Oligosaccharides Isolated from MGO™ Manuka Honey Inhibit the Adhesion of Pseudomonas aeruginosa, Escherichia Coli O157:H7 and Staphylococcus Aureus to Human HT-29 cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Oligosaccharide From Manuka Honey
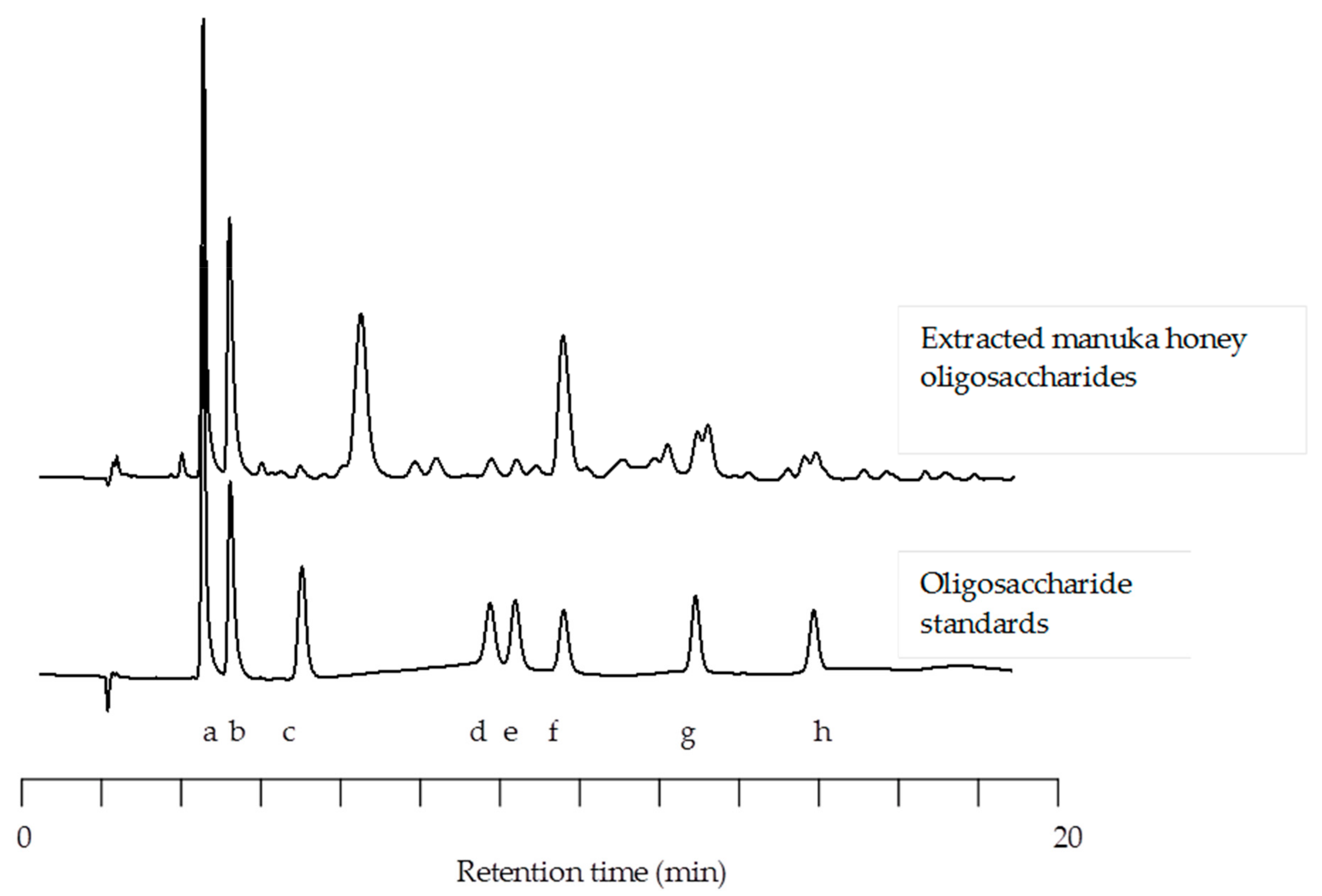
2.2. Analysis of Honey Oligosaccharides
2.2.1. Oligosaccharide Standards
2.2.2. High Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection (HPAEC-PAD)
2.3. Inhibition Studies
2.3.1. Bacterial Culture Conditions
2.3.2. Cell Culture Conditions
2.3.3. Anti-Infective Assay
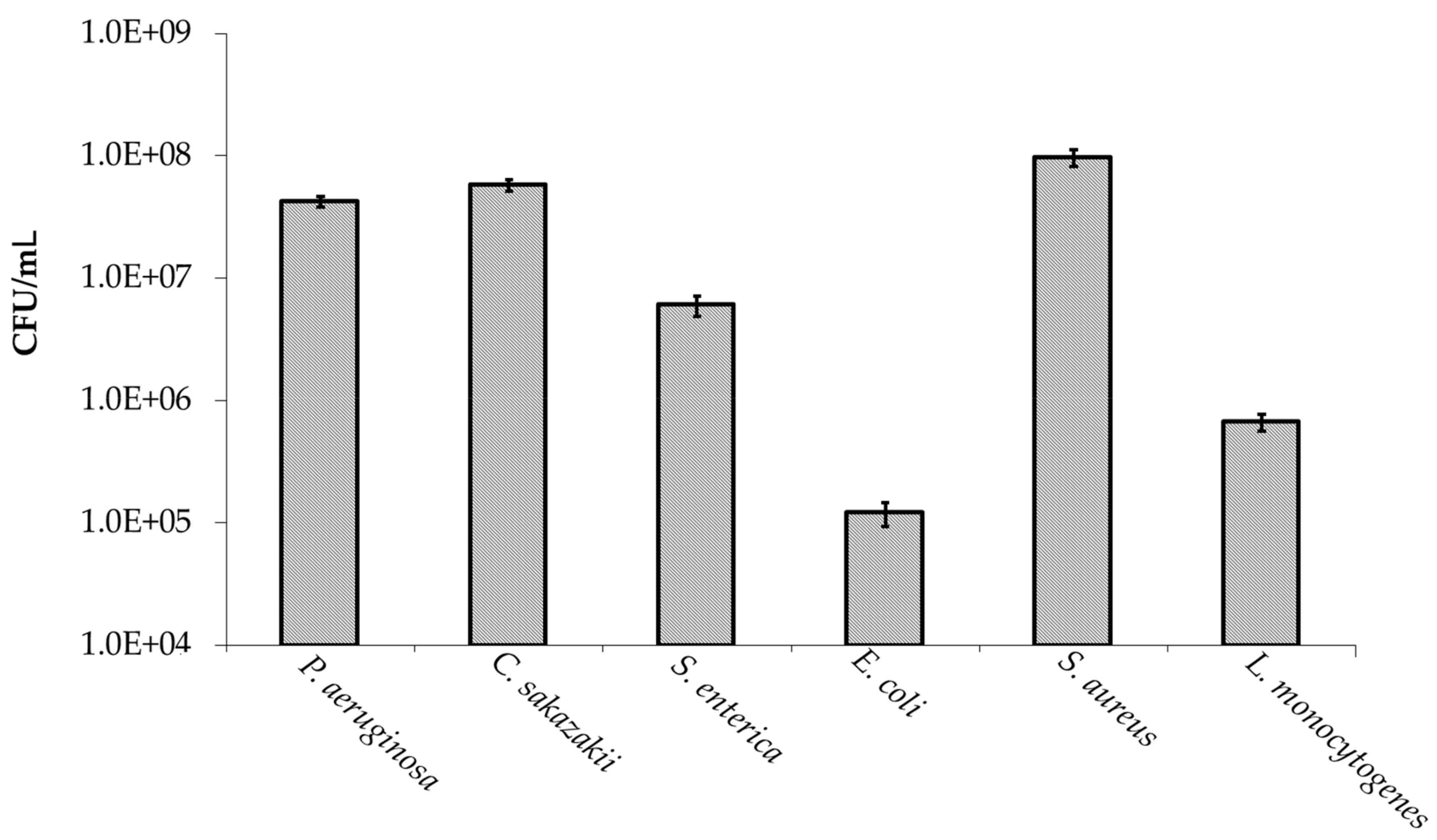
2.4. Effect of Honey Oligosaccharides on Bacterial Growth
2.5. Statistical Analysis
3. Results
3.1. HPLC of Manuka Honey Oligosaccharides
3.2. Anti-Adhesive Activity of Manuka Honey Oligosaccharides
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zumla, A.; Lulat, A. Honey-a remedy rediscovered. J. R. Soc. Med. 1989, 82, 384–385. [Google Scholar] [CrossRef]
- Bromfield, R. Honey for decubitus ulcers. JAMA 1973, 224, 905. [Google Scholar] [CrossRef] [PubMed]
- Bulman, M.W. Honey as a surgical dressing. Middx. Hosp. J. 1955, 55, 188–189. [Google Scholar]
- Cavanagh, D.; Beazley, J.; Ostapowicz, F. Radical operation for carcinoma of the vulva. A new approach to wound healing. J. Obstet. Gynaecol. Br. Commonw. 1970, 77, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Jeddar, A.; Kharsany, A.; Ramsaroop, U.G.; Bhamjee, A.; Haffejee, I.E.; Moosa, A. The antibacterial action of honey. An in vitro study. S. Afr. Med. J. Suid-Afrikaanse tydskrif vir geneeskunde 1985, 67, 257–258. [Google Scholar]
- Al-Waili, N.S.; Lootah, S.A.; Shaheen, W. Mixture of crude honey and olive oil in natural wax to treat chronic skin disorders. FASEB J. 1999, 13, A846. [Google Scholar]
- Al-Waili, N.S.; Lootah, S.A.; Shaheen, W. Honey preparation with olive oil and natural wax to treat skin fungal infections. FASEB J. 1999, 13, A848. [Google Scholar]
- Al-Waili, N.S.; Saloom, K.Y. Crude honey to treat seborrheic dermatitis of the scalp. FASEB J. 1999, 13, A848. [Google Scholar]
- Brudzynski, K.; Sjaarda, C. Honey glycoproteins containing antimicrobial peptides, jelleins of the major royal jelly protein 1, are responsible for the cell wall lytic and bactericidal activities of honey. PLoS ONE 2015, 10, e0120238. [Google Scholar] [CrossRef]
