Pulsed Ultrasound-Assisted Extraction as an Alternative Method to Conventional Maceration for the Extraction of the Polyphenolic Fraction of Ribes nigrum Buds: A New Category of Food Supplements Proposed by The FINNOVER Project
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bud Collection
2.2. Chemicals
2.3. Traditional Preparation of R. nigrum Glyceric Macerates
2.4. Alternative Method to Produce R. nigrum Bud Derivatives: Pulsed Ultrasound-Assisted Extraction (PUAE)
2.5. Untargeted Fingerprints of The R. nigrum Phytocomplex
2.5.1. UV-Visible Spectroscopy
2.5.2. Fluorescence Spectroscopy
2.6. Experimental Design and Multivariate Data Analysis
2.7. Analytical Determinations
2.7.1. Determination of The Total Phenolic Compounds (TPC)
2.7.2. Determination of Radical Scavenging Activity (RSA)
2.8. HPLC Analysis
3. Results and Discussion
3.1. Optimization of The PUAE Experimental Conditions by DoE Using Untargeted Phytochemical Fingerprint
3.2. Analytical Characterization of The Most Promising PUAE Extract (RN8) and the Corresponding RNGM
3.2.1. Determination of the Total Phenolic Compounds (TPC) and the Radical Scavenging Activity (RSA)
3.2.2. Targeted Phytochemical Fingerprint
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FINNOVER Interreg Alcotra Project 2017–2020. Available online: http://www.interreg-finnover.com/ (accessed on 29 July 2019).
- Turrini, F.; Donno, D.; Boggia, R.; Beccaro, G.L.; Zunin, P.; Leardi, R.; Pittaluga, A.M. An innovative green extraction and re-use strategy to valorize food supplement by-products: Castanea sativa bud preparations as case study. Food Res. Int. 2019, 115, 276–282. [Google Scholar] [CrossRef]
- The EU Food Supplements Directive 2002/46/EC. Available online: http://data.europa.eu/eli/dir/2002/46/oj (accessed on 20 July 2019).
- Pharmacopée Française. Codex Medicamentarius Gallicus, Codex Français: Monographie, Préparations Homéopathiques; Ordre National des Pharmaciens: Paris, France, 1965; Available online: http://ansm.sante.fr/Mediatheque/Publications/Pharmacopee-francaise-Plan-Preambule-index (accessed on 26 July 2019).
- Donno, D.; Beccaro, G.L.; Cerutti, A.K.; Mellano, M.G.; Bounous, G. Bud Extracts as New Phytochemical Source for Herbal Preparations—Quality Control and Standardization by Analytical Fingerprint. In Phytochemicals—Isolation, Characterisation and Role in Human Health, 1st ed.; Rao, A.V., Rao, L.G., Eds.; InTech: Rijeka, Croatia, 2015; pp. 187–218. [Google Scholar]
- Donno, D.; Mellano, M.G.; Cerutti, A.K.; Beccaro, G.L. Biomolecules and Natural Medicine Preparations: Analysis of New Sources of Bioactive Compounds from Ribes and Rubus spp. Buds. Pharmaceuticals 2016, 9, 7. [Google Scholar] [CrossRef]
- Donno, D.; Boggia, R.; Zunin, P.; Cerutti, A.K.; Guido, M.; Mellano, M.G.; Prgomet, Z.; Beccaro, G.L. Phytochemical fingerprint and chemometrics for natural food preparation pattern recognition: An innovative technique in food supplement quality control. J. Food Sci. Technol. 2016, 53, 1071–1083. [Google Scholar] [CrossRef]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Rubus, Ribes, and Aronia. Food Chem. Toxicol. 2004, 69, 164–169. [Google Scholar] [CrossRef]
- Paunović, S.M.; Nikolić, M.; Miletić, R.; Mašković, P.; Milinković, M.; Karaklajić-Stajić, Z. Phytochemical Screening and Biological Activity of Extract Berries of Black Currant (Ribes nigrum L.). Erwerbs-Obstbau 2019, 61, 71–78. [Google Scholar] [CrossRef]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Marconi, V.; Bounous, G. Botanicals in Ribes nigrum bud-preparations: An analytical fingerprinting to evaluate the bioactive contribution to total phytocomplex. Pharm. Biol. 2013, 51, 1282–1292. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.S.; Abert-Vian, M. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar]
- Green Chemistry’s 12 Principles, United States Environmental Protection Agency. Available online: https://www.epa.gov/greenchemistry/basics-green-chemistry#twelve (accessed on 26 July 2019).
