Abstract
Wattle seed (Acacia spp.) is a well-known staple food within indigenous communities in Australia. A detailed investigation of the overall nutritional and sensory profile of four abundant and underutilized Acacia species—A. coriacea, A. cowleana, A. retinodes and A. sophorae—were performed. Additionally, molecular weight of protein extracts from the wattle seeds (WS) was determined. The seeds are rich in protein (23–27%) and dietary fibre (33–41%). Relatively high fat content was found in A. cowleana (19.3%), A. sophorae (14.8%) and A. retinodes (16.4%) with oleic acid being the predominant fatty acid. The seeds contained high amounts of essential amino acids (histidine, lysine, valine, isoleucine and leucine). A. coriacea is rich in iron (43 mg/kg), potassium (10 g/kg) and magnesium (1.7 g/kg). Pentose (xylose/arabinose), glucose, galactose and galacturonic acids were the major sugars found in the four species. Raw seeds from A. sophorae, A. retinodes and A. coriacea have the highest protein molecular weight, between 50–90 kDa, 80 kDa and 50–55 kDa, respectively. There was variation in the sensory profile of the WS species. This study showed that the four WS species have good nutritional value and could be included in human diet or used in food formulations.
1. Introduction
As the world population increases and natural resources diminish, there has been a serious concern on available sustainable nutritious foods [1]. In addition, a majority of people from developing countries suffer from protein malnutrition, famine and different kinds of diseases due to inadequate food supply and poor quality food [2]. In order to meet these continued population growth and nutritional requirements, studies are required to examine and discover new sources of food. In the past few years, researchers have focused on the use of underutilized plant products as human food and animal feed [3,4,5].
The genus Acacia, commonly known as wattle, belongs to the family Fabaceae and it is a large group of woody species comprising of shrubs. Acacia subgenus Phyllodineae are naturally the most common Acacia species found in Australia and are among the most promising native leguminous plants [6,7]. These Acacias have been reported to exhibit significant potential to lower poverty in semi-arid regions of Africa [8,9]. Moreover, the seeds from various Acacia species, which were used traditionally as source of food by Australian Indigenous population, have been economically revived as food additives, such as emulsifying and flavouring agents [10,11,12]. Acacia victoriae Bentham is the most common species of Acacia with high water-soluble carbohydrates and protein contents and, thus, have been reported to have significant functional properties in food systems [13,14]. Additionally, Acacia plants have been frequently used to treat diseases, such as fever, leucorrhoea, throat infection, diarrhoea and haemoptysis [15].
However, several Acacia species which are also widely cultivated by indigenous people in different regions of Australia have not been fully utilized in food formulations or incorporated in human diets. These includes A. coriacea and A. cowleana which occurs throughout Northern Australia as well as A. retinodes and A. sophorae that are found in Southern and Southeastern Australia. These Acacia plants, particularly A. retinodes, are mainly used for gum production and ornamental purposes [16]. Nevertheless, information on the nutritive value of these Acacia species is sparse which may limit their use in foods. Therefore, this study investigated the overall nutritional value of seeds of these abundant and native Australian Acacia species. Furthermore, the seeds were roasted and the molecular weight profiles of protein extracts before and after treatment were examined. In addition, a preliminary sensory profiling of the four wattle seed species was carried out. This study will provide information on whether or not it is advisable to incorporate these seeds into the human diet and indicate the sensory characteristics of these species.
2. Materials and Methods
2.1. Materials
Mature seeds of four different species of Australian Acacia species (A. coriacea, A. cowleana, A. retinodes and A. sophorae) used in this study are shown in Figure 1. A. coriacea and A. cowleana were sourced from NATIF Australian Native Superfoods, Fruits Herbs Spices and Mixes, Victoria, Australia, and A. retinodes and A. sophorae were supplied by Valley Seeds Pty Ltd., Victoria, Australia. The seeds from each species were separately ground using a coffee grinder (power: 200 W, time: 30 s) and stored in the refrigerator until further analysis. Additionally, parts of the whole seeds were roasted at 180 °C for 5 min and used to determine the molecular weight profile of the seeds protein extracts for comparison with that obtained from raw seeds. All samples were analysed at least in duplicate and the average for each parameter was reported.