- Brudzynski, K.; Sjaarda, C.; Lannigan, R. Mrjp1-containing glycoproteins isolated from honey, a novel antibacterial drug candidate with broad spectrum activity against multi-drug resistant clinical isolates. Front. Microbiol. 2015, 6, 711. [Google Scholar] [CrossRef]
- Robson, V.; Cooper, R. Using leptospermum honey to manage wounds impaired by radiotherapy: A case series. Ostomy/Wound Manag. 2009, 55, 38–47. [Google Scholar]
- Robson, V.; Dodd, S.; Thomas, S. Standardized antibacterial honey (medihoney) with standard therapy in wound care: Randomized clinical trial. J. Adv. Nurs. 2009, 65, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Hentzer, M.; Eberl, L.; Nielsen, J.; Givskov, M. Quorum sensing—A novel target for the treatment of biofilm infections. Biodrugs 2003, 17, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Starkey, M.; Hazan, R.; Rahme, L.G. Honey’s ability to counter bacterial infections arises from both bactericidal compounds and QS inhibition. Front. Microbiol. 2012, 3, 144. [Google Scholar] [CrossRef]
- Gheldof, N.; Wang, X.H.; Engeseth, N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Ndip, R.N.; Clarke, A.M. Volatile compounds in honey: A review on their involvement in aroma, botanical origin determination and potential biomedical activities. Int. J. Mol. Sci. 2011, 12, 9514–9532. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Q.X. Chemical composition, characterization, and differentiation of honey botanical and geographical origins. Adv. Food Nutr. Res. 2011, 62, 89–137. [Google Scholar]
- Ball, D.W. The chemical composition of honey. J. Chem. Educ. 2007, 84, 1643–1646. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Mazzoni, L.; Giampieri, F. The composition and biological activity of honey: A focus on manuka honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Battino, M. Honey as a source of dietary antioxidants: Structures, bioavailability and evidence of protective effects against human chronic diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A. Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules 2014, 19, 2497–2522. