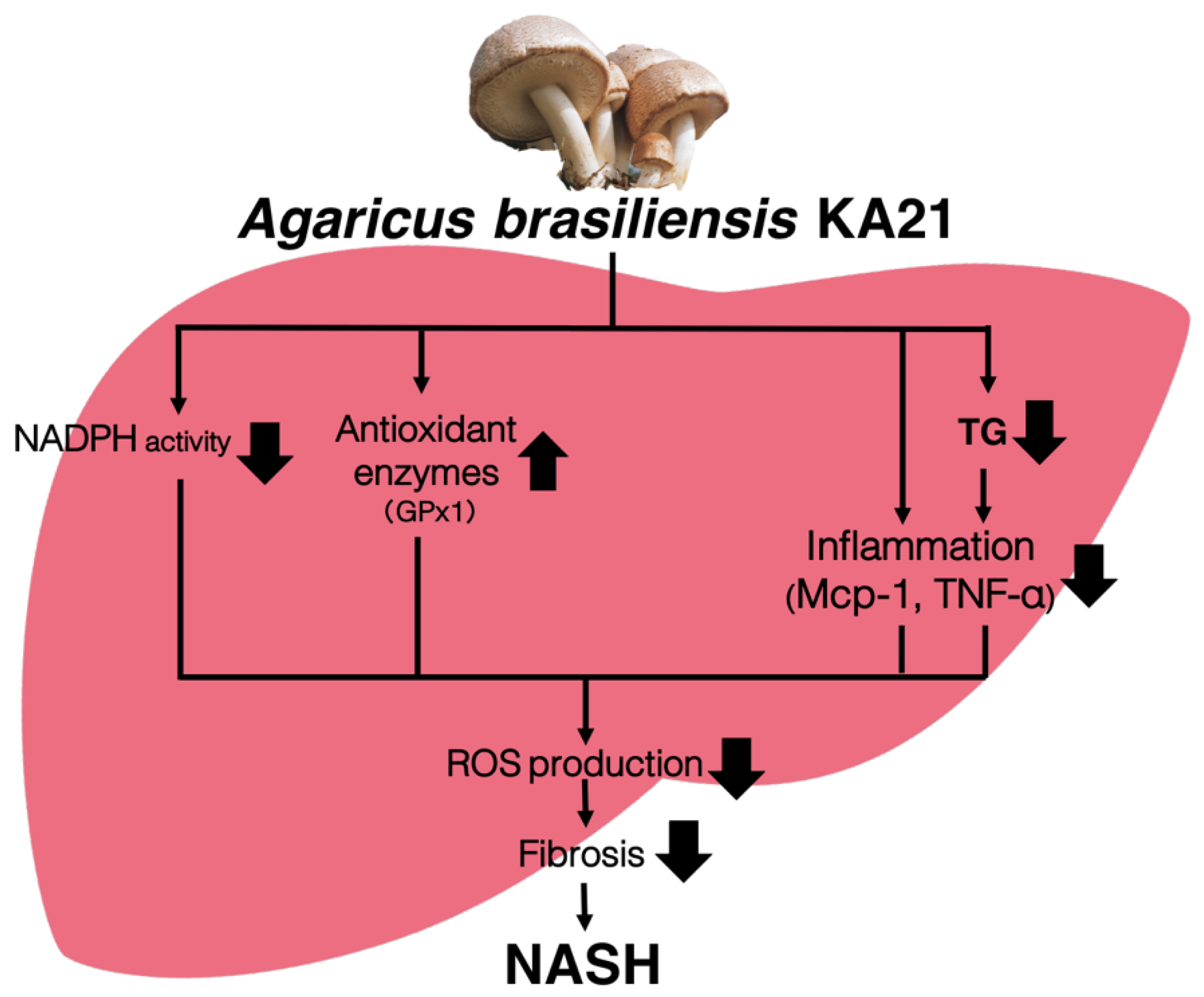
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lidia Popescu, M.; Costea, T.; Elena Gîrd, C.; Fierăscu, I.; Dalila Balaci, T.; Claudiu Fierăscu, R. Antioxidant activity of romanian Agaricus blazei Murrill. and Agaricus bisporus JE. lange mushrooms. FARMACIA 2017, 65, 329–335. [Google Scholar]
- Mourão, F.; Umeo, S.H.; Takemura, O.S.; Linde, G.A.; Colauto, N.B. Antioxidant activity of Agaricus brasiliensis basidiocarps on different maturation phases. Braz. J. Microbiol. 2011, 42, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.L.; Roma, E.H.; Gomes-Santos, A.C.; Aguilar, E.C.; Cisalpino, D.; Fernandes, L.R.; Vieira, A.T.; Oliveira, D.R.; Cardoso, V.N.; Teixeira, M.M.; et al. Pro-inflammatory effects of the mushroom Agaricus blazei and its consequences on atherosclerosis development. Eur. J. Nutr. 2012, 51, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Padilha, M.M.; Avila, A.A.L.; Sousa, P.J.C.; Cardoso, L.G.V.; Perazzo, F.F.; Carvalho, J.C.T. Anti-Inflammatory Activity of Aqueous and Alkaline Extracts from Mushrooms (Agaricus blazei Murill). J. Med. Food 2009, 12, 359–364. [Google Scholar] [CrossRef]
- Saiki, P.; Kawano, Y.; Van Griensven, L.J.L.D.; Miyazaki, K. The anti-inflammatory effect of Agaricus brasiliensis is partly due to its linoleic acid content. Food Funct. 2017, 8, 4150–4158. [Google Scholar] [CrossRef]
- Author, C.; Hwang, K.-C.; Choi, E.; Ham, O.; Lee, S.-Y.; Song, B.-W.; Cha, M.-J.; Youn Lee, C.; Park, J.-H.; Lee, J.; et al. Mushrooms and cardiovascular disease. Curr. Top. Nutraceutical Res. 2012, 10, 43–52. [Google Scholar]
- Oh, T.W.; Kim, Y.A.; Jang, W.J.; Byeon, J.I.; Ryu, C.H.; Kim, J.O.; Ha, Y.L. Semipurified Fractions from the Submerged-Culture Broth of Agaricus blazei Murill Reduce Blood Glucose Levels in Streptozotocin-Induced Diabetic Rats. J. Agric. Food Chem. 2010, 58, 4113–4119. [Google Scholar] [CrossRef]
- Mazzutti, S.; Ferreira, S.R.S.; Riehl, C.A.S.; Smania, A.; Smania, F.A.; Martínez, J. Supercritical fluid extraction of Agaricus brasiliensis: Antioxidant and antimicrobial activities. J. Supercrit. Fluids 2012, 70, 48–56. [Google Scholar] [CrossRef]
- Vincent, M.; Philippe, E.; Everard, A.; Kassis, N.; Rouch, C.; Denom, J.; Takeda, Y.; Uchiyama, S.; Delzenne, N.M.; Cani, P.D.; et al. Dietary supplementation with Agaricus blazei murill extract prevents diet-induced obesity and insulin resistance in rats. Obesity 2013, 21, 553–561. [Google Scholar] [CrossRef]