Figure 1.
Four species of Australian wattle seeds—appearance and size comparison.
2.2. Proximate Analysis
The four different species of Acacia seeds were sent to Symbio Alliance Lab Pty Ltd., Eight Mile Plains, Queensland, Australia. A complete proximate analysis was performed at this accredited National Association of Testing Authorities (NATA) laboratory using AOAC [17] standard methods. The following analysis were measured: moisture (AOAC 925.10) by air oven with a measurement of uncertainty (MU) of ±15%, ash (AOAC 923.03), crude protein (AOAC 990.03) by Dumas combustion with a MU of ±10%, crude fat (AOAC 991.36) with a MU of ±15% and dietary fibre (985.29) with a MU of ±15%, carbohydrate and energy by calculation using information from the Food Standards Code.
2.3. Sugar Analysis
A combination of fast liquid chromatography coupled to UV and electrospray ionization trap detection (LC-UV-ESI-MS/MS) was used for the quantification of various sugars [18]. The hydrolysis was performed in duplicates by adding 6 mL of 2 M TFA to 12 mg of grounded Acacia seeds into 15 mL glass tubes. The tubes were incubated in a heating block (VLM GmbH, EC-Model, Heideblümchenweg, Bielefeld, Germany) for 90 min at 121°C. After cooling to room temperature, the hydrolysates were neutralized to pH ~8 by adding an aqueous solution of 3.2% NH4OH, since light alkaline conditions are required for the subsequent derivatization of monosaccharides. A 25 µL of neutralized hydrolysate supernatant were derivatized via the high throughput 1 phenyl-3-methyl-5-pyrazolone (HT-PMP) method [18]. The calibration standards were diluted with neutralized TFA-matrix to compensate the influence on the derivatization process. Each sample was derivatized in triplicates and the carbohydrate fingerprint was analysed.
2.4. Fatty Acid Profiles
About 1 g of finely chopped seed samples were taken for initial extraction with chloroform and methanol (2:1) followed by agitation at room temperature for 1 h and centrifuged for 5 min at 3500× g. Fatty acid profiling was performed at the School of Agriculture and Food sciences, University of Queensland laboratory. The GC-MS (Shimadzu QP2010, Shimadzu Coporation, Tokyo, Japan) was used at oven temperature of 100 °C, injector temperature 250 °C, total program time was 39 min, and helium used as the carrier gas. The inlet pressure used for gas chromatography was 0.4 kPa, at linear gas velocity of 42.7 cm/s, column (Restek stabilwax capillary column; 30 m × 0.25 mm ID × 0.5 µm film thickness) flow 1.10 mL/min with a split ratio of 1:1 and injection volume of 0.2 µL. For mass spectrometry, the ion source temperature used was 200 °C, the interface temperature was 250 °C and the mass range was 35–500 atomic mass units. Identification of the compounds was done by comparing their retention times and mass spectra with corresponding data from a standard food industry FAME Mix (Restek Corporation, Bellefonte, PA, USA).
2.5. Amino Acid Analysis
The samples (100 mg per replicate) were first hydrolysed with 6 M HCl at 110 °C for 24 h. As asparagine is hydrolysed to aspartic acid and glutamine to glutamic acid, the reported amount of these acids is the sum of those respective components. After hydrolysis, all amino acids were analysed at the Department of Molecular Science, Australian Proteome Analysis Facility, Macquarie University, NSW, Australia, using the Waters AccQTag Ultra chemistry on a Waters Acquity UPLC. Samples were analysed in duplicate and results are expressed as an average. The coefficient of variation (CV) of the UPLC analysis of amino acids was less than 5%.
2.6. Mineral Analysis
A detailed description of method used for mineral analysis is outlined as described by Carter et al. [19]. The ground seed samples were accurately weighed (0.3 g) into digestion Teflon vessels and concentrated nitric acid (4 mL) was added. The samples were digested using a microwave digestion system (MarsXpress, CEM, Matthews, NC, USA) programmed to three steps: step 1 (400 W power, 85 °C, 14 min), step 2 (800 W power, 110 °C, 20 min), and step 3 (1600 W power, 160 °C, 10 min) and the analysis was performed using inductively coupled plasma mass spectrometry (ICP-MS 7500a, Agilent, Tokyo, Japan) and optical emission spectrometry (ICP-OES, Varian Australia, VIC, Australia).
