3.1. Effects of TCA/Acetone and N-Hexane Defatting on the Protein Concentration of Gastric and Gastrointestinal Digests of Whole Peanuts
We compared two defatting treatments, n-hexane and TCA/acetone, with the full, non-defatted liquid gastric and gastrointestinal digestion phases of raw, boiled, and roasted peanuts. The methodological prerequisite for assessing the extraction efficacy and digestibility of peanut proteins in simulated juices is a defatting procedure. According to our results, the choice of defatting procedure alters the results when examining protein concentration, extraction, and gastrointestinal digestion of differently thermally treated peanuts. Protein concentration was assessed by BCA assay and densitometry tests (
Figure 1). Both tests have their advantages and disadvantages when examining complex proteomes and digestomes, such as the full food matrix of non-defatted, gastric digestion phase of raw, boiled, and roasted peanuts being electrophoretically resolved and are presented in
Figure 2. The BCA test, on the other hand, is suitable for digestomic/peptide concentration measurements, and achieves a better performance compared to other colorimetric tests in resisting the interference to other substances, though it is not devoid of interferences [
13], and many times we have found falsely high results with the BCA test in complex protein mixtures rich in polyphenolics and sugars (unpublished data).
In
Figure 1A, it can be seen that the protein concentrations as measured by BCA assay in non-defatted control peanut samples are 21.0 mg/mL for raw and 13.0 mg/mL for boiled and roasted, the former being similar to the 16.6 mg/mL of extracted peanut proteins at pH 3.0 [
15]. The trend we observed is in agreement with the one described by Schmitt and colleagues in 2010 [
3]. Lower protein concentrations in boiled peanut samples can be only partially attributed to the leaching of peanut proteins into the cooking water [
11,
16,
17]. These results were supported by our experiments, in which 5 mg of proteins leached into 50 ml of water during 20 min of boiling, representing 2.6% of the maximal amount of extracted boiled peanut proteins under the conditions set for gastric digestion. Different extraction conditions (neutral pH, different buffers, longer time) and longer boiling time were employed (30 min) in other studies [
16,
17], resulting in an increased percent of leached proteins of 4% and 5.2% out of total extracted boiled peanut proteins, respectively. A decrease in protein concentration in the case of non-defatted digested roasted peanut is, most probably, the result of diminished protein solubility due to the excessive protein aggregation promoted by the Maillard reaction during roasting [
11,
18]. The protein content in the differently thermally treated peanut samples subjected to digestion showed a similar trend to the control samples, but with lower values (16.0 mg/mL, 9.0 mg/mL, and 6.0 mg/mL for the raw, boiled, and roasted peanuts, respectively). In addition, higher protein concentrations in the control compared to digested samples were observed in all full liquid phases, regardless of the assessment methodology (
Figure 1A,B). We assume that this phenomenon is a consequence of the decreased content of peptide bonds imposed by pepsin action and, hence, a diminished ability to form colored complexes with BCA molecules (e.g., single amino acids and dimers are out of the test sensitivity when 60 °C heating is applied during incubation). On the other hand, small peptides (below four amino acids) easily migrate after the dye front (bromophenol blue line), with a possibility of running out of SDS-PAGE and are thus excluded from densitometry measurements.
In contrast to the case of simulated gastric digestion, the opposite trend was observed across thermally treated peanuts in simulated intestinal digestion; the raw peanut samples exhibited the lowest protein concentrations and the roasted samples the highest (BCA assay,
Figure 1A,B). TCA/acetone defatting treatment replicated the trends imposed by digestion and thermal processing seen in non-defatted gastric digestion samples, with a change of trend in the intestinal digestion, whereby digested samples of boiled and roasted peanuts had higher protein concentrations compared to their control counterparts as detected by both techniques (
Figure 1A,B). Interestingly, n-hexane defatting of gastric digests diminished the protein concentrations in control samples to levels lower than the concentrations in the digested samples. However, n-hexane treatment did not alter the protein concentration trends when thermal treatments were compared (
Figure 1A). In addition, the absolute values of protein concentrations were higher in all TCA/acetone defatted samples compared to the respective counterparts of n-hexane defatted samples (
Figure 1A,B). Therefore, even at this stage of the assessment, a preference for the choice of TCA/acetone as the more suitable defatting treatment of peanut digests was noted.
