Metabolic Stability of D-Allulose in Biorelevant Media and Hepatocytes: Comparison with Fructose and Erythritol
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Assay Media for The Stability Study
2.2.1. FaSSIF and SGF
2.2.2. Hepatocytes
2.3. Preliminary Stability Assay
2.4. Metabolic Stability in Simulated Gastrointestinal Fluids
2.5. Metabolic Stability in Primary Hepatocytes
2.6. In Vivo Systemic Pharmacokinetic Study in Rats
2.7. LC-MS/MS Analysis
2.8. Data Analysis
(where t1/2 = elimination half-life)
(Where Qh = Hepatic blood flow, fu,b = unbound fraction in blood, assumed as 1)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Analytical Methods Using LC-MS/MS
3.2. Metabolic Stability Study
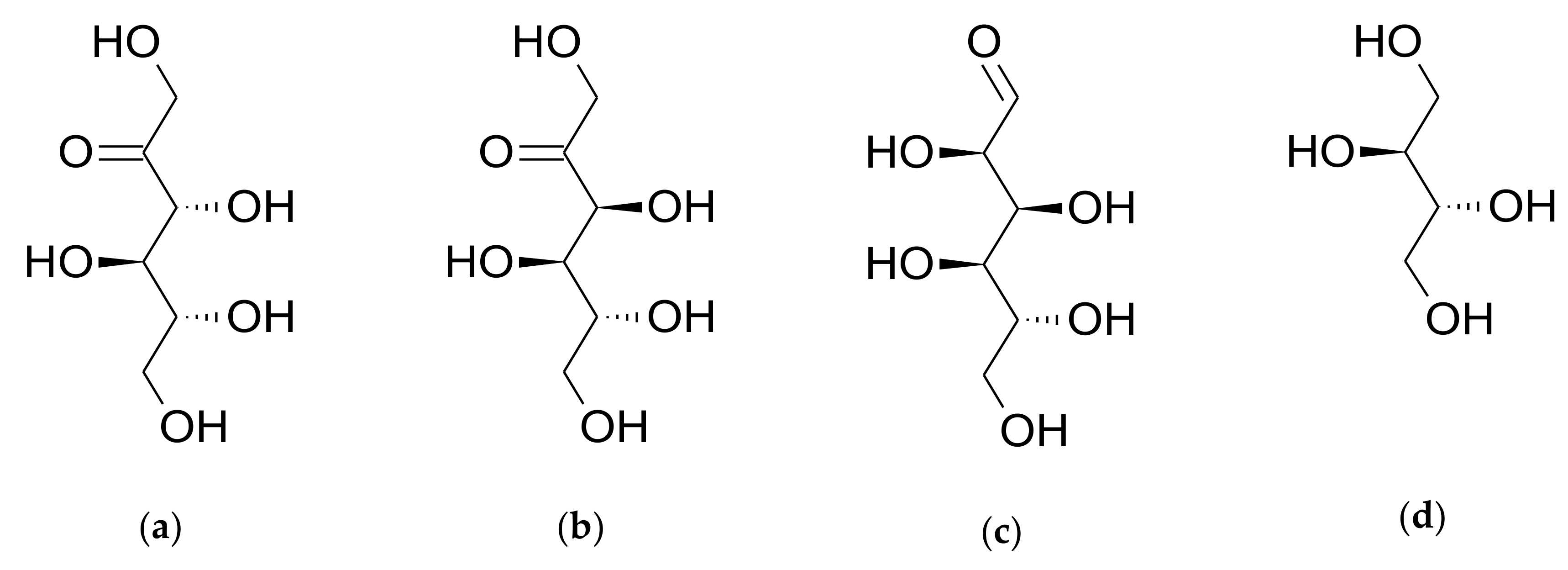
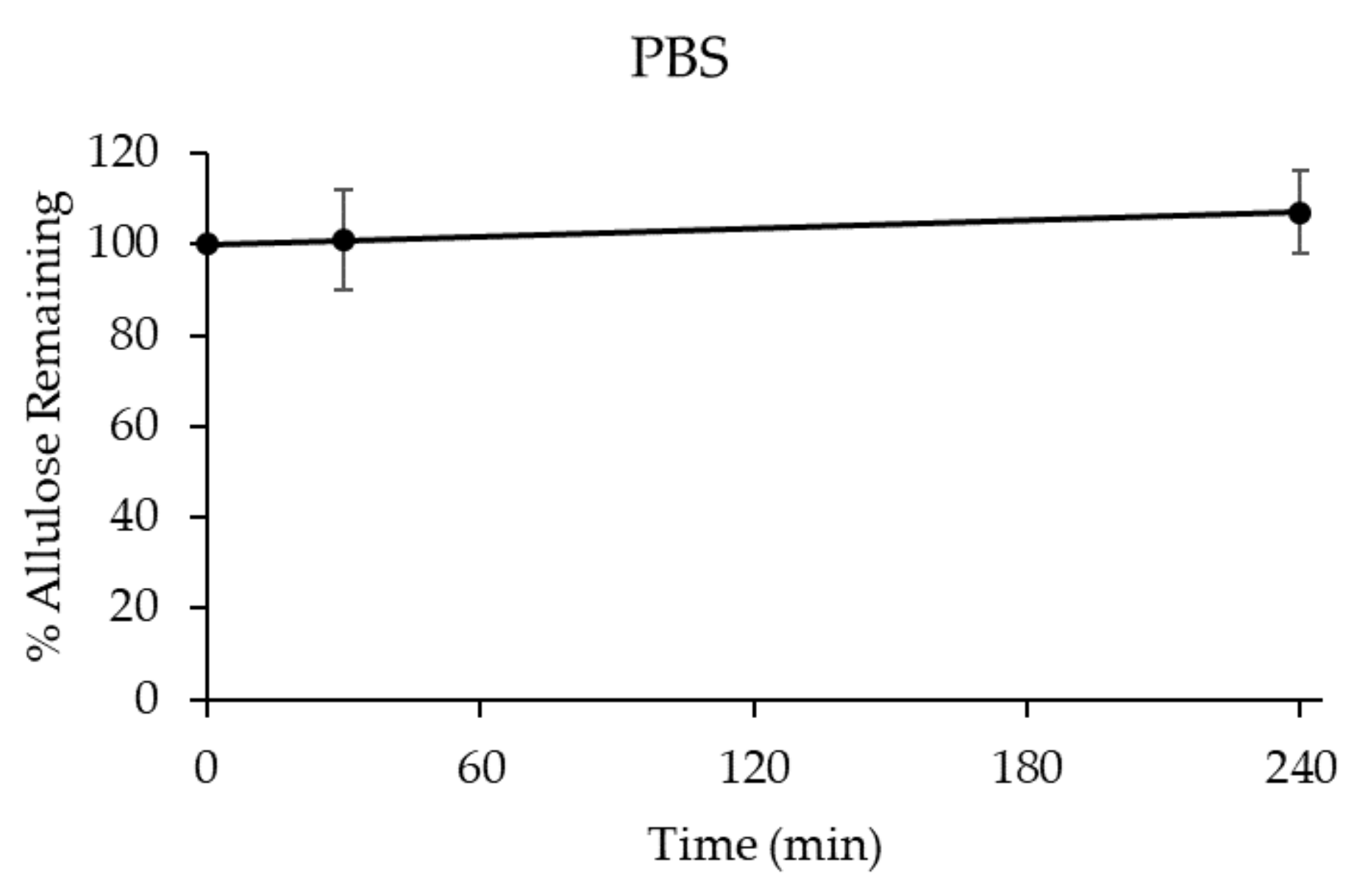
3.2.1. Stability of D-allulose in PBS (pH 7.4)