- de Miranda, A.M.; Rossoni Júnior, J.V.; Souza e Silva, L.; dos Santos, R.C.; Silva, M.E.; Pedrosa, M.L. Agaricus brasiliensis (sun mushroom) affects the expression of genes related to cholesterol homeostasis. Eur. J. Nutr. 2017, 56, 1707–1717. [Google Scholar] [CrossRef]
- Tsubone, H.; Makimura, Y.; Hanafusa, M.; Yamamoto, Y.; Tsuru, Y.; Motoi, M.; Amano, S. Agaricus brasiliensis KA21 Improves Circulatory Functions in Spontaneously Hypertensive Rats. J. Med. Food 2014, 17, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-W.; Kim, K.-H.; Choi, H.-J.; Lee, D.-S. Anti-diabetic activity of β-glucans and their enzymatically hydrolyzed oligosaccharides from Agaricus blazei. Biotechnol. Lett. 2005, 27, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Liao, Y.-L.; Lin, S.-C.; Hwang, K.-C.; Chou, P. The Mushroom Agaricus Blazei Murill in Combination with Metformin and Gliclazide Improves Insulin Resistance in Type 2 Diabetes: A Randomized, Double-blinded, and Placebo-Controlled Clinical Trial. J. Altern. Complement. Med. 2007, 13, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Johnson, E.; Lyberg, T.; Kvalheim, G. The Mushroom Agaricus blazei Murill Elicits Medicinal Effects on Tumor, Infection, Allergy, and Inflammation through Its Modulation of Innate Immunity and Amelioration of Th1/Th2 Imbalance and Inflammation. Adv. Pharmacol. Sci. 2011, 2011, 157015. [Google Scholar] [PubMed]
- Jumes, F.M.D.; Lugarini, D.; Pereira, A.L.B.; de Oliveira, A.; de Christoff, A.O.; Linde, G.A.; do Valle, J.S.; Colauto, N.B.; Acco, A. Effects of Agaricus brasiliensis mushroom in Walker-256 tumor-bearing rats. Can. J. Physiol. Pharmacol. 2010, 88, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Johnson, E.; Lyberg, T.; Bernardshaw, S.; Tryggestad, A.M.A.; Grinde, B. Effects of the Medicinal Mushroom Agaricus blazei Murill on Immunity, Infection and Cancer. Scand. J. Immunol. 2008, 68, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Sumiyoshi, Y.; Hashine, K.; Shirato, A.; Kyo, S.; Inoue, M. Phase I Clinical Study of the Dietary Supplement, Agaricus blazei Murill, in Cancer Patients in Remission. Evid. Based. Complement. Alternat. Med. 2011, 2011, 192381. [Google Scholar] [CrossRef]