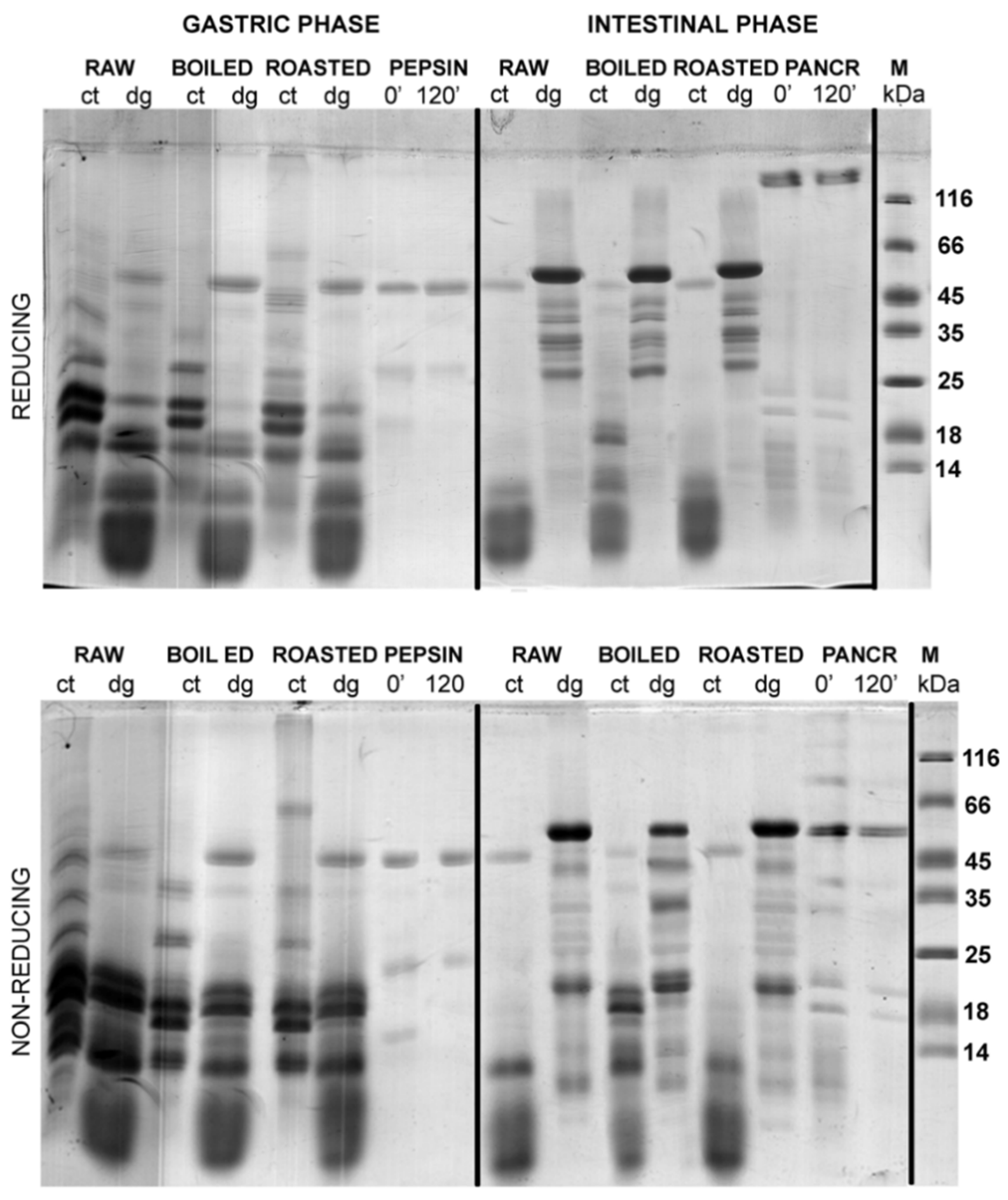
3.2. Effects of TCA/Acetone and N-Hexane Defatting on SDS-PAGE Profiles of Gastric and Gastrointestinal Digests of Whole Peanuts
A comparison of the efficacy of defatting procedures in preserving the proteins in defatted gastric digests and corresponding controls was undertaken by densitometry analysis (
Figure 1A,
Table S1) that included the results following normalizing against non-defatted samples (
Figure 2,
Figure 3 and
Figure 4). In raw, boiled, and roasted peanut gastric digestion controls, protein concentrations were reduced to 76%, 76%, and 85% of their value upon TCA precipitation, respectively. In the case of gastric digests of raw, boiled, and roasted peanuts, protein loss upon TCA/acetone defatting was 68%, 66%, and 81% of the protein concentration without defatting, respectively. The same calculation was applied to the n-hexane treatment and, in this case, the situation was slightly more unfavorable: for the gastric digestion control of raw, boiled, and roasted peanut samples, protein loss was 84%, 83%, and 90%, respectively. The samples of raw, boiled, and roasted peanuts digested in conditions simulating gastric digestion lost 55%, 50%, and 75% of their protein content, respectively, upon n-hexane defatting. However, this was a consequence of the inverse trend between the control and digested samples upon n-hexane defatting, observed in the gastric phase (
Figure 1A). This is also an additional argument favoring the TCA/acetone defatting step over n-hexane defatting when this step is mandatory for downstream applications.