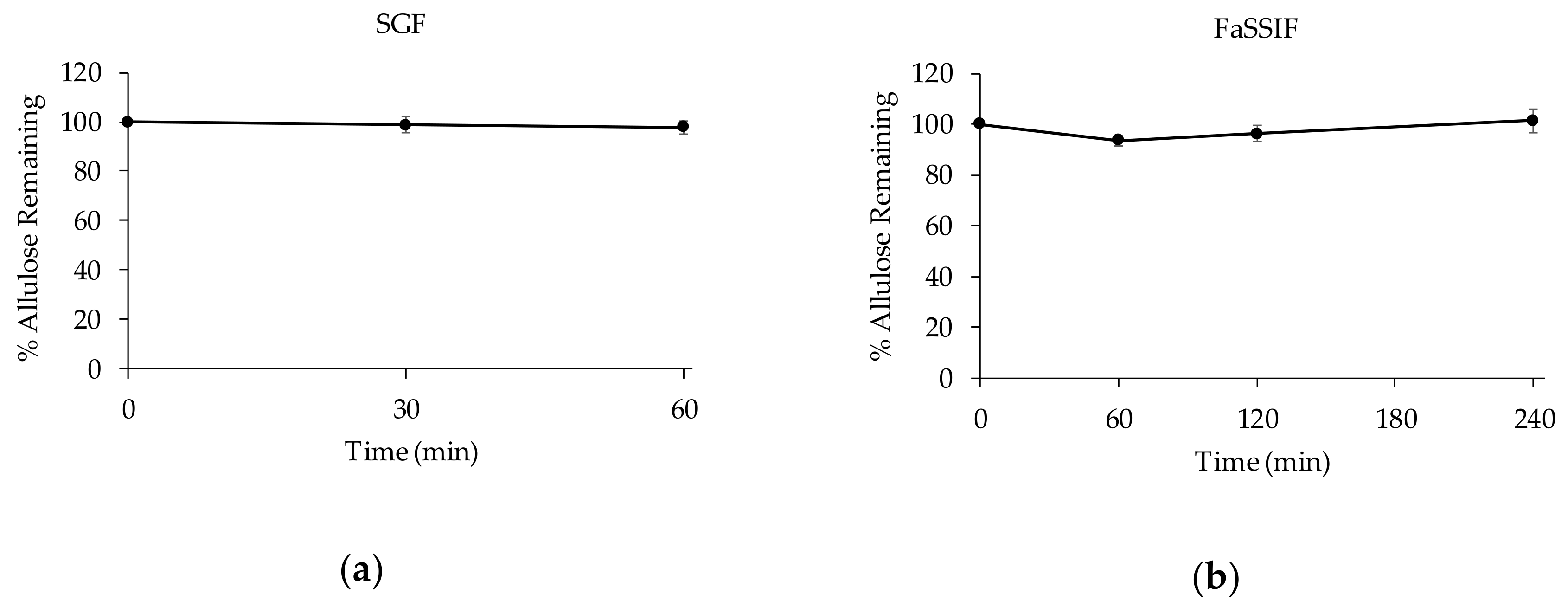
3.2.2. Stability of D-allulose in Biorelevant Media
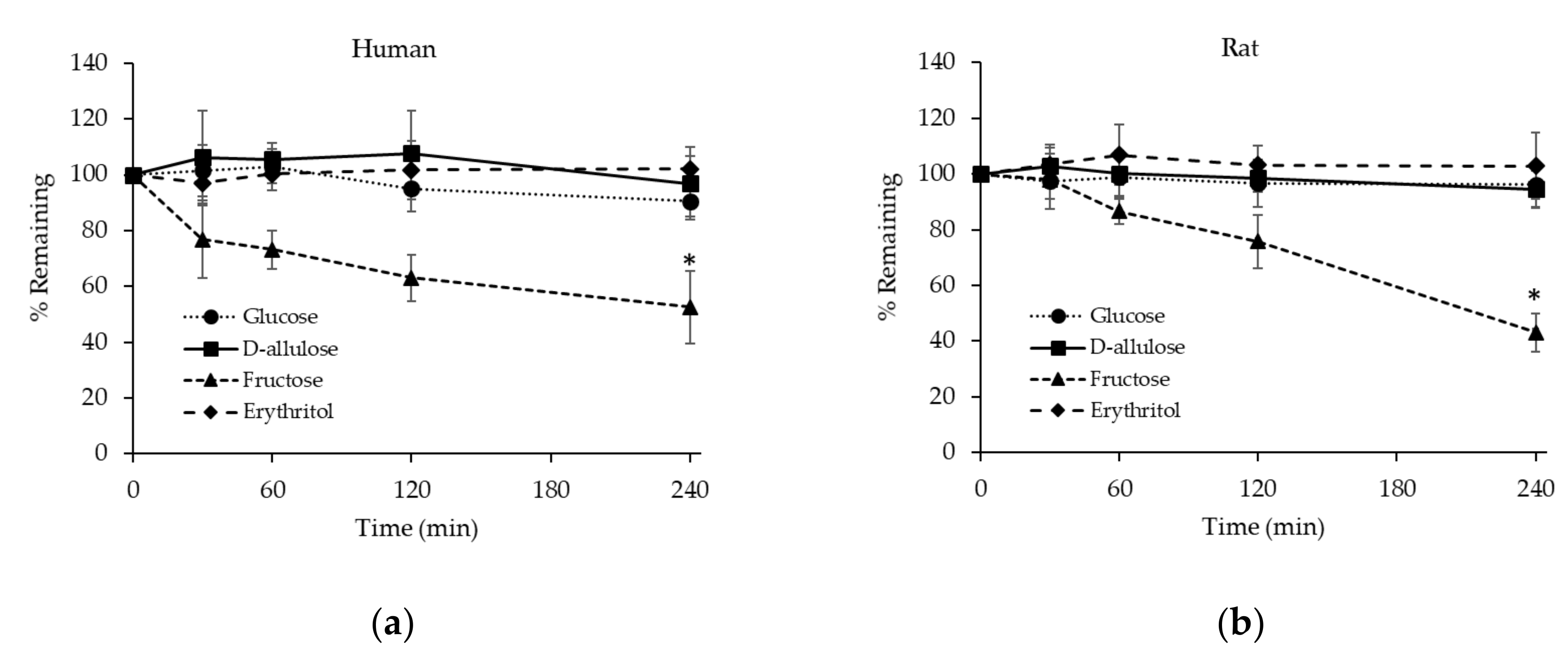
3.2.3. Stability of D-allulose, Glucose, Fructose, and Erythritol in Hepatocytes
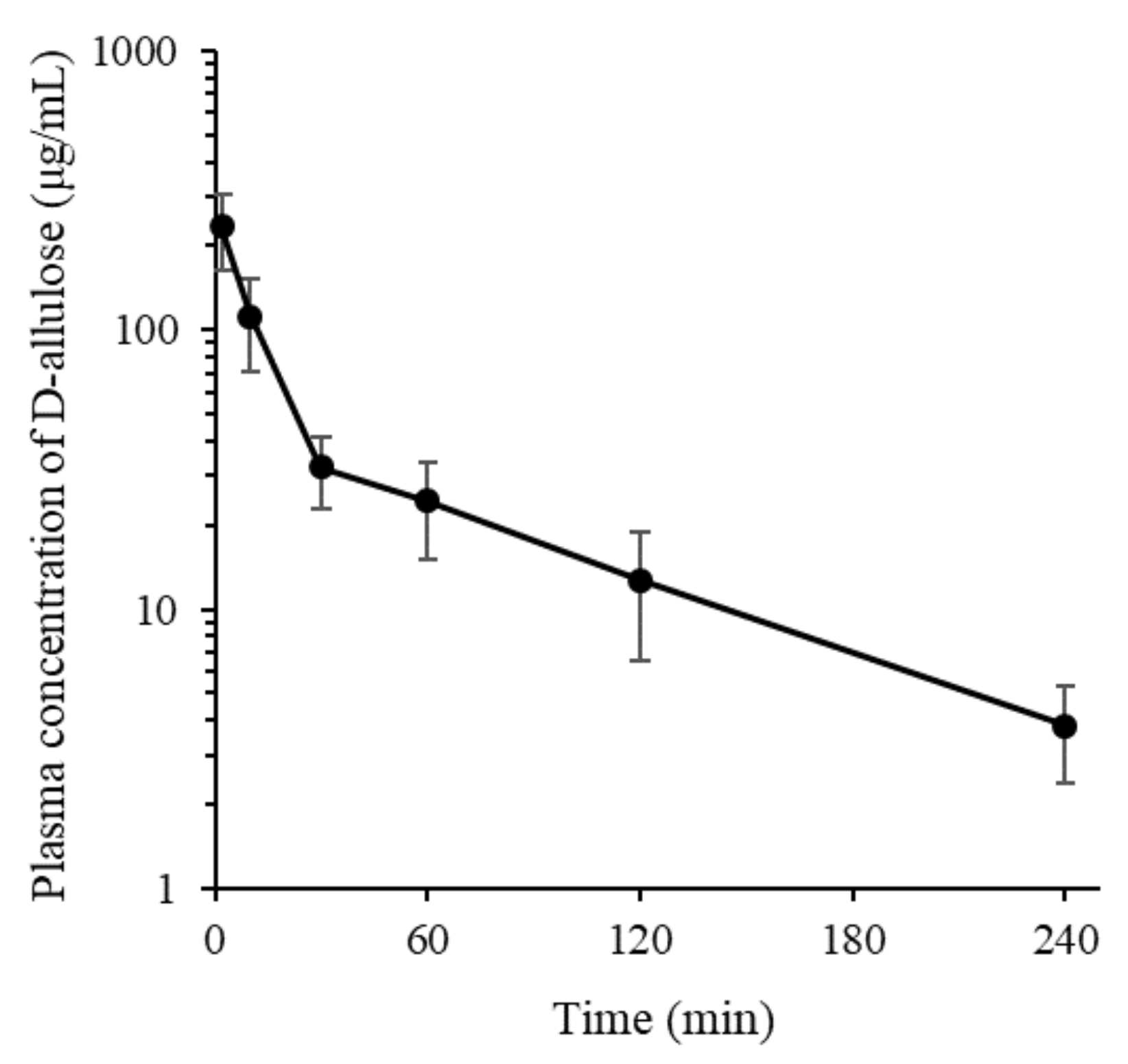
3.3. In Vivo Systemic Pharmacokinetic Study in Rats
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhurosy, T.; Jeewon, R. Overweight and obesity epidemic in developing countries: A problem with diet, physical activity, or socioeconomic status? Sci. World J. 2014, 2014, 964236. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Lê, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 79, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef]
- Moreno-Fernández, S.; Garcés-Rimón, M.; Vera, G.; Astier, J.; Landrier, J.F.; Miguel, M. High fat/high glucose diet induces metabolic syndrome in an experimental rat model. Nutrients 2018, 10, 1502. [Google Scholar] [CrossRef]