- Li, Y.; Fabiano-Tixier, A.S.; Tomao, V.; Cravotto, G.; Chemat, F. Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason. Sonochem. 2013, 20, 12–18. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Cravotto, G.; Binello, A.; Orio, L. Green extraction techniques: For high-quality natural products. Agro Food Ind. Hi Tech 2011, 22, 24–36. [Google Scholar]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Vegetable Sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Boggia, R.; Turrini, F.; Anselmo, M.; Zunin, P.; Donno, D.; Beccaro, G.L. Feasibility of UV–VIS-Fluorescence Spectroscopy combined with pattern recognition techniques to authenticate a new category of plant food supplements. J. Food Sci. Tech. 2017, 54, 2422–2432. [Google Scholar] [CrossRef]
- Leardi, R. Experimental design in chemistry: A tutorial. Anal. Chim. Acta 2009, 652, 161–172. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.; Hassan, S.; De Biaggi, M.; Riondato, I.; Gamba, G.; Giacoma, C.; Beccaro, G.L. Assessing Nutritional Traits and Phytochemical Composition of Artisan Jams Produced in Comoros Islands: Using Indigenous Fruits with High Health-Impact as an Example of Biodiversity Integration and Food Security in Rural Development. Molecules 2018, 23, 2707. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Prgomet, Z.; Beccaro, G.L. Advances in Ribes x nidigrolariaRud. Bauer and A. Bauer fruits as potential source of natural molecules: A preliminary study on physico-chemical traits of an underutilized berry. Sci. Hortic. 2018, 237, 20–27. [Google Scholar] [CrossRef]
- Turrini, F.; Zunin, P.; Catena, S.; Villa, C.; Alfei, S.; Boggia, R. Traditional or hydro-diffusion and gravity microwave coupled with ultrasound as green technologies for the valorization of pomegranate external peels. Food Bioprod. Process. 2019, 117, 30–37. [Google Scholar] [CrossRef]
- Boggia, R.; Turrini, F.; Villa, C.; Lacapra, C.; Zunin, P.; Parodi, B. Green extraction from pomegranate marcs for the production of functional foods and cosmetics. Pharmaceuticals 2016, 9, 63–74. [Google Scholar] [CrossRef]
- Turrini, F.; Boggia, R.; Leardi, R.; Borriello, M.; Zunin, P. Optimization of the Ultrasonic-Assisted Extraction of Phenolic Compounds from Oryza Sativa L. ‘Violet Nori’ and Determination of the Antioxidant Properties of its Caryopses and Leaves. Molecules 2018, 23, 844. [Google Scholar] [CrossRef]
- Sadeckà, J.; Tòthovà, J. Fluorescence spectroscopy and chemometrics in the food classification—A review. Czech J. Food Sci. 2007, 25, 159–173. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Italian Chemical Society. Division of Analytical Chemistry-Group of Chemometrics. CAT Chemometric Agile Tool. Available online: http://www.gruppochemiometria.it/index.php/software (accessed on 29 July 2019).