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.L.; Panyoyai, N.; Kasapis, S.; Pang, E.; Mantri, N. Honey and its role in relieving multiple facets of atherosclerosis. Nutrients 2019, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.L.; Polemis, N.; Morales, V.; Corzo, N.; Drakoularakou, A.; Gibson, G.R.; Rastall, R.A. In vitro investigation into the potential prebiotic activity of honey oligosaccharides. J. Agric. Food Chem. 2005, 53, 2914–2921. [Google Scholar] [CrossRef]
- Jan Mei, S.; Mohd Nordin, M.S.; Norrakiah, A.S. Fructooligosaccharides in honey and effects of honey on growth of Bifidobacterium longum Bb 536 . Int. Food Res. J. 2010, 17, 557–561. [Google Scholar]
- Gluck, U.; Gebbers, J.O. Ingested probiotics reduce nasal colonization with pathogenic bacteria (Staphylococcus aureus, Streptococcus pneumoniae, and beta-hemolytic streptococci). Am. J. Clin. Nutr. 2003, 77, 517–520. [Google Scholar] [CrossRef]
- Johnson-Henry, K.C.; Mitchell, D.J.; Avitzur, Y.; Galindo-Mata, E.; Jones, N.L.; Sherman, P.M. Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Dig. Dis. Sci. 2004, 49, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Su, B.H.; Chen, A.C.; Lin, T.W.; Tsai, C.H.; Yeh, T.F.; Oh, W. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2005, 115, 1–4. [Google Scholar] [CrossRef]
- Lane, J.A.; Marino, K.; Naughton, J.; Kavanaugh, D.; Clyne, M.; Carrington, S.D.; Hickey, R.M. Anti-infective bovine colostrum oligosaccharides: Campylobacter jejuni as a case study. Int. J. Food Microbiol. 2012, 157, 182–188. [Google Scholar] [CrossRef]
- Mysore, J.V.; Wigginton, T.; Simon, P.M.; Zopf, D.; Heman-Ackah, L.M.; Dubois, A. Treatment of Helicobacter pylori infection in rhesus monkeys using a novel antiadhesion compound. Gastroenterology 1999, 117, 1316–1325. [Google Scholar] [CrossRef]
- Newburg, D.S.; Pickering, L.K.; Mccluer, R.H.; Cleary, T.G. Fucosylated oligosaccharides of human-milk protect suckling mice from heat-stabile enterotoxin of escherichia-coli. J. Infect. Dis. 1990, 162, 1075–1080. [Google Scholar] [CrossRef]
- Ruiz-Palacios, G.M.; Cervantes, L.E.; Ramos, P.; Chavez-Munguia, B.; Newburg, D.S. Campylobacter jejuni binds intestinal H(O) antigen (Fucα1, 2Galβ1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 2003, 278, 14112–14120. [Google Scholar] [CrossRef] [PubMed]