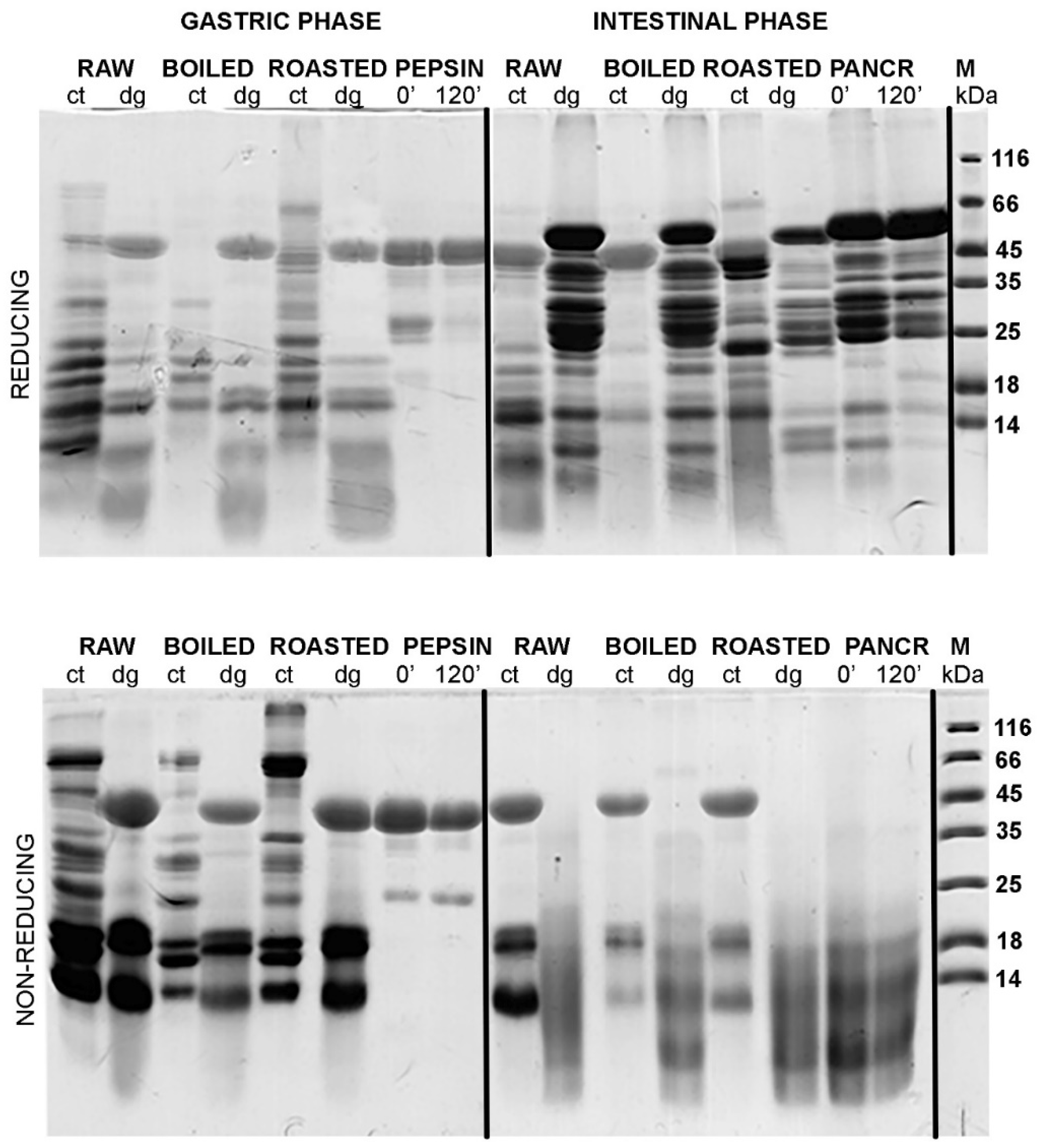
Figure 3 and
Figure 4 show the protein profiles of the thermally treated control and digested peanut samples in reducing and non-reducing conditions after defatting with n-hexane and TCA/acetone, respectively. Generally, TCA/acetone defatting provided samples with similar SDS-PAGE profiles (
Figure 4) to the ones observed in the non-defatted gastric digests in
Figure 2. Within all lanes in both
Figure 3 and
Figure 4, the usual smears of lipids in lower molecular masses are absent when compared to the non-defatted control samples (
Figure 2), facilitating observance of lower molecular mass proteins, such as Ara h 2 and Ara h 6, which are present as triplets in the range of 15–18 kDa.
A study conducted by Wacyzk and colleagues [
19] on the defatting of raw and thermally processed peanuts followed by protein extraction showed that the defatting procedure affects subsequent extraction of proteins, with defatting with n-hexane providing a higher yield of Ara h 1 and Ara h 2 allergens in the peanut extract. However, in our case, when defatting was undertaken upon extraction, Ara h 1 was more preserved in the sample if TCA/acetone defatting was applied (
Figure 3 and
Figure 4).
The additional reason we opted for TCA/acetone precipitation of proteins as a favorable defatting procedure for the follow-up of this study was that our main focus was to investigate the digestion effects on larger fragments of molecular masses higher than 10 kDa. We are aware that for the investigation of a whole peanut digestome, it is necessary to identify small digestion-resistant fragments [
8]. Since TCA/acetone precipitation is not quantitative and leads to the loss of small fragments, this may mislead us in concluding whether changes come from the difference in digestibility of differently treated peanuts or from washing out small peptides. However, TCA/acetone precipitation was exploited in our study because the amount of recovered larger digestion fragments was higher than upon defatting with n-hexane.