- Mukai, H.; Watanabe, T.; Ando, M.; Katsumata, N. An Alternative Medicine, Agaricus blazei, May Have Induced Severe Hepatic Dysfunction in Cancer Patients. Jpn. J. Clin. Oncol. 2006, 36, 808–810. [Google Scholar] [CrossRef]
- Firenzuoli, F.; Gori, L.; Lombardo, G. The Medicinal Mushroom Agaricus blazei Murrill: Review of Literature and Pharmaco-Toxicological Problems. Evid. Based. Complement. Alternat. Med. 2008, 5, 3–15. [Google Scholar] [CrossRef]
- Lima, C.U.J.O.; Souza, V.C.; Morita, M.C.; Chiarello, M.D.; de Oliveira Karnikowski, M.G. Agaricus blazei Murrill and Inflammatory Mediators in Elderly Women: A Randomized Clinical Trial. Scand. J. Immunol. 2012, 75, 336–341. [Google Scholar] [CrossRef]
- Fujimiya, Y.; Suzuki, Y.; Oshiman, K.; Kobori, H.; Moriguchi, K.; Nakashima, H.; Matumoto, Y.; Takahara, S.; Ebina, T.; Katakura, R. Selective tumoricidal effect of soluble proteoglucan extracted from the basidiomycete, Agaricus blazei Murill, mediated via natural killer cell activation and apoptosis. Cancer Immunol. Immunother. 1998, 46, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.-J.; Lin, Y.-C.; Shih, H.-D.; Yang, J.-S.; Liu, K.-C.; Yang, S.-T.; Lin, C.-Y.; Wu, R.S.-C.; Yu, C.-S.; Ko, Y.-C.; et al. Crude Extracts of Agaricus brasiliensis Induce Apoptosis in Human Oral Cancer CAL 27 Cells through a Mitochondria-dependent Pathway. In Vivo (Brooklyn). 2011, 25, 355–366. [Google Scholar]
- Tsuchida, T.; Lee, Y.A.; Fujiwara, N.; Ybanez, M.; Allen, B.; Martins, S.; Fiel, M.I.; Goossens, N.; Chou, H.-I.; Hoshida, Y.; et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J. Hepatol. 2018, 69, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fukuwatari, Y.; Okumura, K.; Takeda, K.; Ishibashi, K.-I.; Furukawa, M.; Ohno, N.; Mori, K.; Gao, M.; Motoi, M. Immunomodulating Activity of Agaricus brasiliensis KA21 in Mice and in Human Volunteers. Evid. Based. Complement. Alternat. Med. 2008, 5, 205–219. [Google Scholar]
- Wang, H.; Fu, Z.; Han, C. The Medicinal Values of Culinary-Medicinal Royal Sun Mushroom (Agaricus blazei Murrill). Evid. Based. Complement. Alternat. Med. 2013, 2013, 842619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oseini, A.M.; Sanyal, A.J. Therapies in non-alcoholic steatohepatitis (NASH). Liver Int. 2017, 37, 97–103. [Google Scholar] [CrossRef] [PubMed]
- NASH prevalence: Key figures | The NASH Education ProgramTM. Available online: https://www.the-nash-education-program.com/what-is-nash/key-figures/ (accessed on 28 October 2019).