- Pita-Calvo, C.; Vazquez, M. Honeydew honeys: A review on the characterization and authentication of botanical and geographical origins. J. Agric. Food Chem. 2018, 66, 2523–2537. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Coppa, G.V.; Zampini, L.; Galeazzi, T.; Facinelli, B.; Ferrante, L.; Capretti, R.; Orazio, G. Human milk oligosaccharides inhibit the adhesion to Caco-2 cells of diarrheal pathogens: Escherichia coli, Vibrio cholerae, and Salmonella fyris. Pediatr. Res. 2006, 59, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Li, G.; Zhang, X.; Xu, H.; Abraham, S.N. A trp channel senses lysosome neutralization by pathogens to trigger their expulsion. Cell 2015, 161, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Molan, P.C. Authenticity of honey. In Food Authentication; Ashurst, P.R., Dennis, M.J., Eds.; Springer: New York, NY, USA, 1996; pp. 259–303. [Google Scholar]
- Mateo, R.; Bosch-Reig, F. Sugar profile of spanish unifloral honeys. Food Chem. 1997, 60, 33–41. [Google Scholar] [CrossRef]
- Morales, V.; Sanz, M.L.; Olano, A.; Corzo, N. Rapid separation on activated charcoal of high oligosaccharides in honey. Chromatographia 2006, 64, 233–238. [Google Scholar] [CrossRef]
- Ouchemoukh, S.; Schweitzer, P.; Bey, M.B.; Djoudad-Kadji, H.; Louaileche, H. HPLC sugar profiles of algerian honeys. Food Chem. 2010, 121, 561–568. [Google Scholar] [CrossRef]
- Weston, R.J.; Brocklebank, L.K. The oligosaccharide composition of some New Zealand honeys. Food Chem. 1999, 64, 33–37. [Google Scholar] [CrossRef]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef]
- Bentabol Manzanares, A.; Hernández García, Z.; Rodríguez Galdón, B.; Rodríguez Rodríguez, E.; Díaz Romer, C. Differentiation of blossom and honeydew honeys using multivariate analysis on the physicochemical parameters and sugar composition. Food Chem. 2011, 126, 664–672. [Google Scholar] [CrossRef]
- Golob, T.; Plestenjak, A. Quality of slovene honey. Food Tech. Biotechnol. 1999, 37, 195–202. [Google Scholar]