3.3. Effects of the Lipid Content in the Food Matrices on Gastric Digestion
In
Figure S2, various fresh and processed foods are presented as examples of different food matrices that were digested according to the in vitro static INFOGEST protocol for gastric digestion: fresh spinach, pasteurized milk, raw walnuts, raw hazelnuts, and boiled chicken breasts. Upon digestion, the samples were defatted with TCA/acetone in the same way as the raw and thermally processed peanuts. Even though the majority of the protein content was lost, as in the peanuts (
Figure 1,
Figure 2, and
Figure 4), similar SDS-PAGE profiles were obtained when compared with the corresponding non-defatted samples (data not shown).
Figure S2 represents the capabilities of different food matrices to protect proteins present in them from degradation. In our experimental setup, walnut and, to a slightly lesser extent, milk matrices were the most efficient in preserving proteins during gastric digestion, as the largest protein fragments are seen in these digests. This observance is probably due to the higher content of lipids present, especially in walnuts compared to chicken breasts and spinach. The SDS-PAGE profiles of peanuts, walnuts, and hazelnuts (
Figure 2,
Figure 3 and
Figure 4, and
Figure S2) indicate that the lipids are an important factor in hampering simulated gastrointestinal digestion. Though the use of gastric lipase is proposed by the novel, updated INFOGEST 2.0 digestion protocol, we did not use it since, at the time of conductance of this research, this protocol had not been published [
10]. However, the inclusion of gastric lipase in the simulated gastric digestion is highly recommended, since it will contribute to more realistic digestion conditions, especially in lipid-rich food samples. The hydrolysis of lipids might also indirectly affect pepsin digestion. Moreover, the use of lipase would probably ease the manipulation of digested samples, since the prerequisite defatting steps might be able to be omitted.
When the food samples digested in simulated gastric and gastrointestinal conditions were thoroughly defatted in preparation for SDS-PAGE analysis, the loss of some proportion of the allergenic fragments and intact proteins was hard to avoid. We believe that the different defatting approaches for food samples and food digests are an important cause of the discrepancy observed in peanut allergen digestion resistance reported by different research groups, even when the same INFOGEST protocol is applied for the peanut digestion [
7,
8,
9]. In our view, the most reproducible results were obtained when non-repetitive defatting with TCA/acetone was used on digested samples. In our current work on hazelnuts, we have succeeded in developing a 2D SDS-PAGE protocol with non-defatted samples. We strongly recommend opting for the optimized electrophoretic protocols instead of defatting the samples, whenever possible. In addition, it is possible to run shotgun proteomics and safeguards against excessive loss of peptides analyzing big and small fragments at once, as shown in the study of whole peanut grain digestome by Di Stasio and colleagues [
9].
3.4. Effect of Thermal Processing on Protein Extraction and Digestion in the Gastric and Gastrointestinal Digestion Conditions
Our data show that the thermal processing of peanuts (i.e., boiling and roasting) decreases the solubility of peanut proteins and hampers their 120 min extraction by simulated gastric fluid (control samples in
Figure 1A,
Figure 3, and
Figure 4). This conclusion is supported by the statistical evaluation of protein concentrations of the gastric digestion control samples, where the protein concentrations of the samples extracted from raw peanuts were significantly higher than the protein concentrations of boiled and roasted peanuts. Though there is no statistically significant difference among the protein concentrations in the samples extracted from the boiled and the roasted peanuts, it is obvious from our data that the roasted samples had a slightly higher concentration of extracted proteins compared to the boiled samples (
Figure 1A).
However, this is not the general conclusion regarding the solubility and efficacy of the extraction of the peanut proteins upon thermal treatment. After extraction in simulated intestinal fluid digestion, the opposite—an increasing trend of solubility/extraction of the peanut proteins—was observed, with the proteins from roasted peanuts being the most easily extracted and providing the highest concentrations in the samples (
Figure 1B). We assume that the peanut proteins of the thermally treated samples are extracted with a delay in comparison to the raw peanut proteins. Moreover, we suggest that alkaline solutions (mimicking intestinal conditions) facilitate the extraction of the peanut proteins more than the acidic conditions of simulated gastric fluid. This phenomenon might be particularly relevant for understanding the sensitizing potential and the allergenicity of peanuts. Stemming from studies performed on animal models, limited proteolysis of proteins is crucial for allergenicity [
20]. Here, we have observed that the thermal treatment of peanuts enhances solubility/extraction of large fragments and digestion-resistant peptides in the intestines, where sensitization occurs through the interaction of large proteinaceous entities with the immune system.
Generally, the protein extraction trend of the digestion control samples across the thermal treatments was replicated in the digested samples (
Figure 1). Overall, gastric digestion efficacy, assessed by BCA tests and densitometry (
Figure 1A,
Table S1), ranges between 30–55% for raw and boiled samples and 40–50% for roasted peanut samples, depending on the applied protein estimation approach. The differences in digestion efficacy of the proteins of raw, boiled, and roasted peanuts is not statistically significant (
Figure 1A). However, upon completion of the intestinal phase of digestion, significant changes in the digestion efficacy are observed; the overall efficacy was 50%, 18%, and 16% for the raw, boiled, and roasted peanut samples, respectively (
Figure 1B,
Table S1). Pepsin possesses superior digestion efficacy for raw peanuts compared to thermally treated peanuts. However, we should not forget that the extraction of proteins from these two preparations was also higher than from raw peanuts. Finally, the effect observed through the difference of gastrointestinal efficacy is significant, because the absolute concentration values representing the digested portions are 8.5 mg/mL, 3.5 mg/mL, and 3.7 mg/mL for the raw, boiled, and roasted samples, respectively, as assessed by densitometry (
Figure 1B,
Table S1).