- Arrese, M.; Feldstein, A.E. NASH-related cirrhosis: An occult liver disease burden. Hepatol. Commun. 2017, 1, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Canbay, A.; Sowa, J.-P.; Syn, W.-K.; Treckmann, J. NASH Cirrhosis—the New Burden in Liver Transplantation: How Should It Be Managed? Visc. Med. 2016, 32, 234–238. [Google Scholar] [CrossRef]
- Farrell, G.C.; Larter, C.Z. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology 2006, 43, S99–S112. [Google Scholar] [CrossRef]
- Schuster, S.; Cabrera, D.; Arrese, M.; Feldstein, A.E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 349–364. [Google Scholar] [CrossRef]
- Koruk, M.; Taysi, S.; Savas, M.C.; Yilmaz, O.; Akcay, F.; Karakok, M. Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steatohepatitis. Ann. Clin. Lab. Sci. 2004, 34, 57–62. [Google Scholar] [PubMed]
- Ore, A.; Akinloye, O.A.; Ore, A.; Akinloye, O.A. Oxidative Stress and Antioxidant Biomarkers in Clinical and Experimental Models of Non-Alcoholic Fatty Liver Disease. Medicina (B. Aires). 2019, 55, 26. [Google Scholar] [CrossRef] [PubMed]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.M.; Aziz, R. Mushrooms: A potential natural source of anti-inflammatory compounds for medical applications. Mediators Inflamm. 2014, 2014, 805841. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.C.; van Rooyen, D.; Gan, L.; Chitturi, S. NASH is an Inflammatory Disorder: Pathogenic, Prognostic and Therapeutic Implications. Gut Liver 2012, 6, 149–171. [Google Scholar] [CrossRef] [PubMed]
- Harmon, R.C.; Tiniakos, D.G.; Argo, C.K. Inflammation in Nonalcoholic Steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Wree, A.; Inzaugarat, M.E.; Feldstein, A.E. Transmembrane BAX Inhibitor motif-containing 1, a novel anti-inflammatory approach for nonalcoholic steatohepatitis treatment. Hepatology 2018, 67, 438–441. [Google Scholar] [CrossRef]
- Kessoku, T.; Imajo, K.; Honda, Y.; Kato, T.; Ogawa, Y.; Tomeno, W.; Kato, S.; Mawatari, H.; Fujita, K.; Yoneda, M.; et al. Resveratrol ameliorates fibrosis and inflammation in a mouse model of nonalcoholic steatohepatitis. Sci. Rep. 2016, 6, 22251. [Google Scholar] [CrossRef]
- Al-Busafi, S.A.; Bhat, M.; Wong, P.; Ghali, P.; Deschenes, M. Antioxidant therapy in nonalcoholic steatohepatitis. Hepat. Res. Treat. 2012, 2012, 947575. [Google Scholar] [CrossRef]