- Cotte, J.F.; Casabianca, H.; Chardon, S.; Lheritier, J.; Grenier-Loustalot, M.F. Application of carbohydrate analysis to verify honey authenticity. J. Chromat. A 2003, 1021, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Swallow, K.W.; Low, N.H. Analysis and quantitation of the carbohydrates in honey using high-performance liquid chromatography. J. Agric. Food Chem. 1990, 38, 1828–1832. [Google Scholar] [CrossRef]
- Lerrer, B.; Zinger-Yosovich, K.D.; Avrahami, B.; Gilboa-Garber, N. Honey and royal jelly, like human milk, abrogate lectin-dependent infection-preceding Pseudomonas aeruginosa adhesion. ISME J. 2007, 1, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.J.; Lowy, F.D. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 2008, 46, S350–S359. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.A.; Mehra, R.K.; Carrington, S.D.; Hickey, R.M. Development of biosensor-based assays to identify anti-infective oligosaccharides. Anal. Biochem. 2011, 410, 200–205. [Google Scholar] [CrossRef]
- Farrokh, C.; Jordan, K.; Auvray, F.; Glass, K.; Oppegaard, H.; Raynaud, S.; Thevenot, D.; Condron, R.; De Reu, K.; Govaris, A.; et al. Review of shiga-toxin-producing Escherichia coli (STEC) and their significance in dairy production. Int. J. Food Microbiol. 2013, 162, 190–212. [Google Scholar] [CrossRef]
- Callaway, T.R.; Carr, M.A.; Edrington, T.S.; Anderson, R.C.; Nisbet, D.J. Diet, Escherichia coli O157:H7, and cattle: A review after 10 years. Curr. Issues Mol. Biol. 2009, 11, 67–79. [Google Scholar]
- Martin-Sosa, S.; Martin, M.J.; Hueso, P. The sialylated fraction of milk oligosaccharides is partially responsible for binding to enterotoxigenic and uropathogenic Escherichia coli human strains. J. Nutr. 2002, 132, 3067–3072. [Google Scholar] [CrossRef]
- Feeney, S.; Ryan, J.T.; Kilcoyne, M.; Joshi, L.; Hickey, R. Glycomacropeptide reduces intestinal epithelial cell barrier dysfunction and adhesion of entero-hemorrhagic and entero-pathogenic Escherichia coli in vitro. Foods 2017, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Tamura, N.; Kobayashi-Hattori, K.; Yoshida, T.; Hara-Kudo, Y.; Ikedo, M.; Sugita-Konishi, Y.; Hattori, M. Prevention of intestinal infection by glycomacropeptide. Biosci. Biotechnol. Biochem. 2005, 69, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
 absence and
absence and  presence of manuka honey oligosaccharides. * p ≤ 0.05 was considered significant.