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; De Biaggi, M.; Riondato, I.; Rakotoniaina, E.N.; Beccaro, G.L. New Findings in Prunuspadus L. Fruits as a Source of Natural Compounds: Characterization of Metabolite Profiles and Preliminary Evaluation of Antioxidant Activity. Molecules 2018, 23, 725. [Google Scholar] [CrossRef]
- Mok, D.K.W.; Chau, F.T. Chemical information of Chinese medicines: A challenge to chemist. Chemometr. Intell. Lab. 2006, 82, 210–217. [Google Scholar] [CrossRef]
- Jagota, S.K.; Dani, H.M. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Cerutti, A.K.; Beccaro, G.L. Nutraceuticals in Alternative and Underutilized Fruits as Functional Food Ingredients: Ancient Species for New Health Needs. In Alternative and Replacement Foods, 1st ed.; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 261–282. [Google Scholar]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Rocchetti, G.; Blasi, F.; Montesano, D.; Ghisoni, S.; Marcotullio, M.C.; Sabatini, S.; Cossignani, L.; Lucini, L. Impact of conventional/non-conventional extraction methods on the untargeted phenolic profile of Moringa oleifera leaves. Food Res. Int. 2019, 115, 319–327. [Google Scholar] [CrossRef] [PubMed]
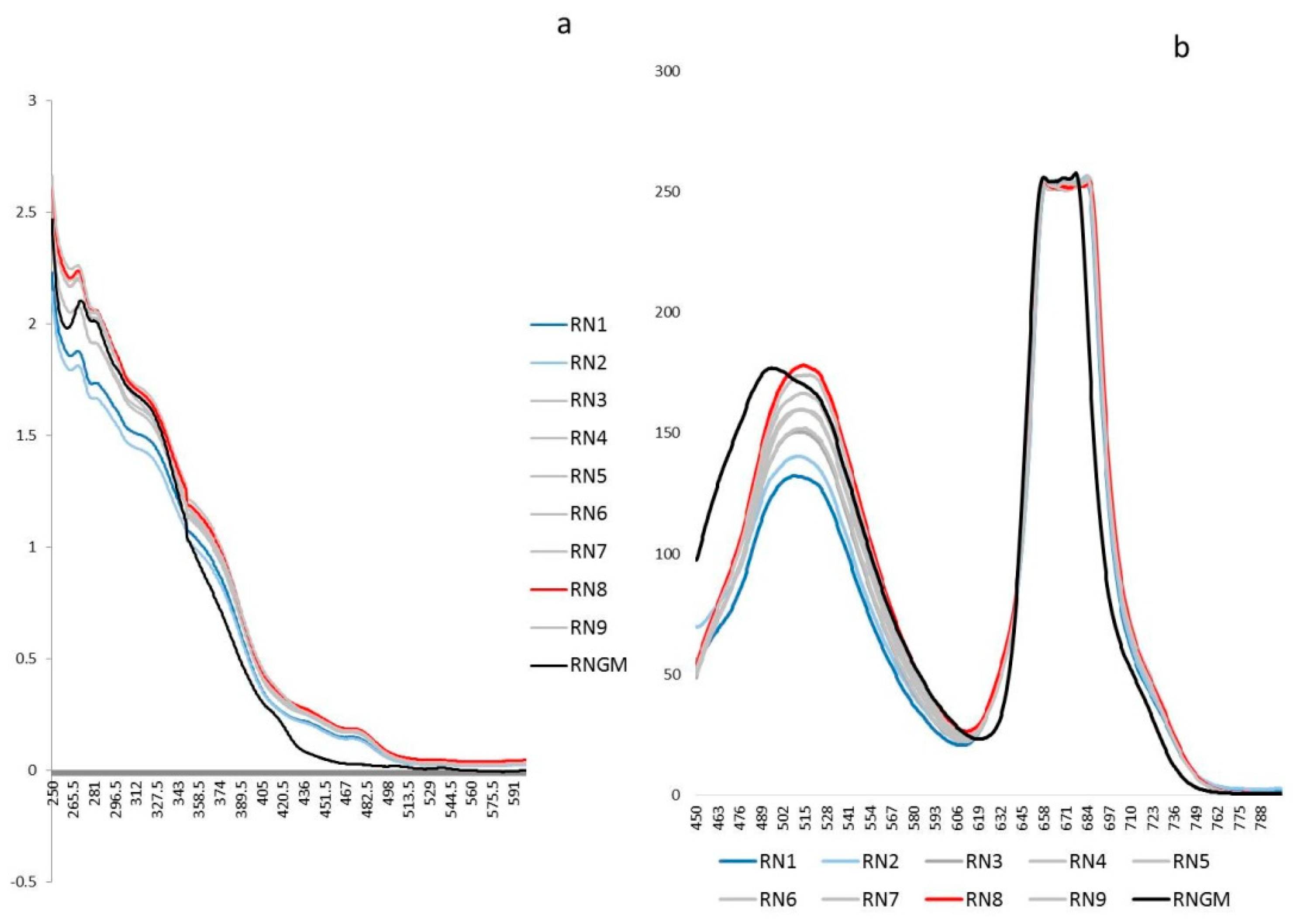
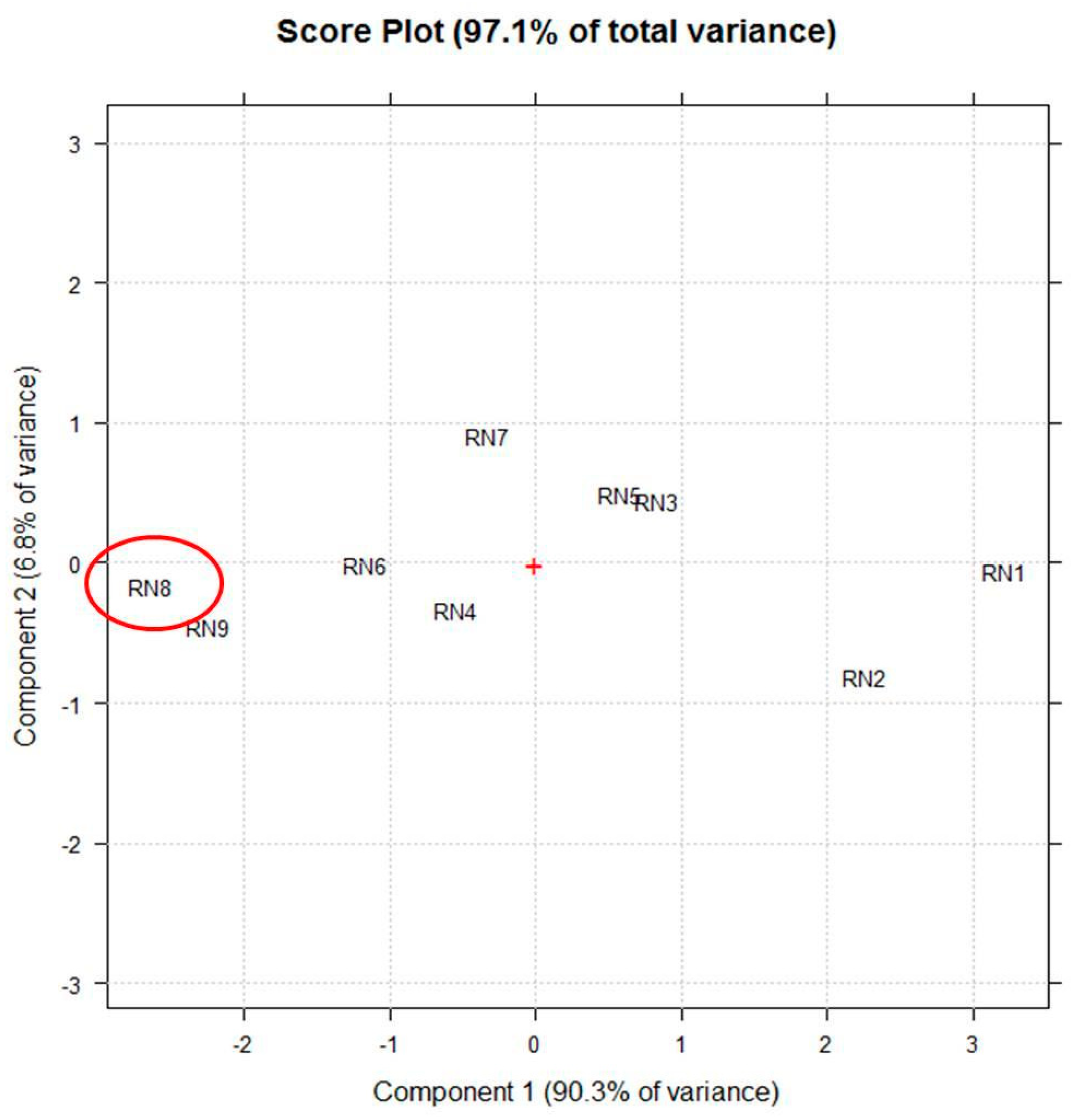
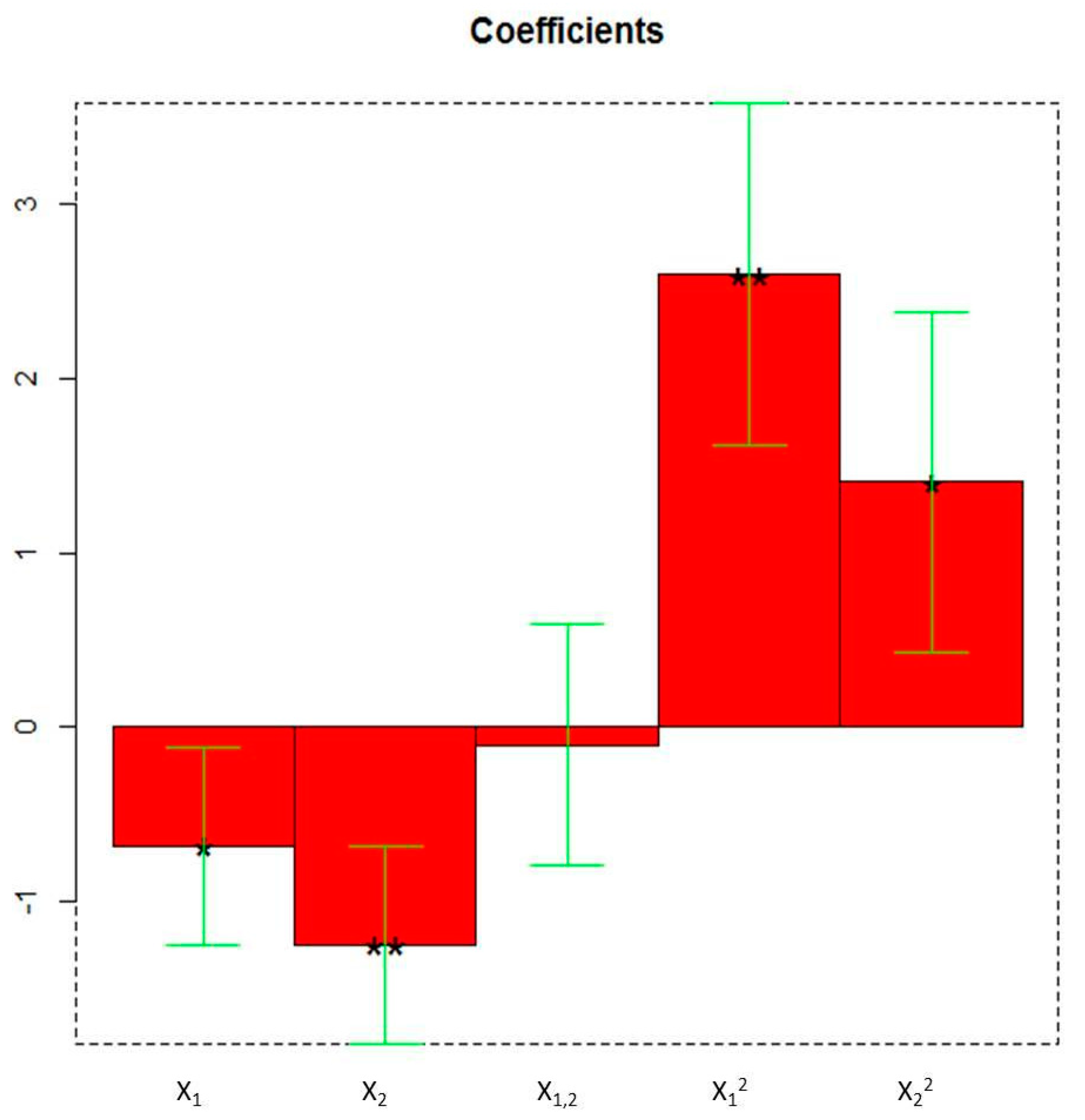
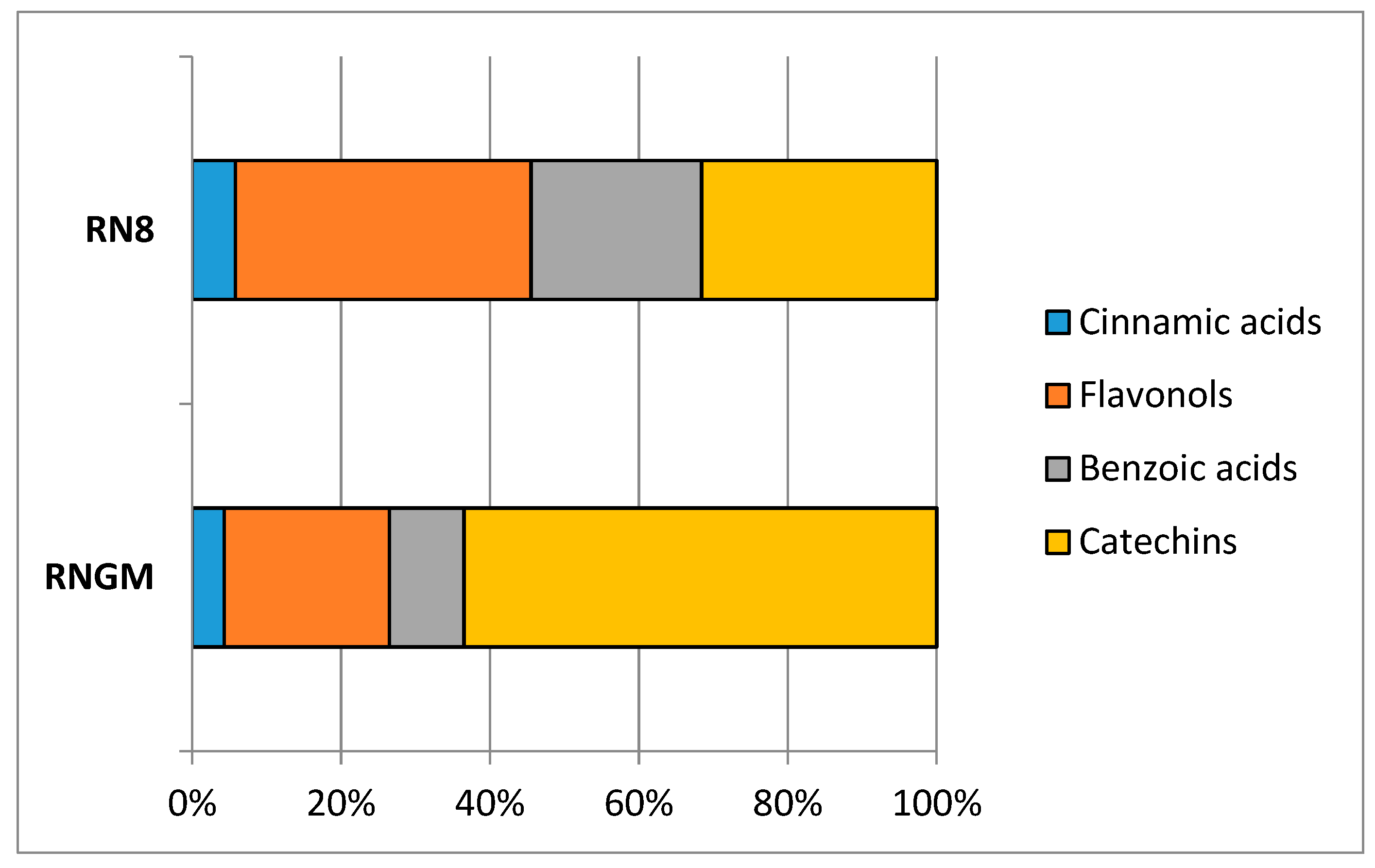

| Experiment | Experimental Conditions | Response Variable | |
|---|---|---|---|
| X1 Duty Cycle (%) | X2 Extraction Time (min) | Y PC1 Scores | |
| RN1 | −1 (50) | −1 (10) | 3.222515159 |
| RN2 | +1 (80) | −1 (10) | 2.256792019 |
| RN3 | −1 (50) | +1 (20) | 0.834250201 |
| RN4 | +1 (80) | +1 (20) | −0.535350397 |
| RN5 | −1 (50) | 0 (15) | 0.587361402 |
| RN6 | +1 (80) | 0 (15) | −1.165491045 |
| RN7 | 0 (65) | −1 (10) | −0.327200585 |