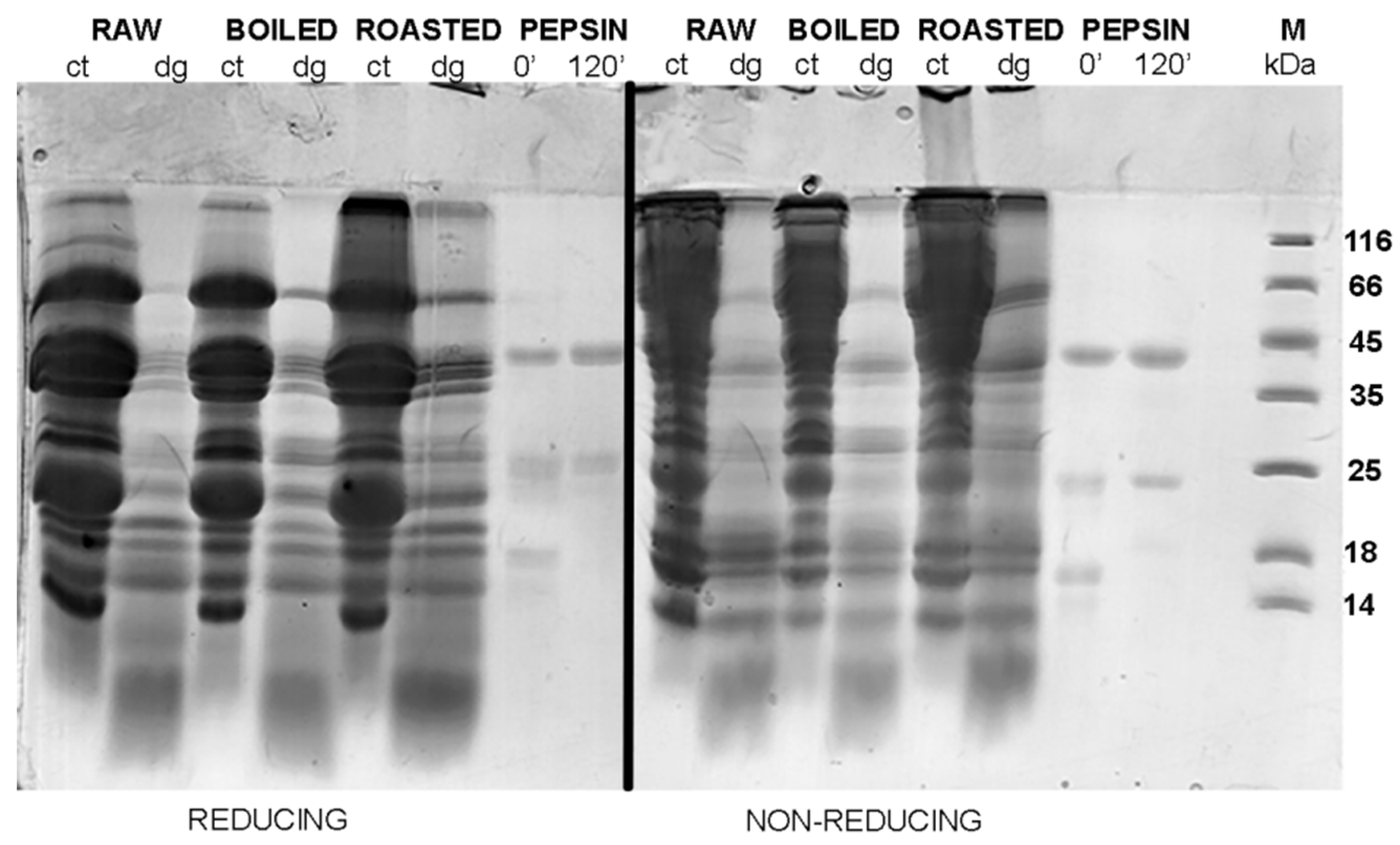
In the non-defatted control samples of the gastric digestion of roasted peanuts, protein bands on the molecular weights higher than 100 kDa were observed in the SDS-PAGE profiles (
Figure 2); these bands were more prominent in non-reducing conditions than in reducing conditions. The identity of these bands in boiled and roasted peanut preparations has not been confirmed with mass spectrometry; however, these bands could contain oligomers, as reported by Maleki and colleagues upon the roasting of the peanuts, because of the corresponding molecular weight (2000) [
18]. The abundance of these protein bands with potential oligomers decreased upon both defatting treatments (
Figure 3 and
Figure 4). In addition, these high molecular mass bands are susceptible to the action of pepsin, since they disappear after simulated gastric digestion in all defatting preparations and electrophoretic conditions (
Figure 2,
Figure 3 and
Figure 4).