- Bril, F.; Biernacki, D.M.; Lomonaco, R.; Kalavalapalli, S.; Subbarayan, S.K.; Lai, J.; Tio, F.O.; Suman, A.; Orsak, B.K.; Hecht, J.; et al. Role of Vitamin E for the Treatment of Nonalcoholic Steatohepatitis (NASH) in Patients with T2DM—A Randomized, Controlled Trial. Diabetes 2018, 67, 1223-P. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Nagashimada, M.; Zhuge, F.; Zhan, L.; Nagata, N.; Tsutsui, A.; Nakanuma, Y.; Kaneko, S.; Ota, T. Astaxanthin prevents and reverses diet-induced insulin resistance and steatohepatitis in mice: A comparison with vitamin E. Sci. Rep. 2015, 5, 17192. [Google Scholar] [CrossRef] [PubMed]
- Zou, A.; Magee, N.; Deng, F.; Lehn, S.; Zhong, C.; Zhang, Y. Hepatocyte nuclear receptor SHP suppresses inflammation and fibrosis in a mouse model of nonalcoholic steatohepatitis. J. Biol. Chem. 2018, 293, 8656–8671. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Toshimitsu, K.; Matsuura, B.; Ohkubo, I.; Niiya, T.; Furukawa, S.; Hiasa, Y.; Kawamura, M.; Ebihara, K.; Onji, M. Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition 2007, 23, 46–52. [Google Scholar] [CrossRef]
| Ingredient | /100 g | Ingredient | /100 g |
|---|---|---|---|
| Energy | 288.0 kcal | Selenium | 88.0 μg |
| Protein | 38.5 g | Arsenicum | 0 .5 ppm |
| Fat | 2.6 g | Cadmium | 2.0 ppm |
| Carbohydrate | 27.7 g | Plumbum | 0.1 ppm |
| β-glucan | 12.4 g | Mercury | 0.2 ppm |
| Fiber | 20.6 g | Vitamin B (total caronene) | |
| Sodium | 8.4 mg | Vitamin B1 (Thiamin) | 0.6 mg |
| Calcium | 22.5 mg | Vitamin B2 (Riboflavin) | 3.0 mg |
| Iron | 10.1 mg | Vitamin B6 | 0.5 mg |
| Potassium | 2920.0 mg | Niacin | 33.5 mg |
| Phosphorus | 952.0 mg | Pantothenic acid | 22.9 mg |
| Magnesium | 96.5 mg | Folic acid | 230.0 μg |
| Zinc | 7.9 mg | Biotin | 123.0 μg |
| Copper | 7.7 mg | ||
| Manganese | 0.8 mg | ||
| Vitamin D | 56.7 μg | ||
| Agaritine | 15.3 ppm |
| Product | High-Fat/Cholesterol Diet (D09100301) | Control Diet (D09100304) | ||
|---|---|---|---|---|
| gm% | kcal% | gm% | kcal% | |
| Protein | 22.5 | 20.0 | 19.2 | 20.0 |
| Carbohydrate | 44.9 | 40.0 | 67.3 | 70.0 |
| Fat | 19.9 | 40.0 | 4.3 | 10.0 |
| Total | 100.0 | 100.0 | ||
| kcal/gm | 4.5 | 3.9 | ||
| Ingredient | gm | kcal | gm | kcal |
| Casein, 80 Mesh | 200.0 | 800.0 | 200.0 | 800.0 |