presence of manuka honey oligosaccharides. * p ≤ 0.05 was considered significant.
 absence and
absence and  presence of manuka honey oligosaccharides. * p ≤ 0.05 was considered significant.
presence of manuka honey oligosaccharides. * p ≤ 0.05 was considered significant.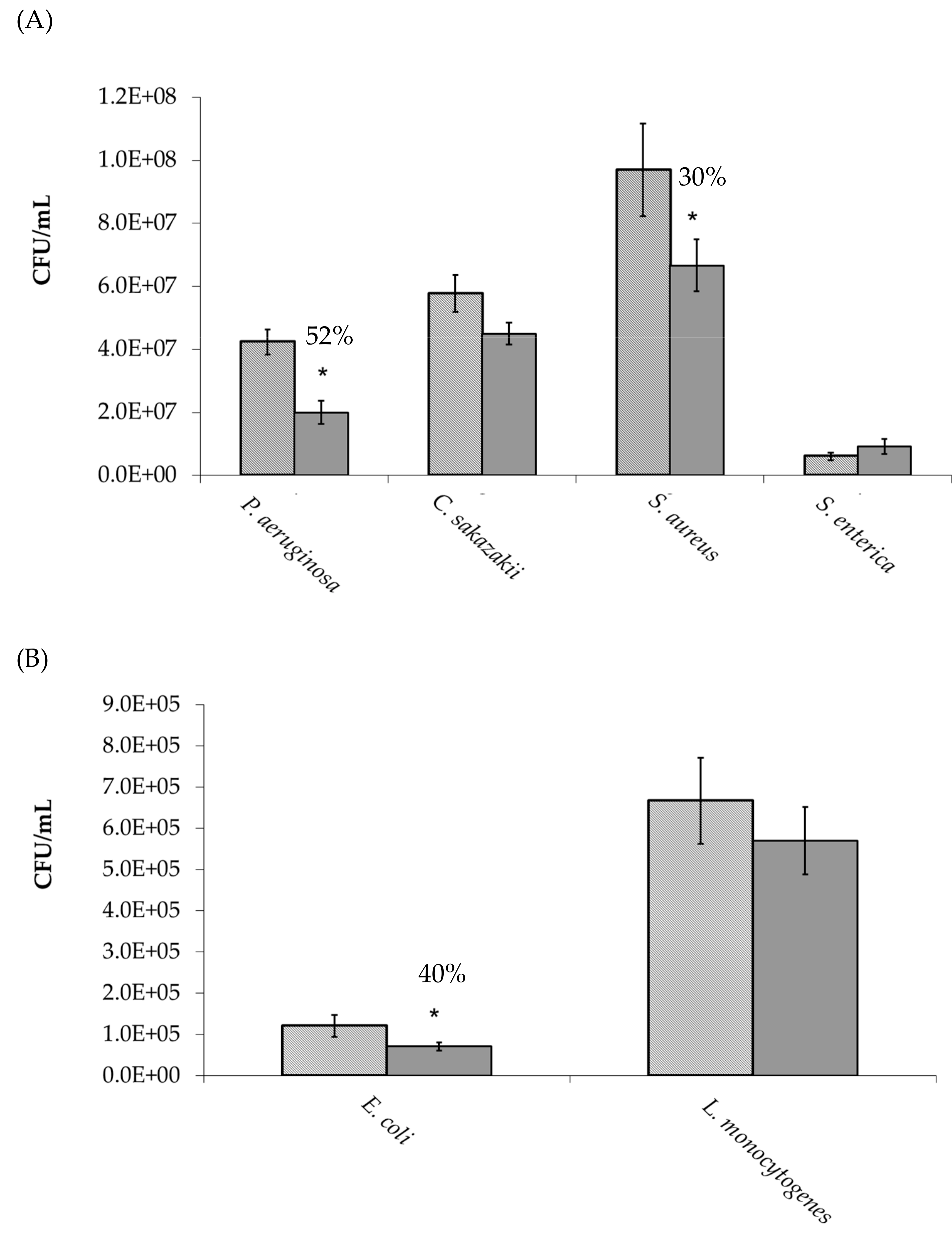
 absence and
absence and  presence of manuka honey oligosaccharides where no pre-incubation step was performed. * p ≤ 0.05 was considered significant.