- Cree, G.M.; Perlin, A.S. O-isopropylidene derivatives of D-allulose (D-psicose) and D-erythro-hexopyranos-2,3-diulose. Can. J. Biochem. 1968, 46, 765–770. [Google Scholar] [CrossRef]
- Mu, W.; Zhang, W.; Feng, Y.; Jiang, B.; Zhou, L. Recent advances on applications and biotechnological production of D-psicose. Appl. Microbiol. Biotechnol. 2012, 94, 1461–1467. [Google Scholar] [CrossRef]
- Matsuo, T.; Suzuki, H.; Hashiguchi, M.; Izumori, K.J. D-psicose is a rare sugar that provides no energy to growing rats. J. Nutr. Sci. Vitaminol. 2002, 48, 77–80. [Google Scholar] [CrossRef]
- Matsuo, T.; Izumori, K. Effects of dietary D-psicose on diurnal variation in plasma glucose and insulin concentrations of rats. Biosci. Biotechnol. Biochem. 2006, 70, 2081–2085. [Google Scholar] [CrossRef]
- Hossain, A.; Yamaguchi, F.; Matsuo, T.; Tsukamoto, I.; Toyoda, Y.; Ogawa, M.; Nagata, Y.; Tokuda, M. Rare sugar D-allulose: Potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol. Ther. 2015, 155, 49–59. [Google Scholar] [CrossRef]
- Iida, T.; Kishimoto, Y.; Yoshikawa, Y.; Hayashi, N.; Okuma, K.; Tohi, M.; Yagi, K.; Matsuo, T.; Izumori, K. Acute D-psicose administration decreases the glycemic responses to an oral maltodextrin tolerance test in normal adults. J. Nutr. Sci. Vitaminol. 2008, 54, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Zhang, W.; Feng, Y.; Jiang, B.; Zhou, L. Hypoglycemic health benefits of D-psicose. J. Agric. Food Chem. 2012, 60, 863–869. [Google Scholar]
- Hayashi, N.; Iida, T.; Yamada, T.; Okuma, K.; Takehara, I.; Yamamoto, T.; Yamada, K.; Tokuda, M. Study on the postprandial blood glucose suppression effect of D-psicose in borderline diabetes and the safety of long-term ingestion by normal human subjects. Biosci. Biotechnol. Biochem. 2010, 74, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Kitagaki, S.; Nakano, D.; Nishiyama, A.; Funamoto, Y.; Matsunaga, T.; Tsukamoto, I.; Yamaguchi, F.; Kamitori, K.; Dong, Y.; et al. Rare sugar D-psicose improves insulin sensitivity and glucose tolerance in type 2 diabetes Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biochem. Biophys. Res. Commun. 2011, 405, 7–12. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Memorandum to the File: Scientific Review of the Evidence on the Metabolism, Caloric Value, Glycemic Response, and Cariogenic Potential of Allulose. 2018. Available online: https://newfoodeconomy.org/wp-content/uploads/2019/05/FDA-2019-D-0725-0012.pdf (accessed on 16 May 2019).