2.7. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
The SDS-PAGE analysis of lyophilized protein extracts from raw and roasted wattle seeds were conducted at an accredited Protein Expression Facility (PEF), University of Queensland, St Lucia, Queensland, Australia. The extracts were resuspended in PBS to a final concentration of 2 mg/mL. Samples were loaded onto a 4–12% Bis-Tris SDS-PAGE gel and run under denatured and reduced conditions, except where noted. Analysis was performed using a Bio-Rad Chemi-DocTM XRS + imaging system.
2.8. Rapid Sensory Profiling
A sensory tasting session was carried out to develop sensory descriptors of four WS species: A. coriacea, A. retinodes, A. sophorae and A. cowleana. Twelve trained assessors (nine females; three males) with an average age of 50 years old participated in a two-hour session where they provided descriptors for aroma, flavour and aftertaste. For sensory evaluation the seeds of the four species were roasted in the oven for 7 min at 180 °C and ground when cooled. The wattle seeds were presented in two forms for sensory evaluation: as ground seeds on their own and the ground seeds mixed with semolina paste to make them more palatable for the assessors. One gram of ground seeds was presented for aroma assessment in a 30 mL plastic cup covered with a lid and labelled with a three digit blinding code. The ground seeds mixed with semolina where used to conduct a second assessment of aroma and to assess flavour and after taste. Each assessor was presented with 6 g of 1:20 ground wattle seeds and hydrated semolina mix in a 30 mL plastic cup covered with a lid and labelled with the same three digit blinding code used for the non-mixed sample. Green apple and water were used as palate cleansers. Upon completion of the session tasting component the panel leader facilitated session where sensory descriptors of each category for four wattle seed species were summarised and a consensus reached. This resulted in a preliminary sensory profile for each variety.
2.9. Statistical Analysis
The data were calculated using Microsoft Excel 2013 and the results are expressed as mean of the triplicate experiments unless otherwise stated. Statistical analysis of the data was done by one-way analysis of variance procedure (ANOVA) using SPSS software (Version 23.0 IBM Corporation, Armonk, NY, USA), and means comparison was done using Duncan’s multiple range test at p < 0.05.
3. Results and Discussion
3.1. Proximate Composition
As shown in Table 1, all of the species had low moisture content (less than 9%) which are comparable with the 6.9% recorded for A. victoriae Bentham [20] and the range (6.3–8.0%) for A. tumida and A. colei [8]. The four species of wattle seeds (WS) showed high protein content, ranging from 22.5% in A. coriacea to 27.5% in A. retinodes. The values for crude protein obtained in this study were higher than that of A. victoriae Bentham [20] but lower than those found in different subspecies of A. saligna (28.6–32.6%) [3]. However, the protein contents were within the range (23.4–34.1%) reported for A. tumida and A. colei [8]. The results indicate that WS can serve as a source of protein in the diets of the Aboriginal population in Australia. Moreover, it can be included in food formulations as a source of protein. The crude fat content varied among the four species with relatively high values observed in A. cowleana followed by A. retinodes while A. coriacea had the least value (9.8%). These results showed that A. cowleana seed would be a good source of energy due to its relatively high crude fat content. All the four species studied had similar ash contents (3.4–3.9%) which were comparable to that found in A. victoriae Bentham [20] and A. tortilis [21]. All seeds also showed high amounts of dietary fibre ranging from 33.7% in A. cowleana to 41.4% in A. coriacea. These values were higher than that recorded for A. tortilis [21] and A. victoriae Bentham [20]. Therefore, the seeds from these Acacia species can be considered as a good source of dietary fibre. Dietary fibre has a vital function in human nutrition by maintaining the health of the gastrointestinal tract, however, in excess may bind to iron and zinc, thereby lowering their bioavailability [22]. Regarding the carbohydrate content, the seeds have a range of 12.8–15.6% carbohydrate. These levels of carbohydrate were lower than that reported for A. victoriae Bentham [20] and A. tumida [2], due to the greater amounts of crude fat, fibre and protein in the seeds. Traditionally, A. coriacea is known to lower postprandial glucose level [23,24], higher dietary fibre and protein content could be the reason for these potential health benefits. This suggest that using wattle seed flour can be a healthier option. Therefore, the chemical composition of these wattle seed species was found to be nutritious and adding these seeds into the human diets will enhance the nutrition status.