presence of manuka honey oligosaccharides where no pre-incubation step was performed. * p ≤ 0.05 was considered significant.
 absence and
absence and  presence of manuka honey oligosaccharides where no pre-incubation step was performed. * p ≤ 0.05 was considered significant.
presence of manuka honey oligosaccharides where no pre-incubation step was performed. * p ≤ 0.05 was considered significant.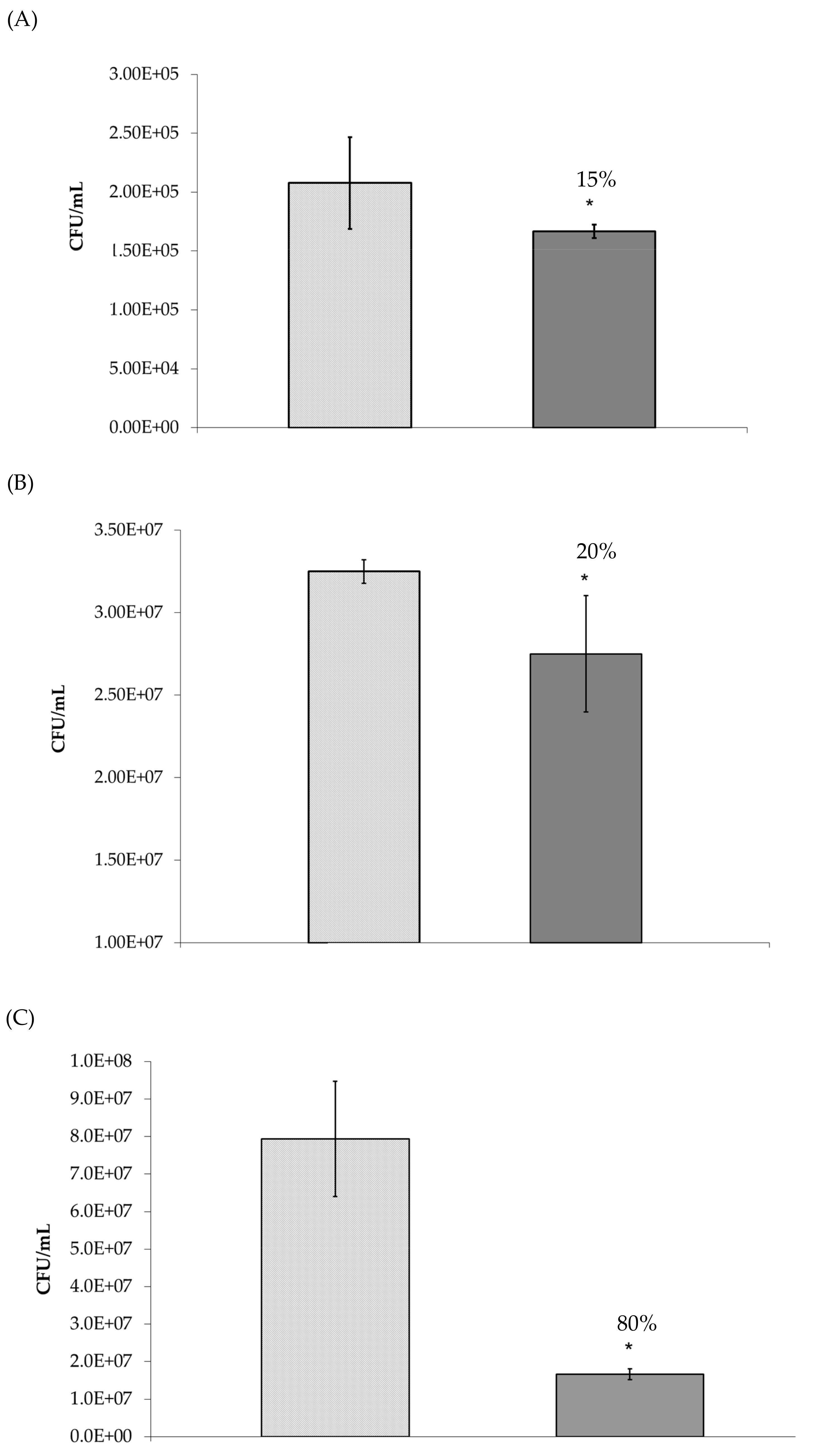
| Pathogens | Growth Media | Strain information |
|---|---|---|
| Staphylococcus aureus ATCC 29213 | BHI ** | Human wound isolate |
| Escherichia coli DPC *P1432 | BHI ** | Non-toxigenic E. coli O157:H7 strain |
| Salmonella enterica serovar Typhimurium ATCC BAA-185 | BHI ** | Pig isolate |
| Cronobacter sakazakii NCTC 08155 | BHI ** | Infant formula isolate |
| Listeria monocytogenes NCTC 5348 | BHI ** | Isolated from mammal cerebrospinal fluid |
| Pseudomonas aeruginosa ATCC 33354 | LB ** | Serotype 6 |
| Concentration | ||||
|---|---|---|---|---|
| 5 mg/mL | 2.5 mg/mL | 1.25 mg/mL | 0.625 mg/mL | |
| Pseudomonas aeruginosa | 46 ± 5.7 | 42 ± 10 | 34 ± 15 | - |
| Staphylococcus aureus | 51 ± 8.2 | 31 ± 10 | - | - |