A protein band around 65 kDa, corresponding to the protein Ara h 1, was visible in the control samples of the gastric digestion of raw and roasted peanuts, in both reducing and non-reducing conditions (
Figure 4). Like other proteins from the cupins family, Ara h 1 is thermostable and undergoes irreversible denaturation and extensive aggregation after passing through the dominant endothermal transition at temperatures above 80 °C [
21]. For our experiments, we used peanuts roasted for 20 min at 170 °C, which represents extensive thermal treatment. SDS-PAGE analysis indicated that Ara h 1 is one of the proteins that were most affected by roasting (
Figure 4). We anticipate that the thermal treatment we applied for roasting the peanuts not only favored the aggregation of Ara h 1, but also might enhance the IgE-binding capacity of this protein since the critical temperature for alteration of the IgE-binding properties is reported to be 140 °C [
17].
The basic subunit of Ara h 3 (25 kDa) is present in all digestion control samples of raw, boiled, and roasted peanuts (
Table S2) [
22]. However, it is easily digested by pepsin, since the corresponding band is missing in the digested samples (
Figure 4,
Table S2). It is easy to identify the acidic subunit of Ara h 3 in the digestion control samples of all three peanut protein preparations since it is represented as a myriad of bands in the 25–45 kDa region (
Figure 2,
Figure 3 and
Figure 4, and Figure 7, and
Table S2.) [
23]. In the samples obtained upon gastric/gastrointestinal digestion, only one band in that region is distinguished (
Figure 2,
Figure 3 and
Figure 4, and Figure 7). Moreover, it is obvious that the thermal processing dramatically affected the pepsin resistance of the acidic subunit of Ara h 3, since the 35 kDa bands are visible in the digests of the raw peanuts, but not in the digests of boiled peanuts (
Figure 4). The roasting of peanuts affected Ara h 3 in a similar manner as Ara h 1; aggregates were formed and the ability to bind the IgE antibodies was increased [
24].
Ara h 2 is seen in the SDS-PAGE as a doublet of bands of 17 kDa and 19 kDa [
25], and we observed it in all control samples of gastric/gastrointestinal digestions of peanuts, regardless of thermal treatment or electrophoretic (reducing or non-reducing) conditions (
Figure 4). Hence, our findings support the previously reported thermostable nature of this protein. However, a lower abundance of Ara h 2 was noticed in the boiled peanut preparations, at least in part due to the leaching of proteins from the peanuts during cooking [
16,
17], and the effect increased with the duration of boiling [
16].
Figure S3 shows the protein profile of peanut proteins that leaked into cooking water (water was ten times concentrated before the SDS-PAGE). Only fragments of 25 kDa and lower are observed (
Figure S3), with a prominent Ara h 2 band at 18 kDa, which is in accordance with previous studies [16, 17].
Peanut conglutins (i.e., Ara h 2 and Ara h 6) are pepsin-digestion resistant not only due to their conserved globular structure but also due to the protective features of the lipid-rich peanut matrix. This specificity of the peanut matrix results in the delayed extraction of the proteins and makes conglutins unavailable for digestion by pepsin at the early onset of gastric digestion. Our results are completely in line with widely accepted knowledge on Ara h 6 as an extremely stable and proteolytically-resistant protein since this protein is detected in all control and gastric-digested samples of raw, boiled, and roasted peanuts. In contrast to gastric digestion, intestinal digestion of Ara h 2 and Ara h 6 by pancreatin results in significant degradation of these two proteins.