Table 1.
Proximate composition of four different species of wattle seeds (%, dry weight).
3.2. Fatty Acid Composition
The fatty acid composition of the four species of wattle seed are presented in Table 2. The unsaturated fatty acids (UFA) constitute the bulk of the fatty acids, as in the case of certain edible legumes, such as peanut [25]. Palmitic acid (17.69–23.83%) was found to be the predominant saturated fatty acid (SFA) in all the seeds, followed by stearic acid (4.15–10.12%) with A. coriacea and A. retinodes having significantly (p < 0.05) higher values in the two fatty acids, respectively. Monounsaturated fatty acids (MUFA) contributed the larger percentage of the total UFA with values more than twice that of polyunsaturated fatty acids (PUFA) in A. coriacea, A. sophorae and A. retinodes, unlike in other legumes and wattle seed species [3,26]. The C18:1 was found to be the most abundant of all the MUFAs with similar values (50.82–57.57%) obtained in all the species except A. cowleana (36.24%) (p < 0.05). However, greatest (p < 0.05) amount of PUFA particularly linoleic acid was found in A. cowleana (34.77%) as compared to other species, with A. sophorae having significantly (p < 0.05) lowest PUFA value (7.17%). These four species have lower linoleic acids as compared to that of other legumes such as broad beans (48.3%), chickpeas (56.7%) and small lentils (52.3%) [26], as well as other WS species such as A. saligna [27] and A. tortilis [21]. Oils rich in linoleic acid can have an important role in lowering the levels of blood cholesterol [28], thus the oil obtained from A. cowleana is highly nutritious when consumed regularly as a part of diet. Moreover, only trace amount of n-3 fatty acids was identified in all the seeds. Overall, the high degree of unsaturation found in all the four WS species agrees with those of common vegetable oils indicating that WS composite flour can be a healthy option for human consumption.

Table 2.
Fatty acid profile of four different species of wattle seeds expressed as percentage (± SD) of the total fatty acid profile as determined by FAME GC-MS analysis.
3.3. Amino Acid Composition
The essential amino acid analysis of four different species of WS showed all four of them contained about 13–15% of glutamic acid, followed by about 9–11.5% of aspartic acid, 6.8–7.4% of lysine, 8.0–8.6% of leucine and about 7% of arginine, serine and alanine (Table 3). Moreover, methionine is the limiting amino acids found in all four seeds and this agrees with that reported in other Acacia seed species [21,22]. This is the first time that a complete detailed analysis of all essential amino acids of these four different species of wattle seeds have been investigated. Due to high amounts of protein in all these species, it is important to look at different types of amino acids available in these seeds. It is observed that the glutamic acid content is slightly lower than that of about 18% in mung bean flour [29]. The amount of lysine in all four seeds is comparable to that of soybean [30]. The amount of arginine is very similar to that of winged bean and soy beans [31]. Presence of high amount of arginine potentially can help to increase physiological pool of L-arginine and may have positive impact on cardiovascular health [32]. Comparing the amino acid profile of the seed proteins with FAO reference pattern [33] can be used to justify the potential food value of the proteins. The amino acid profile of the four WS species showed that histidine, lysine, valine, isoleucine and leucine had higher levels than those listed in FAO/WHO reference pattern.

Table 3.
Amino acid (mol % dry weight) profile of four different species of wattle seeds.