- Iida, T.; Hayashi, N.; Yamada, T.; Yoshikawa, Y.; Miyazato, S.; Kishimoto, Y.; Okuma, K.; Tokuda, M.; Izumori, K. Failure of d-psicose absorbed in the small intestine to metabolize into energy and its low large intestinal fermentability in humans. Metabolism 2010, 59, 206–214. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. The Declaration of Allulose and Calories from Allulose on Nutrition and Supplement Facts Labels: Guidance for Industry. 2019. Available online: https://www.fda.gov/media/123342/download (accessed on 16 May 2019).
- Tsukamoto, I.; Hossain, A.; Yamaguchi, F.; Hirata, Y.; Dong, Y.; Kamitori, K.; Sui, L.; Nonaka, M.; Ueno, M.; Nishimoto, K.; et al. Intestinal absorption, organ distribution, and urinary excretion of the rare sugar D-psicose. Drug Des. Dev. Ther. 2014, 8, 1955–1964. [Google Scholar]
- Matsuo, T.; Tanaka, T.; Hashiguchi, M.; Izumori, K.; Suzuki, H. Metabolic effects of D-psicose in rats: Studies on faecal and urinary excretion and caecal fermentation. Asia Pac. J. Clin. Nutr. 2003, 12, 225–231. [Google Scholar] [PubMed]
- Betley, S.; Alberti, K.G.; Agius, L. Regulation of fatty acid and carbohydrate metabolism by insulin, growth hormone and tri-iodothyronine in hepatocyte cultures from normal and hypophysectomized rats. Biochem. J. 1989, 258, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Aiston, S.; Trinh, K.Y.; Lange, A.J.; Newgard, C.B.; Agius, L. Glucose-6-phosphatase overexpression lowers glucose 6-phosphate and inhibits glycogen synthesis and glycolysis in hepatocytes without affecting glucokinase translocation. Evidence against feedback inhibition of glucokinase. J. Biol. Chem. 1999, 274, 24559–24566. [Google Scholar] [CrossRef]
- Hengstler, J.G.; Utesch, D.; Steinberg, P.; Platt, K.L.; Diener, B.; Ringel, M.; Swales, N.; Fischer, T.; Biefang, K.; Gerl, M.; et al. Cryopreserved primary hepatocytes as a constantly available in vitro model for the evaluation of human and animal drug metabolism and enzyme induction. Drug Metab. Rev. 2000, 32, 81–118. [Google Scholar] [CrossRef]
- Gebhardt, R.; Hengstler, J.G.; Müller, D.; Glöckner, R.; Buenning, P.; Laube, B.; Schmelzer, E.; Ullrich, M.; Utesch, D.; Hewitt, N.; et al. New hepatocyte in vitro systems for drug metabolism: Metabolic capacity and recommendations for application in basic research and drug development, standard operation procedures. Drug Metab. Rev. 2003, 35, 145–213. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Yu, J.; Mho, S.I.; Lee, H.L.; Lee, W.; Yee, S.T.; Lee, G.; Paik, M.J. Method optimization for rapid measurement of carbohydrate in plasma by liquid chromatography tandem mass chromatography. Bull. Korean Chem. Soc. 2013, 34, 1571–1574. [Google Scholar] [CrossRef][Green Version]
- Smith, C.M.; Nolan, C.K.; Edwards, M.A.; Hatfield, J.B.; Stewart, T.W.; Ferguson, S.S.; Lecluyse, E.L.; Sahi, J. A comprehensive evaluation of metabolic activity and intrinsic clearance in suspensions and monolayer cultures of cryopreserved primary human hepatocytes. J. Pharm. Sci. 2012, 101, 3989–4002. [Google Scholar] [CrossRef] [PubMed]