| RN8 | 0 (65) | +1 (20) | −2.638815847 |
| RN9 | 0 (65) | 0 (15) | −2.234060907 |
| Determination | RNGM | RN8 | |||
|---|---|---|---|---|---|
| Mean Value | SD | Mean Value | SD | ||
| TPC | mg GAE/100 mL bud extract | 276.44 | 3.85 | 415.56 | 5.52 |
| RSA | mg AAE/100 mL bud extract | 1137.04 | 38.49 | 1158.58 | 73.24 |
| Bioactive Class | Compound | RNGM | RN8 | ||
|---|---|---|---|---|---|
| Mean Value | SD | Mean Value | SD | ||
| (mg/100 g FW) | (mg/100 g FW) | ||||
| Cinnamic acids | caffeic acid | 22.48 | 0.04 | 20.76 | 0.48 |
| chlorogenic acid | n.d. | / | n.d. | / | |
| coumaric acid | 5.21 | 0.15 | 1.05 | 0.25 | |
| ferulic acid | n.d. | / | n.d. | / | |
| Flavonols | hyperoside | n.d. | / | n.d. | / |
| isoquercitrin | n.d. | / | n.d. | / | |
| quercetin | 49.53 | 0.49 | 80.14 | 1.08 | |
| quercitrin | 30.86 | 0.85 | 48.18 | 0.94 | |
| rutin | 17.25 | 0.22 | 20.88 | 0.48 | |