3.4. Mineral Composition
Table 4 outlines a detailed mineral analysis performed on four different species of Australian WS. As highlighted in the table, all four species are good sources of important essential minerals such as iron, potassium, magnesium, calcium and zinc. Potassium is the most abundant element in all the WS species with A. coriacea having significantly (p < 0.05) highest value (11,000 mg/kg dry weight (DW)). The results obtained agrees with that reported for different WS species [8,21,22]. A significantly (p < 0.05) higher iron content (195.0 mg/kg DW) was found in A. sophorae as compared to other species of WS, showing it is a good source of iron considering the Australian recommended dietary allowance (RDA) of iron is 7 and 12–16 mg/day for men and women during pregnancy, respectively [34,35]. Moreover, heavy metals, such as Hg, are less than 0.005 mg in all four WS species, indicating the use of all these four species of WS is safe for human consumption. As stated in the Food Standards Australia New Zealand (FSANZ), Standard 1.4.1 for contaminants and Natural Toxicants, the maximum level of Pb in legumes is set at 0.2 mg/kg and cadmium in rice is 0.1 mg/kg. The amount of Pb in all four WS species is within this range and in case of A. sophorae and A. cowleana, they have significantly (p < 0.05) higher values (0.1 and 0.175 mg/kg DW, respectively) as compared to other species, but the values still complied with the food standards code [36]. Based on the above results, the WS are a good source for minerals and thus can be incorporated into foods, such as commercial baked products, that are deficient in minerals to enhance their nutritional properties. Further studies should be carried out to investigate the bioavailability of the essentials minerals, particularly potassium in the WS species.

Table 4.
Mineral analysis of four different species of wattle seeds.
3.5. Sugar Profile
The results in Table 5 show that the monosaccharides are the major kind of sugar present in all the four WS species. Pentose sugars (xylose/arabinose) were the predominant monosaccharides found in the seeds and these were followed by galactose and glucose, while a small amount of mannose and fucose were present. Among the species, A. sophorae has a significantly (p < 0.05) higher amount of pentose sugars (84.2%), while significantly (p < 0.05) higher glucose and galactose contents were found in both A. sophorae and A. retinodes. Moreover, the seeds are rich in galacturonic acid but contain lesser amounts of rhamnose. All these sugars were present in substantial amount in all the species, particularly in A. retinodes, A. sophorae and A. coriacea as compared to A. cowleana. To the best of our knowledge, this study was the first to report the sugar composition present in wattle seeds. Overall, the results revealed that these wattle seed species have high amount of reducing sugar and this may be due to action of endogenous enzymes involved in hydrolysing the stored carbohydrate during maturation and storage.

Table 5.
Sugar profile of four different species of wattle seeds.
3.6. SDS-PAGE Profile of Soluble Proteins Extracted from Raw and Roasted Wattle Seed Species
Figure 2 shows the SDS-PAGE gel electrophoretograms of extracts from four species of raw and roasted WS obtained under non-reducing and reducing conditions. For raw WS extracts in the four species, similar protein bands appeared under reducing and non-reducing conditions but protein molecules smaller than 18 kDa were broken down under reducing condition probably due to reduction in disulphide bonds. Moreover, more protein bands were found in raw A. sophorae (lanes 10 and 11) followed by A. coriacea (lanes 6 and 7) and A. retinodes (lanes 16 and 17) while A. cowleana (lanes 1 and 2) showed faint protein bands. Overall, the results suggest that water soluble proteins from raw WS species were mainly polypeptides with less than 80 kDa molecular weights for A. sophorae and A. retinodes and 55 kDa for A. coriacea. This differs from previous study on different wattle seed species where the molecular weight of the protein was found to be lower than 66 kDa [13,27]. After roasting, no protein band was visible in the four wattle seed species with the exception of only two faint protein bands observed in A. coriacea (lanes 8 and 9) at 55 kDa. The results showed that the heat treatment had caused the soluble proteins in WS to be degraded into fragments with molecular weight smaller than 10 kDa. A similar effect of thermal treatment on protein had been reported in previous studies on wattle seeds [27] and peanuts [37].
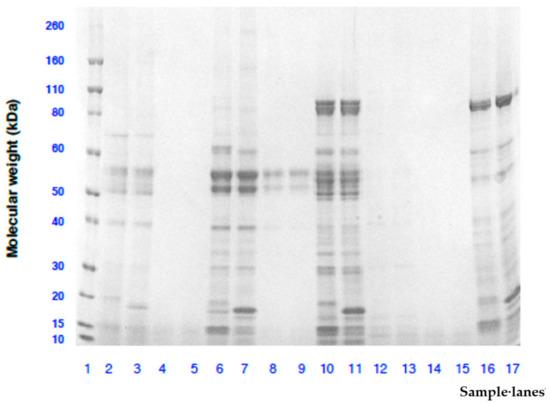
Figure 2.