- Balla, A.; Cho, K.H.; Kim, Y.C.; Maeng, H.-J. Simultaneous determination of procainamide and n-acetylprocainamide in rat plasma by ultra-high-pressure liquid chromatography coupled with a diode array detector and its application to a pharmacokinetic study in rats. Pharmaceutics 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Naritomi, Y.; Terashita, S.; Kagayama, A.; Sugiyama, Y. Utility of hepatocytes in predicting drug metabolism: Comparison of hepatic intrinsic clearance in rats and humans in vivo and in vitro. Drug Metab. Dispos. 2003, 31, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.; Morris, T. Physiological parameters in laboratory animals and humans. Pharm. Res. 1993, 10, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.S.; Rowland, M. Hepatic clearance of drugs. I. Theoretical considerations of a “well-stirred” model and “parallel tube” model. Influence of hepatic blood flow, plasma and blood cell binding, and the hepatocellular enzymatic activity on hepatic drug clearance. J. Pharmacokinet. Biopharm. 1977, 5, 625–653. [Google Scholar] [CrossRef]
- Terra Gde, P.; Vinícius De Farias, M.; Trevisan, M.G.; Garcia, J.S. Evaluation of pancreatin stability through enzyme activity determination. Acta Pharm. 2016, 66, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Bissell, D.M.; Levine, G.A.; Bissell, M.J. Glucose metabolism by adult hepatocytes in primary culture and by cell lines from rat liver. Am. J. Physiol. 1978, 234, C122–C130. [Google Scholar] [CrossRef]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018, 128, 545–555. [Google Scholar] [CrossRef]
- Theytaz, F.; de Giorgi, S.; Hodson, L.; Stefanoni, N.; Rey, V.; Schneiter, P.; Giusti, V.; Tappy, L. Metabolic fate of fructose ingested with and without glucose in a mixed meal. Nutrients 2014, 6, 2632–2649. [Google Scholar] [CrossRef] [PubMed]
- Coss-Bu, J.A.; Sunehag, A.L.; Haymond, M.W. Contribution of galactose and fructose to glucose homeostasis. Metabolism 2009, 58, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Hoele, M.; Ghoos, Y.; Rutgeerts, P.; Vantrappen, G. Metabolism of erythritol in humans: Comparison with glucose and lactitol. Br. J. Nutr. 1993, 69, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Geidl-Flueck, B.; Gerber, P.A. Insights into the hexose liver metabolism-glucose versus fructose. Nutrients 2017, 9, 1026. [Google Scholar] [CrossRef] [PubMed]
- Khitan, Z.; Kim, D.H. Fructose: A key factor in the development of metabolic syndrome and hypertension. J. Nutr. Metab. 2013, 2013, 682673. [Google Scholar] [CrossRef] [PubMed]
- Raushel, F.M.; Cleland, W.W. The substrate and anomeric specificity of fructokinase. J. Biol. Chem. 1973, 248, 8174–8177. [Google Scholar] [PubMed]
- Mooradian, A.D.; Smith, M.; Tokuda, M. The role of artificial and natural sweeteners in reducing the consumption of table sugar: A narrative review. Clin. Nutr. ESPEN 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Pearlman, M.; Obert, J.; Casey, L. The association between artificial sweeteners and obesity. Curr. Gastroenterol. Rep. 2017, 19, 64. [Google Scholar] [CrossRef]