| L-Cystine | 3.0 | 12.0 | 3.0 | 12.0 |
| Corn Starch | 0.0 | 0.0 | 350.0 | 1400.0 |
| Maltodextrin 10 | 100.0 | 400.0 | 85.0 | 340.0 |
| Fructose | 200.0 | 800.0 | 0.0 | 0.0 |
| Glucose | 0.0 | 0.0 | 169.0 | 676.0 |
| Sucrose | 96.0 | 384.0 | 96.0 | 384.0 |
| Cellulose, BW200 | 50.0 | 0.0 | 50.0 | 0.0 |
| Soybean Oil | 25.0 | 225.0 | 25.0 | 225.0 |
| Primex Shortening | 135.0 | 121.0 | 0.0 | 0.0 |
| Lard | 20.0 | 180.0 | 20.0 | 180.0 |
| Mineral Mix S10026 | 10.0 | 0.0 | 10.0 | 0.0 |
| DiCalcium Phosphate | 13.0 | 0.0 | 13.0 | 0.0 |
| Calcium Carbonate | 5.5 | 0.0 | 5.5 | 0.0 |
| Potassium Citrate, 1 H2O | 16.5 | 0.0 | 16.5 | 0.0 |
| Vitamin Mix V10001 | 10.0 | 40.0 | 10.0 | 40.0 |
| Choline Bitartrate | 2.0 | 0.0 | 2.0 | 0.0 |
| Cholesterol | 18.0 | 0.0 | 0.0 | 0.0 |
| FD&C Yellow Dye #5 | 0.1 | 0.0 | 0.0 | 0.0 |
| Total | 904.1 | 4056.0 | 1055.1 | 4057.0 |
| Forward Primer (5′→3′) | Reverse Primer (5′→3′) | |
|---|---|---|
| 16S | CCGCAAGGGAAAGATGAAAGAC | TCGTTTGGTTTCGGGGTTTC |
| 18S | TTCTGGCCAACGGTCTAGACAAC | CCAGTGGTCTTGGTGTGCTGA |
| Acc | ACCCACTCCACTGTTTGTGA | CCTTGGAATTCAGGAGAGGA |
| Atp5g | CACTGCTCATTTCTCCAGCTC | CAGGAAGGCTGCTTAGATGG |
| catalase | CCAGCGACCAGATGAAGCAG | CCACTCTCTCAGGAATCCGC |
| Ccr2 | AGCACATGTGGTGAATCCAA | TGCCATCATAAAGGAGCCA |
| Cd11c | AAGAACTGTGGAGCTGACCA | CCACCAGGGTCTTCAAGTCT |
| CHOP | AGGTGAAAGGCAGGGACTCA | CCACCACACCTGAAAGCAGAA |
| Col1a1 | CCTCAGGGTATTGCTGGACAAC | TTGATCCAGAAGGACCTTGTTTG |
| Col3a1 | TTGATGTGCAGCTGGCATTC | GCCACTGGCCTGATCCATAT |
| Col4a1 | CACATTTTCCACAGCCAGAG | GTCTGGCTTCTGCTGCTCTT |
| Cox6b1 | ATGTCTCCGTGTGTGAGTGG | GATCTTCCCAGGAAATGTGC |
| Cox7a2l | TTTGGTTGGTGTGGCAAATA | AGTTTCACGCAGAAGTTGGC |
| Ctgf | ACCCGAGTTACCAATGACAATACC | CCGCAGAACTTAGCCCTGTATG |
| Cyc1 | GCTTCCAGGTGCAAGTGCT | CAGACTTCGAGGACAAGGACA |
| Cycs | GCAAHCATAAGACTGGACCAAA | TTGTTGGCATCTGTGTAAGAGAATC |
| Cyp2e1 | TCTGAGATATGGGCTCCTGA | ATGCACTACAGCGTCCATGT |
| Fas | TCTGCCAGTGAGTTGAGGAC | CTGCAGAGAAGCGAGCATAC |
| Gp91 | TTGGGTCAGCACTGGCTCTG | TGGCGGTGTGCAGTGCTATC |
| Gpx1 | AGTCCACCGTGTATGCCTTCT | GAGACGCGACATTCTCAATGA |
| Gshp | CCTTGCCAACACCCAGTGA | CCGGAGACCAAATGATGTACTTG |
| Il1b | CTGAACTCAACTGTGAAATGCCA | AAAGGTTTGGAAGCAGCCCT |
| MCP-1 | CCACTCACCTGCTGCTACTCAT | TGGTGATCCTCTTGTAGCTCTCC |
| Nd1 | CTAGCAGAAACAAACCGGGC | CCGGCTGCGTATTCTACGTT |