Molecular weight analysis by SDS-PAGE of protein extracts from raw and roasted wattle seeds species. Lanes 1: Novex Sharp Pre-stained molecular weight ladder; Lanes 2, 3, 4 and 5: Raw and reducing, raw and non-reducing, roasted and reducing, roasted and non-reducing A. cowleana, respectively; Lanes 6, 7, 8 and 9: Raw and reducing, raw and non-reducing, roasted and reducing, roasted and non-reducing A. coriacea, respectively; Lanes 10, 11, 12 and 13: Raw and reducing, raw and non-reducing, roasted and reducing, roasted and non-reducing A. sophorae, respectively; Lanes 14, 15, 16 and 17: Roasted and reducing, roasted and non-reducing, raw and reducing, raw and non-reducing A. retinodes, respectively.
3.7. Rapid Sensory Profiling
The results of the rapid sensory profiling presented in Table 6 showed a wide diversity of sensory characteristics for the four WS species. The summary of the descriptors provided by the trained panel showed that, while all four species had savoury notes, certain descriptors characterised each species. A. cowleana, for instance, was perceived as a complex cultivar where the broth, popcorn and savoury notes where more intense for aroma and flavour. A. coriacea was distinguished by some confectionary and tropical fruit notes. A. sophorae was characterised by more savoury and grassy notes but not very intense. Cultivar A. retinodes was characterised by gravy, lemony earthy and nutty notes. Previous preliminary studies in A. victoriae have shown that this wattle seed cultivar has a savoury note as the four WS species in this study but also its own nutty and wheat biscuit notes [38]. The informal sensory component of this study has shown diversity in sensory profiles between WS seed species, opening the door for different applications of WS species in the food industry. Future work could investigate more rigorously the sensory differences between these four species and other species of the WS, as well as investigate effects of roasting and grinding on flavour.

Table 6.
Rapid sensory profiling of four different species of wattle seeds.
4. Conclusions
This study shows that the four species of wattle seeds are good source of protein and dietary fibre. In addition, the seeds can be considered as a potential dietary source of major and minor minerals, essential amino acids and oleic acids, thus, the seeds have promising nutritional profiles. Nevertheless, further studies should be carried out to investigate the impact of processing on these nutrients and also to evaluate the anti-nutritional components present in these species before and after processing.
Author Contributions
K.J.S., B.R., U.T., S.M.O.M., O.Q.A. performed the experiments, collected, analysed and interpreted the data and drafted the manuscript. Y.S., O.Q.A., H.E.S., S.H. and V.S. conceived and designed the experiments, checked and approved the results and critically revised the manuscript. All authors read and approved the final version of the manuscript.
Funding
This project was funded by the University of Queensland, Australia, The Australian Research Council Transformational Training Centre for Uniquely Australian Foods Grant number IC180100045 and the Innovation Connections Grant number RC52539.
Acknowledgments