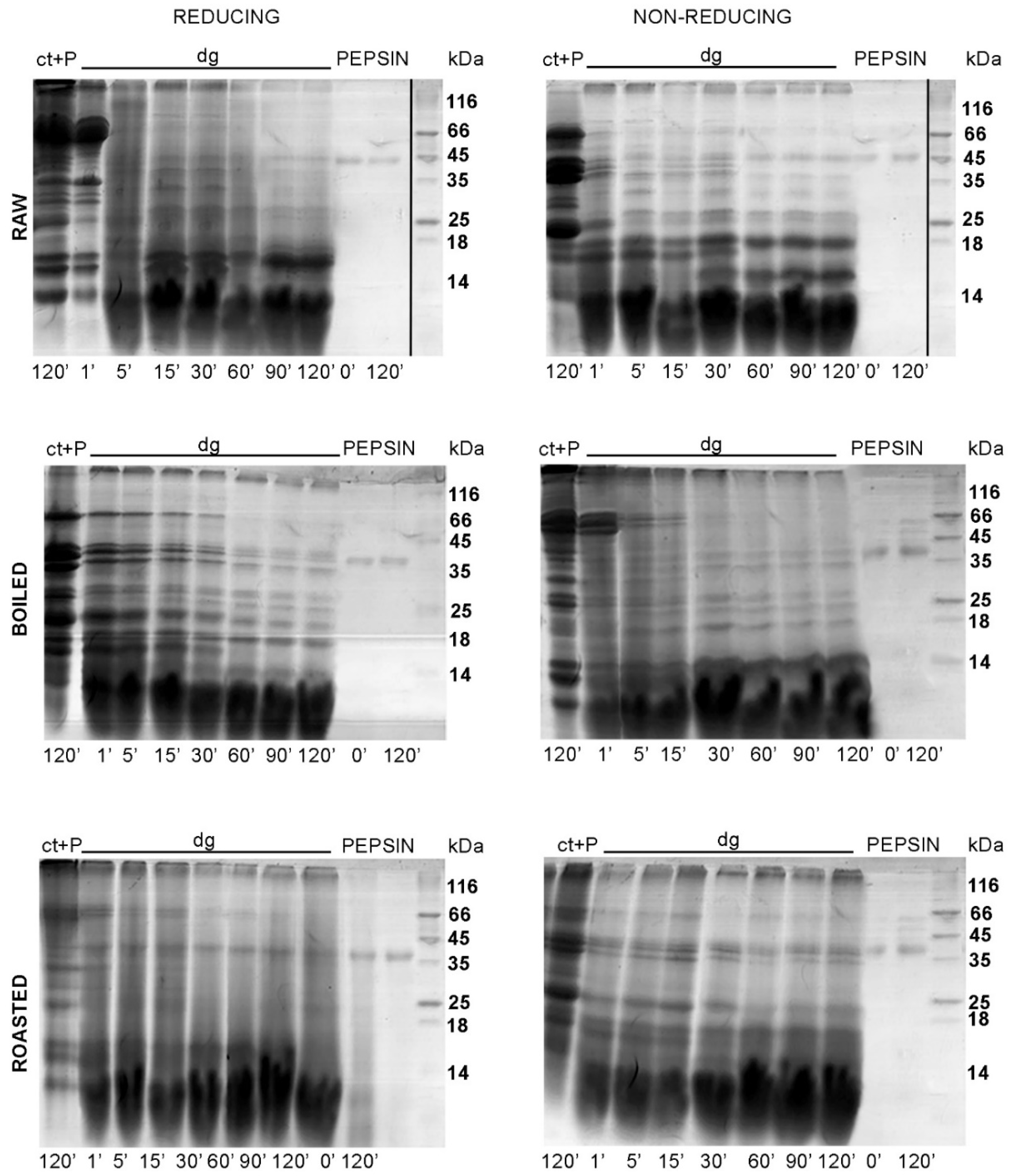
We also aimed to characterize the gastric digestion of thermally treated peanuts over a 2 h time course. Our focus was on peanut proteins of higher molecular weights, such as Ara h 1 and Ara h 3. In raw peanuts, these two proteins were digested by pepsin in between 60 and 90 min (
Figure 5). At those critical time points only Ara h 1 (approximately 65 kDa) was digested in the boiled and roasted peanuts, while the fragments derived from Ara h 3 were still visible in the gastric digests of thermally treated peanuts. Moreover, in all preparations, large protein fragments were visible in the region of 25–45 kDa. Our data suggest that the monitoring of the gastric digestion of peanuts should be extended for a period of 120 min, in contrast to the 10 min gastric digestion in the study by Rao and colleagues [
7].
3.6. Raw Peanut Gastric Digestion Assessment by the Improved NLC-MS/MS Method and Bioinformatics Approach
A thorough assessment of the gastrointestinal digestion efficacy solely by the densitometry analysis of SDS-PAGE profiles is prone to erroneous estimations, due to the possible overlapping of the different protein fragments at the same position in the gel. Moreover, this is of great importance when assessing the digestion efficacy of the proteins of lower molecular weights, since the presence of the fragments derived from the proteins of higher molecular weights in the same position in the gel could cause false readings, and higher quantities could be assigned to the lower molecular-weight protein.