| Escherichia coli O157:H7 | 40 ± 13 | 20 ± 2.0 | 1 ± 9.0 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lane, J.A.; Calonne, J.; Slattery, H.; Hickey, R.M. Oligosaccharides Isolated from MGO™ Manuka Honey Inhibit the Adhesion of Pseudomonas aeruginosa, Escherichia Coli O157:H7 and Staphylococcus Aureus to Human HT-29 cells. Foods 2019, 8, 446. https://doi.org/10.3390/foods8100446
Lane JA, Calonne J, Slattery H, Hickey RM. Oligosaccharides Isolated from MGO™ Manuka Honey Inhibit the Adhesion of Pseudomonas aeruginosa, Escherichia Coli O157:H7 and Staphylococcus Aureus to Human HT-29 cells. Foods. 2019; 8(10):446. https://doi.org/10.3390/foods8100446
Chicago/Turabian StyleLane, Jonathan A., Julie Calonne, Helen Slattery, and Rita M. Hickey. 2019. "Oligosaccharides Isolated from MGO™ Manuka Honey Inhibit the Adhesion of Pseudomonas aeruginosa, Escherichia Coli O157:H7 and Staphylococcus Aureus to Human HT-29 cells" Foods 8, no. 10: 446. https://doi.org/10.3390/foods8100446
APA StyleLane, J. A., Calonne, J., Slattery, H., & Hickey, R. M. (2019). Oligosaccharides Isolated from MGO™ Manuka Honey Inhibit the Adhesion of Pseudomonas aeruginosa, Escherichia Coli O157:H7 and Staphylococcus Aureus to Human HT-29 cells. Foods, 8(10), 446. https://doi.org/10.3390/foods8100446