The authors acknowledge the Traditional Owners of the lands on which the wattle seed species were harvested, and respect the knowledge and experience the Traditional Owners hold regarding the care, harvest and use of these plants. We thank NATIF Australian Native Superfoods, Fruits Herbs Spices and Mixes, Victoria, Australia and Valley Seeds Pty Ltd., Victoria, Australia for supplying the wattle seeds. The authors thank the Australian Government via the Innovation Connections Grant Scheme for funding this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karhagomba, I.B.; Mirindi, T.A.; Mushagalusa, T.B.; Nabino, V.B.; Koh, K.; Kim, H.S. The cultivation of wild food and medicinal plants for improving community livelihood: The case of the Buhozi site, DR Congo. Nutr. Res. Pract. 2013, 7, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Falade, M.S.; Owoyomi, O.; Harwood, C.E.; Adewusi, S.R.A. Chemical composition and starch hydrolysis of Acacia colei and Acacia tumida seeds. Cereal Chem. 2005, 82, 479–484. [Google Scholar] [CrossRef]
- Ee, K.Y.; Yates, P. Nutritional and antinutritional evaluation of raw and processed Australian wattle (Acacia saligna) seeds. Food Chem. 2013, 138, 762–769. [Google Scholar] [CrossRef]
- El-Adawy, T.A.; Khalil, A.H. Characteristics of roselle seeds as a new source of protein and lipid. J. Agric. Food Chem. 1994, 42, 1896–1900. [Google Scholar] [CrossRef]
- Embaby, H.E.S.; Mokhtar, S.M. Chemical composition and nutritive value of lantana and sweet pepper seeds and nabak seed kernels. J. Food Sci. 2011, 76, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.K.; Johnson, K.A. Horticultural development of Australian native edible plants. Aus. J. Bot. 2000, 48, 417–426. [Google Scholar] [CrossRef]
- Seigler, D.S. Economic potential of Western Australian Acacia species: Secondary plant products. Conserv. Sci. West. Aust. 2002, 4, 109–116. [Google Scholar]
- Adewusi, S.R.A.; Falade, O.S.; Harwood, C. Chemical composition of Acacia colei and Acacia tumida seeds—Potential food sources in the semi-arid tropics. Food Chem. 2003, 80, 187–195. [Google Scholar] [CrossRef]
- Rinaudo, A.; Cunningham, P. Australian Acacias as multi-purpose agro-forestry species for semi-arid regions of Africa. Muelleria 2008, 26, 79–85. [Google Scholar]
- Maslin, R.; McDonald, M.W. AcaciaSearch: Evaluation of Acacia as a Woody Crop Option for Southern Australia: Canberra; Rural Industries Research and Development Corporation: Macquaire Street, Barton, ACT, Australia, 2004. [Google Scholar]
- Zhao, J.; Agboola, S. A Report for the Rural Industries Research and Development Corporation: Functional Properties of AUSTRALIAN Bush Food; Canberra, Rural Industries Research and Development Corporation: Canberra, Australia, 2007. [Google Scholar]
- Maslin, B.R.; Thomson, L.A.J.; McDonald, M.W.; Hamilton-Brown, S. Edible Wattle Seeds of Southern Australia: A Review of Species for Use in Semi-Arid Regions; CSIRO Publishing: Collingwood, Australia, 1998. [Google Scholar]
- Agboola, S.; Ee, K.Y.; Mallon, L.; Zhao, J. Isolation, characterization, and emulsifying properties of wattle seed (Acacia victoriae Bentham) extracts. J. Agric. Food Chem. 2007, 55, 5858–5863. [Google Scholar] [CrossRef]
- Ee, K.Y.; Zhao, J.; Rehman, A.; Agboola, S. Characterisation of trypsin and α-chymotrypsin inhibitors in Australian wattle seed (Acacia victoriae Bentham). Food Chem. 2008, 107, 337–343. [Google Scholar] [CrossRef]
- Agrawal, N.K.; Gupta, U. Evaluation of hypoglycemic and antihyperglycemic effects of Acacia tortilis seed extract in normal and diabetic rats. Int. J. Pharm. Technol. Res. 2013, 5, 330–336. [Google Scholar]
- ILDIS. International Legume Database & Information Service: LegumeWeb. Available online: https://ildis.org/cgi-bin/Araneus.pl?version~10.01&LegumeWeb&tno~5839&genus~Acacia&species~retinodes (accessed on 16 June 2018).