- Van Opstal, A.M.; Hafkemeijer, A.; van den Berg-Huysmans, A.A.; Hoeksma, M.; Mulder, T.P.J.; Pijl, H.; Rombouts, S.A.R.B.; van der Grond, J. Brain activity and connectivity changes in response to nutritive natural sugars, non-nutritive natural sugar replacements and artificial sweeteners. Nutr. Neurosci. 2019, 10, 1–11. [Google Scholar] [CrossRef]
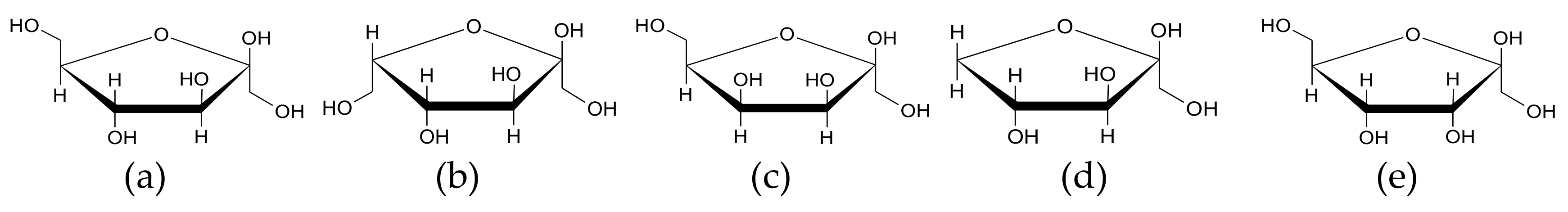
| Composition | SGF | FaSSIF |
|---|---|---|
| NaH2PO4 (mM) | – | 29 |
| NaCl (mM) | 34 | 106 |
| Sodium taurocholate (mM) | 0.08 | 3 |
| Lecithin (mM) | 0.02 | 0.75 |
| Pancreatin (mg/mL) | – | 10 |
| Pepsin (mg/mL) | 3.2 | – |
| pH | 1.6 | 6.5 |
| Parameters | Human | Rat |
|---|---|---|
| Number of hepatocyte (cells/g liver) | 120 × 106 | 120 × 106 |
| Liver weight (g liver/kg body weight) | 25.7 | 40 |
| Liver blood flow (mL/min/kg) | 20.7 | 55.2 |
| Analyte | Molecular Mass | MRM Transition | Collision Energy | Cell Accelerator Voltage |
|---|---|---|---|---|
| D-allulose | 180.156 | 179.0 → 89.0 | 2 | 3 |
| Fructose | 180.156 | 179.0 → 89.0 | 2 | 3 |
| Glucose | 180.156 | 179.0 → 89.0 | 2 | 3 |
| Erythritol | 122.120 | 121.0 → 71.0 | 10 | 3 |
| Salicin (IS) | 286.280 | 285.1 → 123.0 | 18 | 3 |
| Compound | CLint, in vitro (μL/min/106 cells) (Human/Rat) | CLint, in vivo (mL/min/kg) (Human/Rat) | Predicted CLh, in vivo (mL/min/kg) (Human/Rat) | Eh (Human/Rat) |
|---|---|---|---|---|
| D-allulose | NC 1/NC | NC/NC | NC/NC | NC/NC |
| Fructose | 2.46 ± 1.11/3.63 ± 0.60 | 7.58 ± 3.43/17.4 ± 2.89 | 5.37 ± 1.79/13.2 ± 1.70 | 0.260 ± 0.09/0.239 ± 0.03 |
| Erythritol | NC/NC | NC/NC | NC/NC | NC/NC |
| Parameters | D-allulose |
|---|---|
| t1/2 (min) | 72.2 ± 8.6 |
| AUClast (μg min/mL) | 6090 ± 1420 |
| AUCinf (μg min/mL) | 6720 ± 1590 |
| CL (mL/min/kg) | 15.8 ± 5.0 |
| Vss (mL/kg) | 1060 ± 429 |
| MRT (min) | 66.9 ± 14.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeng, H.-J.; Yoon, J.-H.; Chun, K.-H.; Kim, S.T.; Jang, D.-J.; Park, J.-E.; Kim, Y.H.; Kim, S.-B.; Kim, Y.C. Metabolic Stability of D-Allulose in Biorelevant Media and Hepatocytes: Comparison with Fructose and Erythritol. Foods 2019, 8, 448. https://doi.org/10.3390/foods8100448
Maeng H-J, Yoon J-H, Chun K-H, Kim ST, Jang D-J, Park J-E, Kim YH, Kim S-B, Kim YC. Metabolic Stability of D-Allulose in Biorelevant Media and Hepatocytes: Comparison with Fructose and Erythritol. Foods. 2019; 8(10):448. https://doi.org/10.3390/foods8100448
Chicago/Turabian StyleMaeng, Han-Joo, Jin-Ha Yoon, Kwang-Hoon Chun, Sung Tae Kim, Dong-Jin Jang, Ji-Eun Park, Yang Hee Kim, Seong-Bo Kim, and Yu Chul Kim. 2019. "Metabolic Stability of D-Allulose in Biorelevant Media and Hepatocytes: Comparison with Fructose and Erythritol" Foods 8, no. 10: 448. https://doi.org/10.3390/foods8100448
APA StyleMaeng, H.-J., Yoon, J.-H., Chun, K.-H., Kim, S. T., Jang, D.-J., Park, J.-E., Kim, Y. H., Kim, S.-B., & Kim, Y. C. (2019). Metabolic Stability of D-Allulose in Biorelevant Media and Hepatocytes: Comparison with Fructose and Erythritol. Foods, 8(10), 448. https://doi.org/10.3390/foods8100448