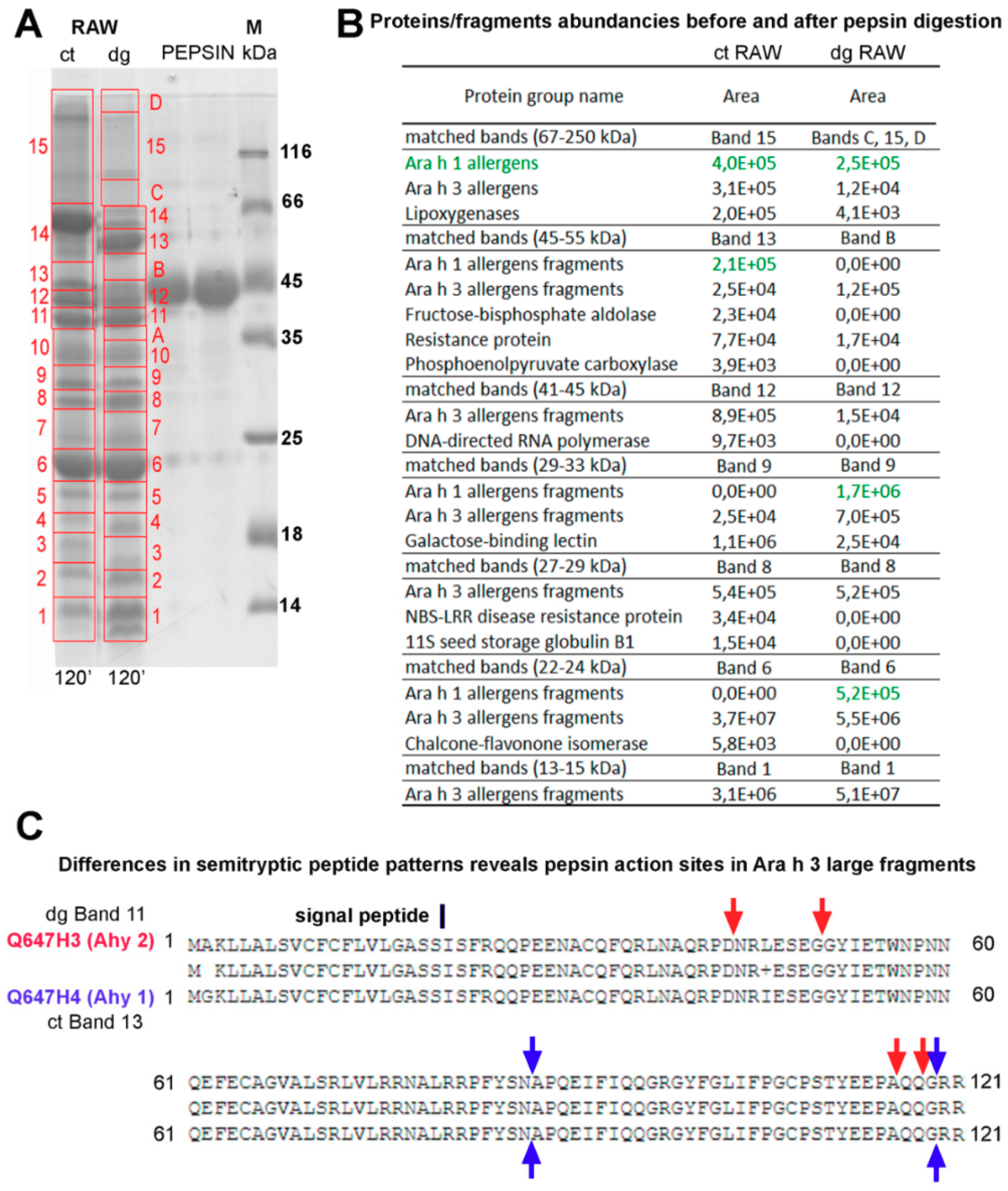
Therefore, we have applied a pilot assessment using high-resolution tandem mass spectrometry and the PEAKS Suite X software’s post-translational modification search engine (PEAKS PTM) to assess the pepsin digestion efficacy of raw peanuts. This approach is, unlike the label-free in solution approach, only semi-quantitative and these results should be taken with caution. However, we have compared the amount of each protein or allergen to matched gel bands (
Figure 7A). Moreover, the comparison of the detected peptide sequence profiles of the same protein and its fragments from the control and digested raw peanuts provided us with information on the specific sites of the pepsin action (
Figure 7B).
The intact form of Ara h 1 (65 kDa) was partially digested and its digestion pattern is given in
Figure 7A (band 15 and green letters). In contrast to Ara h 1, Ara h 3, which was also detected in this region in the control sample, was almost completely digested. We have obtained, also, that lipoxygenase (band 15), galactose-binding lectin (band 9), and 11S seed storage protein (band 8) were almost completely digested by pepsin (
Figure 7A). These findings are in the agreement with the widely accepted consideration of these proteins as being susceptible to pepsin digestion.
Regarding the digestion fate of the major allergen Ara h 1, the accumulation of Ara h 1-derived fragments occurs upon pepsin action. More specifically, Ara h 1-derived fragments were detected in bands nine (approximately 29–33 kDa range) and six (approximately 22–24 kDa range) of the digested sample, while Ara h 1-derived fragments were completely absent in the control within the corresponding regions. In contrast to Ara h 1, which is resistant to pepsin digestion to a decent extent, Ara h 3 was almost completely digested by pepsin, even in the case of band six where it seems to be unaffected by pepsin (
Figure 7A). In this band, we detected digestion fragments of the acidic Ara h 3 subunit and accumulation of other Ara h 3-derived fragments. Finally, band one (13–15 kDa range) of the digested sample shows an accumulation of small Ara h 3 peptides to a high extent. A detailed examination of
Table S2 provides information on fragments accounting for the accumulation in certain gel bands in the digested sample, and fragments that were originally present in the specific mass range, by matching them to the proteins in the control sample band.
Additionally, we succeeded in identifying the pepsin proteolytic sites by comparing homologs N-terminal fragments of the acidic Ara h 3 subunit found in the 25–45 kDa region, and they included peptide bonds between the following amino acids: aspartic acid and asparagine, glycine and glycine, alanine and glutamine, and glutamine and glycine (
Figure 7B). In addition, no difference in the PTM qualitative appearance between the control and digested preparation of raw peanuts was observed (
Table S2).