| Benzoic acids | ellagic acid | 69.66 | 0.08 | 75.37 | 0.30 |
| gallic acid | 0.31 | 0.09 | 0.64 | 0.05 | |
| Catechins | catechin | 95.88 | 0.26 | 55.85 | 2.78 |
| epicatechin | 59.83 | 0.37 | 49.08 | 0.48 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turrini, F.; Donno, D.; Beccaro, G.L.; Zunin, P.; Pittaluga, A.; Boggia, R. Pulsed Ultrasound-Assisted Extraction as an Alternative Method to Conventional Maceration for the Extraction of the Polyphenolic Fraction of Ribes nigrum Buds: A New Category of Food Supplements Proposed by The FINNOVER Project. Foods 2019, 8, 466. https://doi.org/10.3390/foods8100466
Turrini F, Donno D, Beccaro GL, Zunin P, Pittaluga A, Boggia R. Pulsed Ultrasound-Assisted Extraction as an Alternative Method to Conventional Maceration for the Extraction of the Polyphenolic Fraction of Ribes nigrum Buds: A New Category of Food Supplements Proposed by The FINNOVER Project. Foods. 2019; 8(10):466. https://doi.org/10.3390/foods8100466
Chicago/Turabian StyleTurrini, Federica, Dario Donno, Gabriele Loris Beccaro, Paola Zunin, Anna Pittaluga, and Raffaella Boggia. 2019. "Pulsed Ultrasound-Assisted Extraction as an Alternative Method to Conventional Maceration for the Extraction of the Polyphenolic Fraction of Ribes nigrum Buds: A New Category of Food Supplements Proposed by The FINNOVER Project" Foods 8, no. 10: 466. https://doi.org/10.3390/foods8100466
APA StyleTurrini, F., Donno, D., Beccaro, G. L., Zunin, P., Pittaluga, A., & Boggia, R. (2019). Pulsed Ultrasound-Assisted Extraction as an Alternative Method to Conventional Maceration for the Extraction of the Polyphenolic Fraction of Ribes nigrum Buds: A New Category of Food Supplements Proposed by The FINNOVER Project. Foods, 8(10), 466. https://doi.org/10.3390/foods8100466