| Ndufa1 | GTCCACTGCGTACATCCACA | ATCGCGTTCCATCAGATACC |
| Ndufs1 | GGAACTACTCGGTGGGCTC | GCCAGTTGTGCGAACATATC |
| p22 | GTCCACCATGGAGCGATGTG | CAATGGCCAAGCAGACGGT |
| p40 | GCAGGCTCAGGAGGTTCTTC | CGCCGCTATCGCCAGTTCTAC |
| p47 | AGATGTTCCCCATTGAGGCCG | GTTTCAGGTCATCAGGCCGC |
| p67 | CTGGCTGAGGCCATCAGACT | AGGCCACTGCAGAGTGCTT |
| Pai-1 | GCATAGCCAGCACCGAGGA | TCAGCCCTTGCTTGCCTCAT |
| Scd-1 | CTCCTGCTGATGTGCTTCAT | AAGGTGCTAACGAACAGGCT |
| Sdha | ATGACACTGTGAAAGGCTCCGACT | TTCCCAAACTTGAGGCTCTGTCCA |
| Sdhb | GCCGTTCTCGGCAGAGTG | TCTGGGTCCCATCGGTAAAT |
| Sdhc | AGTTTGTGCTTGTCTTCCCG | CACTCCAGACAGCCAGACCT |
| Sdhd | CATGGCGGTTCTCTTAAAGC | TGACACATAAGCGGGTCTGA |
| Sod1 | TGAGGTCCTGCACTGGTAC | CAAGCGGTGAACCAGTTGTG |
| Sod2 | TTAACGCGCAGATCATGCA | GGTGGCGTTGAGATTGTTCA |
| Tfam | ATGTCTCCGGATCGTTTCAC | CCAAAAAGACCTCGTTCAGC |
| Tgfb | TTGCTTCAGCTCCACAGAGA | TGGTTGTAGAGGGCAAGGAC |
| Timp | AGGTGGTCTCGTTGATTTCT | GTAAGGCCTGTAGCTGTGCC |
| Tnfa | CTGGGACAGTGACCTGGACT | GCACCTCAGGGAAGAGTCTG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, A.; Zhu, Q.; Yokoyama, Y.; Kitamura, N.; Uchida, S.; Kumadaki, K.; Tsubota, K.; Watanabe, M. Agaricus brasiliensis KA21 May Prevent Diet-Induced Nash Through Its Antioxidant, Anti-Inflammatory, and Anti-Fibrotic Activities in the Liver. Foods 2019, 8, 546. https://doi.org/10.3390/foods8110546
Nakamura A, Zhu Q, Yokoyama Y, Kitamura N, Uchida S, Kumadaki K, Tsubota K, Watanabe M. Agaricus brasiliensis KA21 May Prevent Diet-Induced Nash Through Its Antioxidant, Anti-Inflammatory, and Anti-Fibrotic Activities in the Liver. Foods. 2019; 8(11):546. https://doi.org/10.3390/foods8110546
Chicago/Turabian StyleNakamura, Anna, Qi Zhu, Yoko Yokoyama, Naho Kitamura, Sena Uchida, Kayo Kumadaki, Kazuo Tsubota, and Mitsuhiro Watanabe. 2019. "Agaricus brasiliensis KA21 May Prevent Diet-Induced Nash Through Its Antioxidant, Anti-Inflammatory, and Anti-Fibrotic Activities in the Liver" Foods 8, no. 11: 546. https://doi.org/10.3390/foods8110546
APA StyleNakamura, A., Zhu, Q., Yokoyama, Y., Kitamura, N., Uchida, S., Kumadaki, K., Tsubota, K., & Watanabe, M. (2019). Agaricus brasiliensis KA21 May Prevent Diet-Induced Nash Through Its Antioxidant, Anti-Inflammatory, and Anti-Fibrotic Activities in the Liver. Foods, 8(11), 546. https://doi.org/10.3390/foods8110546