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Rühmann, B.; Schmid, J.; Sieber, V. Fast carbohydrate analysis via liquid chromatography coupled with ultra violet and electrospray ionization ion trap detection in 96-well format. J. Chromatogr. A 2014, 1350, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.F.; Tinggi, U.; Yang, X.; Fry, B. Stable isotope and trace metal compositions of Australian prawns as a guide to authenticity and wholesomeness. Food Chem. 2015, 170, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.B.; James, K.W.; Maggiore, P.M.A. Tables of Composition of Australian Aboriginal Foods; Aboriginal Studies Press: Canberra, ACT, Australia, 1993. [Google Scholar]
- Embaby, H.E.; Rayan, A.M. Chemical composition and nutritional evaluation of the seeds of Acacia tortilis (Forssk.) Hayne ssp. raddiana. Food Chem. 2016, 200, 62–68. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Vijayakumari, K.; Janardhanan, K. Chemical composition and nutritional evaluation of an underexploited legume, Acacia nilotica (L.). Del. Food Chem. 1996, 57, 385–391. [Google Scholar] [CrossRef]
- Thorburn, A.W.; Brand, J.C.; Cherikoff, V.; Truswell, A.S. Lower postprandial plasma glucose and insulin after addition of Acacia coriacea flour to wheat bread. Aust. N. Z. J. Med. 1987, 17, 24–26. [Google Scholar] [CrossRef]
- Thorburn, A.W.; Brand, J.C.; Truswell, A.S. Slowly digested and absorbed carbohydrate in traditional bushfoods: A protective factor against diabetes? Am. J. Clin. Nutr. 1987, 45, 98–106. [Google Scholar] [CrossRef]
- Mukhtar, A. Oil and fatty acid composition of peanut species grown in Pakistan. Pak. J. Bot. 2012, 44, 627–630. [Google Scholar]
- Kalogeropoulos, N.; Chiou, A.; Ioannou, M.; Karathanos, V.T.; Hassapidou, M.; Andrikopoulos, N.K. Nutritional evaluation and bioactive microconstituents (phytosterols, tocopherols, polyphenols, triterpenic acids) in cooked dry legumes usually consumed in the Mediterranean countries. Food Chem. 2010, 121, 682–690. [Google Scholar] [CrossRef]
- Ee, K.Y.; Zhao, J.; Rehman, A.U.; Agboola, S. Effects of roasting on the characteristics of Australian wattle (Acacia victoriae Bentham) seed and extracts. Int. J. Food Prop. 2013, 16, 1135–1147. [Google Scholar] [CrossRef]
- El Adawy, T.A.; Taha, K.M. Characteristics and composition of different seed oils and flours. Food Chem. 2001, 74, 47–54. [Google Scholar] [CrossRef]
- Coffmann, C.W.; Garciaj, V.V. Functional properties and amino acid content of a protein isolate from mung bean flour. Int. J. Food Sci. Technol. 1977, 12, 473–484. [Google Scholar] [CrossRef]
- Deshpande, S.S. Food legumes in human nutrition: A personal perspective. Crit. Rev. Food Sci. Nutr. 1992, 32, 333–363. [Google Scholar] [CrossRef] [PubMed]
- Ekpenyong, T.E.; Borchers, R.L. Amino acid profile of the seed and other parts of the winged bean. Food Chem. 1982, 9, 175–182. [Google Scholar] [CrossRef]
- Chivandi, E.; Davidson, B.C.; Erlwanger, K.H. Proximate, mineral, fibre, phytate-phosphate, vitamin E, amino acid and fatty acid composition of Terminalia sericea. S. Afr. J. Bot. 2013, 88, 96–100. [Google Scholar] [CrossRef]
- FAO/WHO. Protein quality evaluation. In Report of a Joint FAO/WHO Expert Consultation; Viale delle Terme di Caracalla: Rome, Italy, 1990. [Google Scholar]
- Lehrfeld, J.; Wu, Y.V. Distribution of phytic acid in milled fractions of scout-66 hard red winter-wheat. J. Agric. Food Chem. 1991, 39, 1820–1824. [Google Scholar] [CrossRef]
- National Health and Medical Research Council (NHMRC). Recommended Dietary Intakes for Use in Australia; Australian Government Publishing Service: Canberra, Australia, 1991.
- Arise, A.K.; Alashi, A.M.; Nwachukwu, I.D.; Ijabadeniyi, O.A.; Aluko, R.E.; Amonsou, E.O. Antioxidant activities of bambara groundnut (Vigna subterranea) protein hydrolysates and their membrane ultrafiltration fractions. Food Funct. 2016, 7, 2431–2437. [Google Scholar] [CrossRef]
- Ejigui, J.; Savoie, L.; Marin, J.; Desrosiers, T. Influence of traditional processing methods on the nutritional composition and antinutritional factors of red peanuts (Arachis hypogyea) and small red kidney beans (Phaseolus vulgaris). J. Biol. Sci. 2005, 5, 597–605. [Google Scholar] [CrossRef]
- Smyth, H.E.; Sanderson, J.E.; Sultanbawa, Y. Lexicon for the sensory description of Australian native plant foods and ingredients. J. Sens. Stud. 2012, 27, 471–481. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).